Abstract
The response regulator OmpR is involved in numerous adaptive responses to environmental challenges. The role that OmpR plays in swarming behavior and swarm-cell differentiation in the symbiotic-pathogenic bacterium Xenorhabdus nematophila was examined in this study. Swarming began 4 h sooner in an ompR mutant strain than in wild-type cells. Precocious swarming was correlated with elevated expression of fliC, early flagellation, and cell elongation. The level of flhDC mRNA was elevated during the early period of swarming in the ompR strain relative to the level in the wild type. These findings show that OmpR is involved in the temporal regulation of flhDC expression and flagellum production and demonstrate that this response regulator plays a role in the swarming behavior of X. nematophila.
Xenorhabdus nematophila is a motile gram-negative bacterium belonging to the Proteus clade of the Enterobacteriaceae family (9, 10). Its life cycle is characterized by a symbiotic association with a soil nematode and by pathogenic interactions with different insect hosts. X. nematophila persists in the specialized gut sac of the entomopathogenic nematode Steinernema carpocapsae (10, 20). As X. nematophila transitions from the symbiotic stage in the nematode to the pathogenic stage, it must adapt to the hemocoel environment and the innate immune system of the insect. The regulatory pathways involved in adaptation to the insect hemocoel are not well understood. Bacterial adaptation to different environmental conditions involves two-component signal transduction pathways consisting of histidine kinase sensors and response regulators (22, 23). Histidine kinases sense environmental signals, undergo autophosphorylation, and subsequently transfer a phosphate group to a cognate response regulator. Sensor kinases can also possess phosphatase activity that stimulates the dephosphorylation of the cognate response regulator. In addition, response regulators can be phosphorylated by low-molecular-weight phosphodonors such as acetyl phosphate (21, 31). Modulation of the phosphorylated state of the response regulator controls the expression of the target genes.
The OmpR-EnvZ two-component system has been studied extensively in Escherichia coli and Salmonella. The response regulator OmpR is involved in regulating numerous cell functions, including the expression of ompF and ompC porin genes, motility, biofilm formation, adaptation to acidic conditions, and virulence (2, 18, 23, 25, 26, 30). In E. coli and Salmonella enterica serovar Typhimurium, ompF is expressed under low-osmolarity conditions, while ompF is repressed and ompC expression increases under high-osmolarity conditions. In contrast, expression of opnP, the ompF homolog of X. nematophila, requires OmpR, but OpnP is not osmoregulated (5, 11, 28). A shift to high-osmolarity conditions does not reduce OpnP production or affect the production of other outer membrane proteins (11). An ompC homolog has not been identified in X. nematophila, and inactivation of ompR does not affect the production of other outer membrane proteins besides OpnP (5). In E. coli, OmpR negatively regulates the master flagellar regulatory operon, flhDC (24, 26). However, inactivation of ompR did not affect flhDC expression in S. enterica serovar Typhimurium (17). Taken together, the above results indicate that OmpR may function differently in bacteria with different ecological and host niches.
To begin to elucidate the function of OmpR in the adaptation of X. nematophila to diverse environmental conditions, an ompR mutant strain (ABR2) was recently constructed (5). The ompR strain produced elevated levels of hemolysin activity and exhibited a hyperswarming phenotype. Swarming motility has been extensively studied in Proteus mirabilis (1, 3). Belas et al. (4) have shown that disruption of rsbA in the rcsC-rsbA-rcsB phosphorelay system of P. mirabilis results in precocious swarming behavior. RcsC and RcsB constitute a two-component system involved in the regulation of genes required for production of an extracellular polysaccharide capsule (27). In E. coli, the RsbA homolog, YojN, was found to possess a His-containing phosphotransfer domain which transfers the phosphate group from the histidine kinase RcsC to the response regulator RcsB (7, 29). Inactivation of yojN results in precocious swarming behavior in E. coli (29). The molecular details of how the RcsC-RsbA(YojN)-RcsB phosphorelay system affects swarming behavior remain unresolved. In the present study, we show that the ompR strain of X. nematophila exhibits precocious swarming behavior and enhanced flhDC expression. These results indicate that OmpR is involved in the temporal regulation of flhDC expression and swarming behavior in X. nematophila.
Precocious swarming in the ompR strain ABR2.
