Abstract
Passage through the digestive tract exposes Salmonella enterica to high concentrations of bile salts, powerful detergents that disrupt biological membranes. Mutations in the wecD or wecA gene, both of which are involved in the synthesis of enterobacterial common antigen (ECA), render S. enterica serovar Typhimurium sensitive to the bile salt deoxycholate. Competitive infectivity analysis of wecD and wecA mutants in the mouse model indicates that ECA is an important virulence factor for oral infection. In contrast, lack of ECA causes only a slight decrease in Salmonella virulence during intraperitoneal infection. A tentative interpretation is that ECA may contribute to Salmonella virulence by protecting the pathogen from bile salts.
Bile salts are detergent-like substances that aid in the digestion and absorption of lipids. Bile salts are secreted from the liver, stored in the gall bladder, and released through the bile duct into the intestine during food passage. The most abundant bile salts in humans are cholate and deoxycholate (DOC). Enteric bacteria are intrinsically resistant to bile salts, due both to the low permeability of the outer membrane bilayer to these lipophilic solutes and to active efflux mechanisms. Mutations that impair bile salt resistance in genes for lipopolysaccharide (LPS) synthesis (14), tol genes (16), efflux pump genes (12, 22), regulatory genes such as marAB (20) and phoPQ (25), and the DNA adenine methyltransferase gene (8, 17) have been previously described.
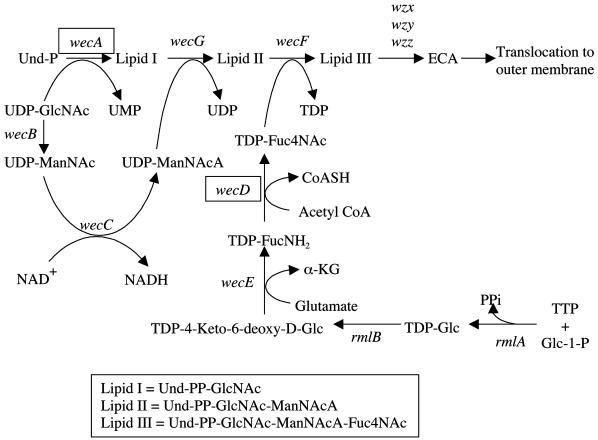
Here we show that mutations in the wecD and wecA genes of Salmonella enterica cause sensitivity to DOC. The wec gene cluster is required for synthesis of the enterobacterial common antigen (ECA), a glycolipid found in the external leaflet of the outer membrane in all species of the family Enterobacteriaceae (reviewed in reference 18). The ECA biosynthetic pathway is diagrammed in Fig. 1. The polysaccharide portion of ECA consists of a linear trimeric repeat with the following structure: →3)α-d-Fuc4NAc-(1→4)-β-d-ManNAcA-(1→4)-α-d-GlcNAc-(1→, where Fuc4NAc is 4-acetamido-4,6-dideoxy-d-galactose, ManNAcA is N-acetyl-d-mannosaminuronic acid, and GlcNAc is N-acetyl-d-glucosamine. The initial step in the synthesis of the repeat unit is the transfer of GlcNAc-1-phosphate from UDP-GlcNAc to undecaprenyl monophosphate to yield GlcNAc-pyrophosphorylundecaprenol (lipid I). The synthesis of lipid I is followed by the successive incorporation of ManNAcA and Fuc4NAc from the donors UDP-ManNAcA and TDP-Fuc4NAc to form lipid II and lipid III, respectively. Subsequent steps involve polymerization, transfer of the polymer to a phospholipid aglycone, and translocation to the outer membrane. Below we show that Salmonella mutants unable to synthesize ECA are highly attenuated in oral infections and slightly attenuated in intraperitoneal infections. Hence, we propose that ECA plays a role in Salmonella virulence. Such a role can be tentatively correlated with bile salt resistance.
FIG. 1.
The ECA biosynthetic pathway (based on reference 2). Individual genetic loci are shown next to each enzymatic reaction. Genes relevant for this work are boxed. Abbreviations: Und-P, undecaprenyl monophosphate; Glc, glucose; ManNAc, N-acetyl-d-mannosamine; Glc-1-P, glucose 1-phosphate; FucNH2, 4-amino-4,6-dideoxy-d-glucose; Fuc4NAc, 4-acetamido-4,6-dideoxy-d-galactose; CoASH, coenzyme A; α-KG, α-ketoglutaric acid; PPi, inorganic pyrophosphate.