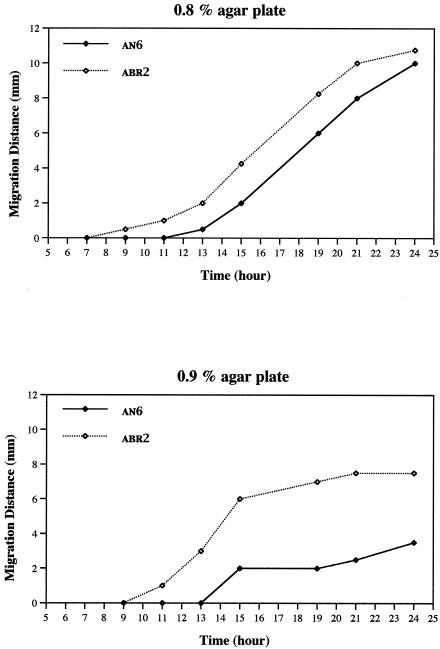
It was shown previously that swarm rings formed by the ompR strain ABR2 (AN6-ompR::Kanr) were significantly larger than those formed by the wild-type strain AN6 (5). The doubling rates of the wild-type strain and the ompR strain, as measured by both optical density and cell counting, were found to be comparable (5). Since the hyperswarming behavior of the ompR strain could be caused by several factors, including early initiation of swarming and/or an increased swarm rate, swarming motility was monitored continually from the initial spotting of the culture onto the agar plate (Fig. 1). Luria-Bertani (LB) agar plates containing 0.8% agar and 0.75% NaCl were found to be optimal for swarming behavior for X. nematophila (5). The agar plates were allowed to sit for 60 min at 29°C before the bacterial cultures were spotted onto the surface of the agar. A 6-μl drop of a stationary-phase culture (optical density at 600 nm, 7.5) was applied to the agar surface and subsequently incubated at 29°C. The number of CFU of the stationary-phase culture of the ompR strain was determined to be slightly less (∼80%) than that of the wild-type strain. A visible bacterial colony forms by approximately 5 h after application of the cultures to the agar surface. Swarming migration was monitored by measuring the movement of the swarm front away from the edge of the bacterial colony.
FIG. 1.
Comparison of the swarming motilities of AN6 and ABR2. The width of the swarm ring for the wild-type strain (AN6) and the ompR strain (ABR2) was measured from the edge of the bacterial colony over a 23-h period. This experiment was repeated four times, giving closely similar results. A representative experiment is shown.
The AN6 strain began to swarm by 13 h after spotting onto the agar plate (Fig. 1). In contrast, ABR2 began swarming by 9 h after spotting, 4 h earlier than the wild-type strain. During the migration period, there was no detectable difference between the swarm rates of the AN6 and ABR2 strains. To determine whether precocious swarming also occurred at higher agar concentrations, cells were spotted onto plates containing 0.9% agar (Fig. 1). The ABR2 strain started to swarm 11 h after spotting, while the AN6 strain began swarming at approximately 14 h. Thus, while swarm migration commenced later and the swarm rings were smaller for both strains when grown on the 0.9% agar plates, the ompR strain initiated swarming earlier than the wild-type cells.
Early swarm-cell differentiation and flagellation in ABR2.
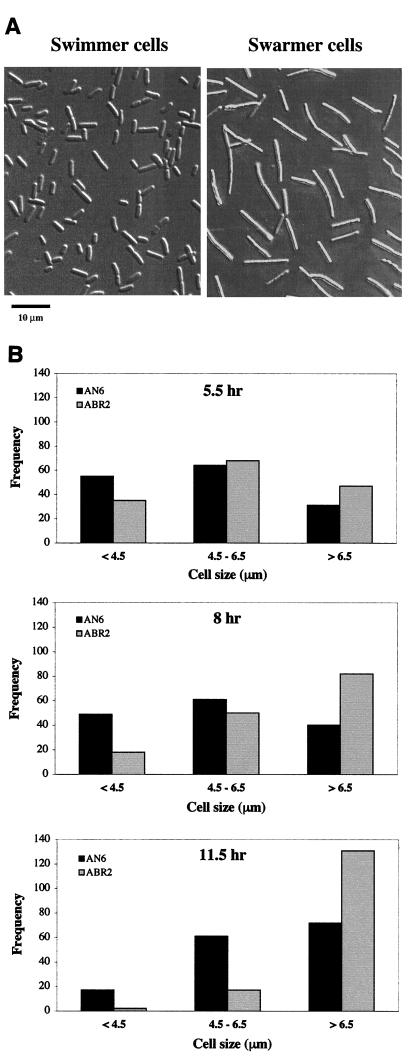
To determine whether precocious swarming in the ompR strain was correlated with early swarm-cell differentiation, the cell morphology of swarmer and swimmer cells was examined by Nomarski microscopy. Since swarmer-cell differentiation had not been characterized in X. nematophila, we first examined the differences in the morphologies of swarmer and swimmer cells in the wild-type strain. To obtain fully differentiated swarmer cells, 6 μl of a stationary-phase culture was spotted onto a 0.8% agar plate and incubated for 15 h. Cells were scraped from the outer edge of the swarm ring and suspended in LB broth. Swimmer cells were collected from late-log-phase LB broth cultures. Wild-type swimmer cells were short rods with an average cell length of 4.0 ± 0.1 μm, while the swarmer cells were elongated, with an average cell length of 10.0 ± 0.6 μm (Fig. 2A).
FIG. 2.
Swarm cell differentiation. (A) Swimming cells and fully differentiated swarm cells were viewed under 630× magnification. (B) The frequency of size distribution of swimmer cells (<4.5 μm),intermediate cells (4.5 to 6.5 μm), and elongated swarmer cells (>6.5 μm) was assessed by measuring 150 individual cells viewed under 630× magnification.
While swarmer-cell elongation is typical in enteric bacteria, the 2.5-fold elongation of the swarmer cells of X. nematophila was not as dramatic as in P. mirabilis, in which swarmer cells are approximately 40-fold longer than the vegetative swimmer cells (12). The cell length of the ompR swarmer cells at the 15-h time point was indistinguishable from that of the wild-type strain (data not shown), indicating that ompR function is not essential for the formation of the fully differentiated swarmer cell in X. nematophila. The cell length of the ompR swimmer cells was also found to be the same as that of the AN6 swimmer cells (data not shown).
To monitor the progression of AN6 and ABR2 cell elongation, the lengths of individual cells obtained from the edge of the swarm colony were viewed over time by Nomarski microscopy (Fig. 2B). During the early phase of growth, the edge of the bacterial colony contains cells that are differentiating from short rods into the elongated swarmer cells, resulting in a heterogeneous mixture of cell lengths. At 5.5 h, more ABR2 than AN6 cells had a cell length of >6.5 μm, while more AN6 cells were <4.5 μm long. By 8 h, the number of ABR2 cells that were >6.5 μm had increased, while the number of shorter rods (<4.5 μm) had decreased. At this time, the proportion of AN6 cells that were <4.5 μm was still larger than the proportion of cells that were >6.5 μm. At 11.5 h, most of the ABR2 cells were elongated (>6.5 μm), while the AN6 cells were distributed between the intermediate (4.5 to 6.5 μm) and elongated size classes. Finally, by 15 h, the AN6 cells had elongated into the swarmer cell type (Fig. 2A). These findings indicate that differentiation into the elongated swarmer cell occurred earlier in the ABR2 cells than in the AN6 cells. Thus, the precocious swarming behavior in the ABR2 strain (Fig. 1) was correlated with early differentiation into the swarmer cell type.
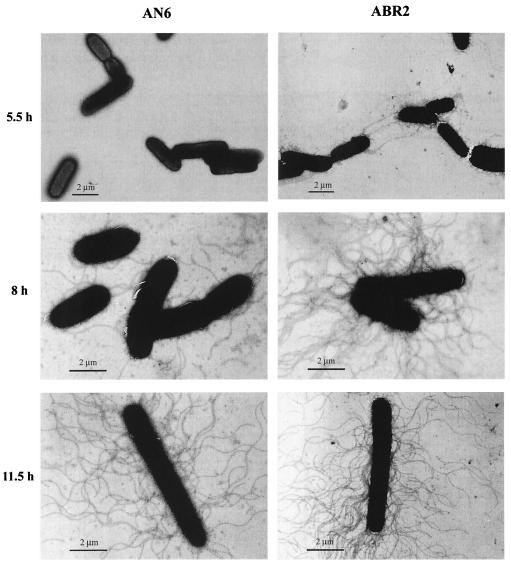
To examine flagellation during swarm-cell differentiation, cells were viewed by transmission electron microscopy. Cells were allowed to settle for 30 s, followed by negative staining with 0.5% phosphotungstic acid for 30 s. Figure 3 shows that ABR2 was flagellated by 5.5 h after spotting onto the agar surface, while very few flagellated AN6 cells were detected at this time point. Approximately 90% of the ABR2 cells viewed possessed flagella. At 8 h, ABR2 cells were heavily flagellated, while the AN6 cells displayed considerably fewer flagella. By 11.5 h, both cells were heavily flagellated to a similar extent. The hyperflagellation of the X. nematophila swarmer cells was similar to the that of P. mirabilis swarmer cells, where flagellum density increases 50-fold relative to that of the vegetative swimmer cell (12, 13).