MudJ mutagenesis identifies wecD as a locus necessary for resistance to DOC.
In an attempt to identify new genes potentially involved in bile salt resistance in S. enterica serovar Typhimurium, we performed a screen for mutants sensitive to sodium deoxycholate in strain 14028, which is virulent in mice. The strains used in this study are described in Table 1. MudJ mutagenesis was achieved by the method of Hughes and Roth (9). Five thousand Kmr colonies from 10 independent mutagenesis trials were patched in grids onto Luria-Bertani (LB) plates containing 1% DOC. Twenty-eight DOC-sensitive (DOCs) isolates were obtained. Reconstruction experiments showed that 19 of 28 DOCs isolates were resistant to lysis by P22 HT phage. These are probably LPS mutants, since P22 adsorbs to the O antigen (15), and Escherichia coli mutants lacking a complete O side chain in LPS (rough mutants) have been shown to display bile salt sensitivity (14). In addition, 3 out of 14 Salmonella mutants sensitive to bile salts obtained by Prouty et al. (16) had a rough LPS phenotype.
TABLE 1.
Strains used in this study
| Strain | Description | Source |
|---|---|---|
| 14028 | Wild type | ATCCb |
| TT10288 | hisD9953::MudJ his-9944::MudI | J. Roth |
| SV4429 | wecD::MudJ | This work |
| SV4759 | wecA::Tn10dTet | This worka |
| SV4801 | wecD::MudJ wecA::Tn10dTet | This work |
This mutant was constructed by P22 HT-mediated transduction of strain 14028 with a lysate from mutant SV2078 (6).
ATCC, American Type Culture Collection.
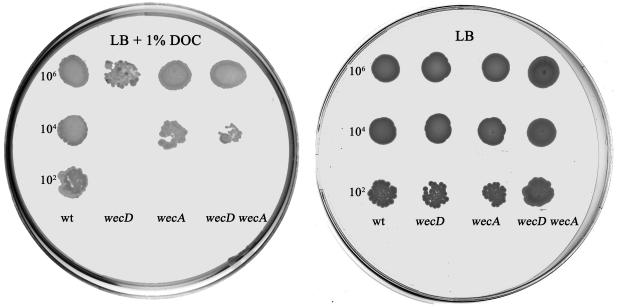
Chromosomal DNA from one DOCs P22-sensitive mutant was prepared as previously described (4), digested with BamHI, and ligated to plasmid pBluescript SK II(+). Transformants were selected on LB plates containing 40 μg of kanamycin/ml. DNA sequencing with the MuL primer (23) revealed that the insertion was located in wecD. This gene is part of the wec gene cluster, which is involved in the biosynthesis and assembly of ECA. Two lines of evidence confirmed that the wecD mutant was sensitive to DOC. (i) Dilutions from exponential cultures of the wecD mutant and the wild-type strain were spread on LB plates supplemented with 1% DOC. Clear-cut differences between the strains under study were found (Fig. 2). (ii) The MIC of DOC was determined for each strain. For MIC determination, samples of 3 × 103 CFU/ml from stationary-phase cultures were subjected to various concentrations of DOC in polypropylene microtiter plates (Greiner, Frickenhausen, Germany). After an overnight incubation at 37°C, the MIC for the wecD mutant was found to be 0.1%, compared to 4% for the wild type.
FIG. 2.
DOC sensitivity assay for wec mutants. Five-microliter portions of the appropriate dilutions of exponential cultures of the wild-type and mutant strains were incubated for 24 h at 37°C in an LB plate (right) or an LB plate containing 1% DOC (left). The approximate numbers of bacteria present in the original drops are indicated on the left of each plate.
DOC sensitivity of wec mutants is due to failure to synthesize ECA.