FIG. 3.
Precocious flagellation of the ABR2 cells. The AN6 and ABR2 cells obtained at the indicated times were viewed by transmission electron microscopy. Over 100 fields were viewed for each time point. A representative field is shown.
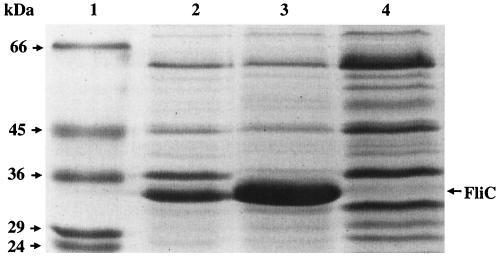
Early flagellation in the ompR strain was examined further by measuring the production of the major flagellar subunit, FliC, in cells grown on 0.8% agar swarm plates (Fig. 4). A total of 40 spots of 6 μl each of either AN6 or ABR2 stationary phase-cells, normalized to equivalent cell density, were applied to the surface of the agar swarm plate and incubated at 29°C for 5.5 h. A flhC strain, which does not produce flagella, was also included as a control. Cells were harvested in 2 ml of phosphate-buffered saline, and 1.5 ml of each strain was vortexed vigorously for 5 min. Cells were pelleted, and the resulting supernatant containing released flagella was centrifuged at high speed (353,000 × g) for 14 min in a TL100 Beckman ultracentrifuge. The flagellar pellets were solubilized in 30 μl of sodium dodecyl sulfate loading buffer, boiled for 5 min, and applied to a sodium dodecyl sulfate-15% polyacrylamide gel. As shown in Fig. 4, the level of FliC produced in the ABR2 strain (lane 3) was significantly greater than that produced in the AN6 strain (lane 2). As expected, FliC was not produced in the flhC strain (lane 4). Taken together, these findings indicate that inactivation of ompR in X. nematophila resulted in early swarm-cell differentiation, as characterized by early flagellation and cell elongation.
FIG. 4.
Overproduction of FliC in the ABR2 strain. Cells were grown on LB swarm plates for 5.5 h. Lanes: 1, molecular mass markers; 2, FliC obtained from AN6; 3, FliC obtained from ABR2; 4, proteins released from the flhC strain (AN6-flhC::kan). FliC has a predicted molecular mass of 33.3 kDa (9). This experiment was repeated five times, with similar results.
Early expression of flhDC and fliC in the ABR2 strain.
To address the question of whether early swarm-cell elongation and flagellation in ABR2 correlated with early expression of flhDC and fliC, a reverse transcriptase (RT)-PCR approach was taken. The nucleotide sequences of the flhDC (14) and fliCD operons (15) of X. nematophila are available. Primers were designed to flhD and fliC to amplify 501- and 657-bp RT-PCR products, respectively. Primers for 16S ribosomal DNA (rDNA) were used as the internal control for the RT-PCR. Total RNA from cells grown for 5.5 h on 0.8% agar was extracted with the RNeasy kit (Qiagen) and subsequently treated with RQ1 RNase-free DNase (Promega). One-step RT-PCR was performed with the AccessQuik RT-PCR Kit (Promega). First-strand cDNA synthesis was performed at 52°C for 45 min with 1 U of AMV-RT polymerase (Promega). The following cycle conditions were used for the PCR: 30 s at 94°C, 30 s at 55°C, and 60 s at 72°C. The number of cycles varied according to the level of expression of the various mRNAs to ensure that the comparison was performed in the linear range of the amplification. The cycles used were as follows: 21 cycles for flhD, 17 cycles for 16S rDNA, and 15 cycles for fliC. The RT-PCR assay was repeated numerous times with serially diluted total RNA to ensure that it yielded quantitative results. A typical reaction contained 600 ng of total RNA.
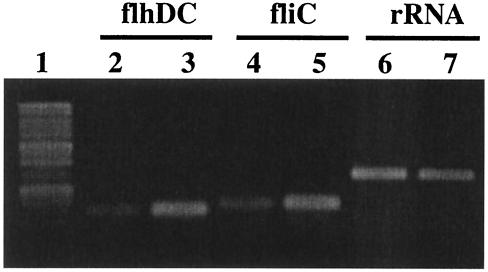
Figure 5 shows the amount of the flhDC and fliC RT-PCR products obtained from total RNA derived from AN6 (lanes 2, 4, and 6) and ABR2 (lanes 3, 5, and 7). The ABR2 strain expressed significantly higher levels of both flhD and fliC mRNA than AN6 at the 5.5-h premigration period. Thus, in the absence of OmpR, flhDC expression is elevated during the premigration period, which in turn promotes precocious synthesis of flagella. The precocious production of flagella would account for the early swarming behavior in the ABR2 strain. Since FlhD has been shown to negatively affect cell division (24), elevated FlhDC levels may also participate in early swarmer cell elongation in the ABR2 strain. It is of interest that expression of flhDC is dramatically elevated in the elongated swarmer cells of P. mirabilis (13).
FIG. 5.
RT-PCR analysis of flhD and fliC levels in AN6 and ABR2 cells. Each reaction contained 600 ng of total RNA. A negative control reaction lacking reverse transcriptase was conducted for each primer set. Lanes: 1, base pair marker; 2, 4, and 6, RT-PCR products derived from AN6; 3, 5, and 7, RT-PCR products derived from ABR2.
Concluding remarks.
The present results show that OmpR is involved in the temporal control of flhDC expression and swarming motility in X. nematophila. FlhDC has been shown to be required for hemolysin and lipase production and full virulence in X. nematophila (6, 14) and for exoenzyme production in several other enteric bacteria (1, 14, 16, 19, 32). Based on these findings, we speculate that early in infection, OmpR-phosphate participates in the negative regulation of flhDC, thereby reducing flagellum production and exoenzyme secretion. Low levels of FlhDC would favor cell proliferation (24). As the bacteria grow, flhDC expression would increase (24), stimulating both motility and the secretion of exoenzymes. Secretion of hemolysins may serve to inactivate insect immunocompetent cells (6, 14), while the exoenzymes may break down macromolecules in the insect hemolymph, providing nutrients essential for continued nematode and bacterial growth. These and previous findings indicate that the EnvZ-OmpR and the RcsC-RsbA(YojN)-RcsB (4, 29) phosphorelay systems negatively regulate swarming behavior and exoenzyme secretion. It was recently shown that OmpR is required for yojN-rcsB expression in S. enterica serovar Typhimurium (8). Thus, besides its role in the regulation of flhDC, OmpR may affect swarming behavior and exoenzyme secretion by controlling the activity of other regulatory pathways.
Acknowledgments
We thank D. Saffarini, B. Prüß, and P. Prohinar for their critical reading of, and helpful comments on, the manuscript. We are grateful to H. Owen for her assistance in the electron microscopic aspects of this study.
This work was supported by National Institutes of Health grant GM58392 to S.F.
REFERENCES
- 1.Allison, C., H.-C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. [DOI] [PubMed] [Google Scholar]
- 2.Bang, I. S., B. H. Kim, J. W. Foster, and Y. K. Park. 2000. OmpR regulates the stationary-phase acid tolerance response of Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:2245-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belas, R. 1997. Proteus mirabilis and other swarming bacteria, p. 183-219. In J. Shapiro and M. Dworkin (ed.), Bacteria as multicellular organisms. Oxford University Press, New York, N.Y.
- 4.Belas, R., R. Schneider, and M. Melch. 1998. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J. Bacteriol. 180:6126-6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan, B., and S. A. Forst. 2002. Characterization of the pleiotropic phenotype of an ompR strain of Xenorhabdus nematophila. Antonie Van Leeuwenhoek 81:43-49. [DOI] [PubMed] [Google Scholar]
- 6.Brillard, J., C. Ribeiro, N. Boemare, M. Brehelin, and A. Givaudan. 2001. Two distinct hemolytic activities in Xenorhabdus nematophila are active against immunocompetent insect cells. Appl. Environ. Microbiol. 67:2515-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke, D. J., S. A. Joyce, C. M. Toutain, A. Jacq, and I. B. Holland. 2002. Genetic analysis of the RscC sensor kinase from Escherichia coli K-12. J. Bacteriol. 184:1204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detweiler, C. S., D. M. Monack, I. E. Brodsky, H. Mathew, and S. Falkow. 2003. virK, somA and rcsC are important for systemic Salmonella enterica serovar Typhimurium infection and cationic peptide resistance. Mol. Microbiol. 48:385-400. [DOI] [PubMed] [Google Scholar]
- 9.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus spp. and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 10.Forst, S., and D. Clarke. 2002. Bacteria-nematode symbiosis, p. 57-78. In R. Gaugler (ed.), Entomopathogenic nematology. CABI Publishing, Wallingford, United Kingdom.