Studies with E. coli have reported that null mutations in wecE or wecF confer sensitivity to MacConkey agar (which contains bile salts) and that this phenotype is caused by accumulation of lipid II, an ECA biosynthetic intermediate (5). According to the same study, the wecE wecA and wecF wecA double mutants, which are unable to synthesize ECA but which do not accumulate lipid II, are able to grow on MacConkey agar. Results for the wecA single mutant were not shown in that study (5). A wecD mutant can be also expected to accumulate lipid II (Fig. 1). To investigate if the absence of ECA without accumulation of lipid II could cause DOC sensitivity in Salmonella, we tested a wecA mutant previously isolated in our laboratory (6, 11). The MIC determined for the wecA mutant was 0.2%, slightly higher than that for the wecD mutant but well below the MIC for the wild type (Fig. 2). A nearly identical MIC for the wecD wecA double mutant was obtained (Fig. 2). These experiments suggest that in Salmonella the absence of ECA, rather than the accumulation of lipid II, is the cause of DOC sensitivity.
S. enterica wec mutants are severely attenuated in the mouse model.
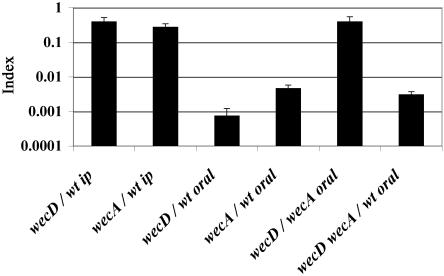
During a natural infection, Salmonella encounters DOC and other bile salts in the gut. Since wec mutants are 40-fold more sensitive to DOC than the wild type, we reasoned that wec mutations might cause attenuation specifically in orally inoculated mice. To test this hypothesis, 8-week-old female BALB/c mice (Charles River Laboratories, Santa Perpetua de Mogoda, Spain) were subjected to mixed infections with wec mutants. Groups of three or four animals were inoculated with a 1:1 ratio of the mutant and the wild type. For oral inoculation, bacteria were grown overnight at 37°C in LB without shaking. For intraperitoneal inoculation, bacteria were grown overnight at 37°C in LB with shaking, diluted into fresh medium (1:100), and grown until an optical density at 600 nm of 0.35 to 0.6 was reached. Oral inoculation was performed by feeding the mice 25 μl of saline containing 0.1% lactose and 108 bacteria. Intraperitoneal inoculation was performed with 0.2 ml of physiological saline containing 105 CFU. Bacteria were recovered from the spleen 48 h after intraperitoneal inoculation or 6 days after oral inoculation, and CFU were enumerated on selective medium (LB with 40 μg of kanamycin/ml for wecD and 20 μg of tetracycline/ml for wecA). A competitive index (CI) for each mutant, the ratio between the mutant and the wild-type strain in the output (bacteria recovered from the host after infection) divided by their ratio in the input (initial inoculum), was calculated (7, 21). The CI is a sensitive measure of the relative degree of virulence attenuation caused by a given mutation (3). wecD and wecA mutants were significantly outcompeted by the wild-type strain in both intraperitoneal and oral infections (Fig. 3). A detailed analysis of the CIs obtained indicates that both mutants are slightly attenuated in intraperitoneal infections (CI, 0.2 to 0.4) but severely attenuated in oral infections (CI < 0.005). The results obtained after intraperitoneal inoculations are consistent with an earlier report suggesting a small but significant difference in virulence between ECA-producing and ECA-deficient strains of S. enterica serovar Typhimurium (24). In that study, the difference in 50% lethal doses was about 10-fold. A subsequent study indicated that the apparently higher virulence of ECA-positive strains was due to their increased survival in mice and that this could be only partially attributed to their ECA-positive character (13). These data, together with the finding that ECA does not possess endotoxin-like activity (10) and the failure of anti-ECA antibodies to protect against salmonellosis (19), prompted the view that ECA was not a significant determinant of virulence (18). In contrast, our results with oral infections show that wec genes have a significant role in virulence. The different behaviors exhibited by wec mutants in oral and intraperitoneal experiments are in agreement with the hypothesis that ECA may be required for resistance to DOC in the small intestine.
FIG. 3.