- 11.Forst, S., J. Waukau, G. Leisman, M. Exner, and R. Hancock. 1995. Functional and regulatory analysis of the OmpF-like porin, OpnP, of the symbiotic bacterium Xenorhabdus nematophilus. Mol. Microbiol. 18:779-789. [DOI] [PubMed] [Google Scholar]
- 12.Fraser, G. M., and C. Hughes. 1999. Swarming motility. Curr Opin. Microbiol. 2:630-635. [DOI] [PubMed] [Google Scholar]
- 13.Furness, R. B., G. M. Fraser, N. A. Hay, and C. Hughes. 1997. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J. Bacteriol. 179:5585-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Givaudan, A., and A. Lanois. 2000. flhDC, the flagellar master operon of Xenorhabdus nematophilus: requirement for motility, lipolysis, extracellular hemolysis, and full virulence in insects. J. Bacteriol. 182:107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Givaudan, A., A. Lanois, and N. Boemare. 1996. Cloning and nucleotide sequence of a flagellin encoding genetic locus from Xenorhabdus nematophilus: phase variation leads to differential transcription of two flagella genes (fliCD). Gene 183:243-253. [DOI] [PubMed] [Google Scholar]
- 16.Givskov, M., L. Eberl, G. Christiansen, M. J. Bendik, and S. Molin. 1995. Induction of phospholipase and flagella synthesis in Serratia liquefaciens is controlled by expression of the master operon flhD. Mol. Microbiol. 15:445-454. [DOI] [PubMed] [Google Scholar]
- 17.Kutsukake, K. 1997. Autogenous and global control of the flagellar master operon, flhD, in Salmonella typhimurium. Mol. Gen. Genet. 254:440-448. [DOI] [PubMed] [Google Scholar]
- 18.Lee, A. K., C. S. Detweiler, and S. Falkow. 2000. OmpR regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J. Bacteriol. 182:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, J., M. Lai, S. Ang, J. Shu, P. Soo, Y. Horng, W. Yi, H. Lai, K. Luh, S. Ho, and S. Swift. 2000. Role of flhDC in the expression of the nuclease gene nucA, cell division and flagella synthesis in Serratia marcescens. J. Biomed. Sci. 7:475-483. [DOI] [PubMed] [Google Scholar]
- 20.Martens, E. C., K. Heugens, and H. Goodrich-Blair. 2003. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J. Bacteriol. 185:3147-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCleary, W. R., J. B. Stock, and A. J. Ninfa. 1993. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkinson, J. S. 1993. Signal transduction schemes of bacteria. Cell 73:857-871. [DOI] [PubMed] [Google Scholar]
- 23.Pratt, L. A., and T. J. Silhavy. 1995. Porin regulon of Escherichia coli, p. 105-127. In J. A. Hoch, and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 24.Prüß, B. M. 1998. Acetyl phosphate and the phosphorylation of OmpR are involved in the regulation of the cell division rate in Escherichia coli. Arch. Microbiol. 170:141-146. [DOI] [PubMed] [Google Scholar]
- 25.Robert, D. L., and S. A. Forst. 1994. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res. Microbiol. 145:363-373. [DOI] [PubMed] [Google Scholar]
- 26.Shin, A., and C. Park. 1995. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 177:4696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stout, V. 1994. Regulation of capsule synthesis includes interactions of the RcsC/RcsB regulatory pair. Res. Microbiol. 145:389-392. [DOI] [PubMed] [Google Scholar]
- 28.Tabatabai, N., and S. A. Forst. 1995. Molecular analysis of the two-component genes, ompR and envZ, in the symbiotic bacterium Xenorhabdus nematophilus. Mol. Microbiol. 17:643-652. [DOI] [PubMed] [Google Scholar]
- 29.Takeda, S., Y. Fujisawa, M. Matsubara, H. Aiba, and T. Mizuno. 2001. A novel feature of the multistep phosphorelay in Escherichia coli: a revised model of the RcsC>YojN>RcsB signaling pathway implicated in capsular synthesis and swarming behavior. Mol. Microbiol. 40:440-450. [DOI] [PubMed] [Google Scholar]
- 30.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanner, B. L., and M. R. Wimes-Riesenberg. 1992. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J. Bacteriol. 174:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagella export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]