CI analysis for wecD and wecA mutants. Shown is a graphical representation of CI analysis of strains carrying mutations in wecD, wecA, or both genes, after intraperitoneal (ip) or oral infections. The strains used in each mixed infection are indicated under the corresponding bars. The CIs are the means from at least three infections. Error bars represent the standard deviations. wt, wild-type strain.
Different degrees of attenuation in wecD and wecA mutants.
The analysis of CIs of wec mutants against the wild-type strain yields another interesting conclusion. Although both wecD and wecA mutants were significantly outcompeted by the wild-type strain in oral infections, the CI of the wecD mutant in oral infections was lower than the CI of the wecA mutant. Statistical analysis (Student's t test) showed that this difference was significant (P = 0.0009). In pursuit of a more precise comparison of wecD and wecA mutants, we tested them in a direct-competition experiment. The CI for the wecD mutant versus the wecA mutant in oral infections was 0.39 (Fig. 3), and statistical analysis indicates that this value is significantly different from 1 (P = 0.0008). This result confirms that a wecD mutant is more attenuated than a wecA mutant. One difference between these mutants is that the wecD strain is expected to accumulate lipid II (5) (Fig. 1). Altogether, these results suggest that ECA is an important virulence factor per se and that the accumulation of the lipid II intermediate might cause further attenuation. If this hypothesis is correct, a double mutant carrying null mutations in wecD and wecA should be as attenuated in oral infections as the wecA single mutant, since the double mutant does not accumulate lipid II. To test this prediction, we constructed a wecD wecA double mutant by P22 HT transduction and determined the CI value for this strain versus the wild type. Data shown in Table 2 and Fig. 3 indicate that the wecD wecA double mutant is highly attenuated in oral infections. However, the CI for this strain is significantly higher than the CI for the wecD single mutant and not significantly different from the CI for the wecA mutant (P values [Student's t test] of 0.0034 and 0.0952, respectively). These data support the view that lack of ECA causes a decrease in virulence and that a further decrease occurs if lipid II is accumulated.
TABLE 2.
CIs for wecD and wecA mutants obtained in mixed infections with the wild-type straina
| Strain | Mutant gene(s) | Intraperitoneal inoculation
|
Oral inoculation
|
||
|---|---|---|---|---|---|
| CI | P | CI | P | ||
| SV4429 | wecD | 0.39 | 0.0137 | 0.0007 | <0.0001 |
| SV4759 | wecA | 0.27 | 0.0030 | 0.0046 | <0.0001 |
| SV4801 | wecD, wecA | ND | ND | 0.003 | <0.0001 |
P values were obtained by Student's t test. The null hypothesis was a CI of 1. A P value ≤0.05 indicates that CI is significantly different from 1. ND, not determined.
Role of ECA in Salmonella virulence.
Despite the unique and universal occurrence of ECA in the family Enterobacteriaceae, its biological function remains unknown. Several lines of evidence presented in this study support a role for ECA in both the resistance to bile salts and the virulence of S. enterica serovar Typhimurium in the mouse model. Our results also suggest that both traits may be related, since the attenuation of wec mutants is more significant in oral than in intraperitoneal infections. A recent study (1) suggested that extracellular polysaccharides of uropathogenic E. coli are virulence determinants in the murine urinary tract. However, the CI displayed by a wecE mutant against the wild-type strain was not significantly different from 1 (which indicates no attenuation) for the bladder or the kidney and around the limit of significance for the urine (P = 0.05) (1). In contrast, the CI's displayed by S. enterica wecA and wecD mutants are extremely low and statistically significant, especially after oral inoculation (P < 0.0001). This high degree of attenuation is consistent with the failure of wec mutants to resist the bactericidal effect of bile salts. Neither defect can be attributed to the accumulation of lipid II since a wecA mutant, in which lipid II does not accumulate, is also attenuated in mice and sensitive to DOC.
Acknowledgments
This work was supported by grants QLRT-PL-00310 from the European Union and BIO2001-0232-C02-02 from the Ministry of Science and Technology of Spain. A stay of F.R.-M. at the Imperial College, London, was partially supported by a grant from the Regional Government of Andalusia. F.R.-M. is an investigator of the Ramón y Cajal program from the Ministry of Science and Technology of Spain.
REFERENCES
- 1.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. [DOI] [PubMed] [Google Scholar]
- 2.Barr, K., J. Klena, and P. D. Rick. 1999. The modality of enterobacterial common antigen polysaccharide chain lengths is regulated by o349 of the wec gene cluster of Escherichia coli K-12. J. Bacteriol. 181:6564-6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 3:1345-1352. [DOI] [PubMed] [Google Scholar]
- 4.Cano, D. A., M. Martinez-Moya, M. G. Pucciarelli, E. A. Groisman, J. Casadesus, and F. Garcia-Del Portillo. 2001. Salmonella enterica serovar Typhimurium response involved in attenuation of pathogen intracellular proliferation. Infect. Immun. 69:6463-6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese, P. N., G. R. Oliver, K. Barr, G. D. Bowman, P. D. Rick, and T. J. Silhavy. 1998. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J. Bacteriol. 180:5875-5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores, A., and J. Casadesus. 1995. Suppression of the pleiotropic effects of HisH and HisF overproduction identifies four novel loci on the Salmonella typhimurium chromosome: osmH, sfiW, sfiX, and sfiY. J. Bacteriol. 177:4841-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freter, R., P. C. O'Brien, and M. S. Macsai. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect. Immun. 34:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes, K. T., and J. R. Roth. 1988. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics 119:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer, H., and G. Schmidt. 1979. Chemistry and biology of the enterobacterial common antigen (ECA). Curr. Top. Microbiol. Immunol. 85:99-153. [DOI] [PubMed] [Google Scholar]
- 11.Mouslim, C., D. A. Cano, and J. Casadesus. 1998. The sfiX, rfe and metN genes of Salmonella typhimurium and their involvement in the Hisc pleiotropic response. Mol. Gen. Genet. 259:46-53. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido, H., M. Basina, V. Nguyen, and E. Y. Rosenberg. 1998. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those beta-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 180:4686-4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nnalue, N. A., and B. A. Stocker. 1987. The effects of O-antigen character and enterobacterial common antigen content on the in vivo persistence of aromatic-dependent Salmonella sp. live-vaccine strains. Microb. Pathog. 3:31-44. [DOI] [PubMed] [Google Scholar]
- 14.Picken, R. N., and I. R. Beacham. 1977. Bacteriophage-resistant mutants of Escherichia coli K-12. Location of receptors within the lipopolysaccharide. J. Gen. Microbiol. 102:305-318. [DOI] [PubMed] [Google Scholar]
- 15.Poteete, A. R. 1988. Bacteriophage P22, p. 647-682. In R. Calendar (ed.), The bacteriophages, vol. 2. Plenum Press, New York, N.Y.
- 16.Prouty, A. M., J. C. Van Velkinburgh, and J. S. Gunn. 2002. Salmonella enterica serovar Typhimurium resistance to bile: identification and characterization of the tolQRA cluster. J. Bacteriol. 184:1270-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pucciarelli, M. G., A. I. Prieto, J. Casadesus, and F. Garcia-del Portillo. 2002. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 148:1171-1182. [DOI] [PubMed] [Google Scholar]
- 18.Rick, P. D., and R. P. Silver. 1996. Enterobacterial common antigen and capsular polysaccharides, p. 104-122. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 19.Saxén, H., and M. Hovi. 1985. The effect of antibodies to the enterobacterial common antigen (ECA) on experimental mouse salmonellosis. FEMS Microbiol. Lett. 27:307-312. [Google Scholar]
- 20.Sulavik, M. C., M. Dazer, and P. F. Miller. 1997. The Salmonella typhimurium mar locus: molecular and genetic analyses and assessment of its role in virulence. J. Bacteriol. 179:1857-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torreblanca, J., and J. Casadesus. 1996. DNA adenine methylase mutants of Salmonella typhimurium and a novel dam-regulated locus. Genetics 144:15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valtonen, M. V., U. M. Larinkari, M. Plosila, V. V. Valtonen, and P. H. Makela. 1976. Effect of enterobacterial common antigen on mouse virulence of Salmonella typhimurium. Infect. Immun. 13:1601-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Velkinburgh, J. C., and J. S. Gunn. 1999. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect. Immun. 67:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]