Abstract
In gram-negative bacteria, coexpression of single- and multicomponent efflux pumps may result in multiplicative enhancement of the level of resistance against cytoplasmically acting antibiotics. Here, a simple model is presented to show that this cooperative effect can be accounted for only if substrate capture by the multicomponent efflux transporter occurs in the periplasm but not the cytosol.
Multicomponent efflux pumps, exemplified by the Escherichia coli AcrAB system (5), provide an important means of drug (9) and solvent (11) resistance in gram-negative bacteria. These systems span both the inner and the outer membranes and the periplasm (14), and they were initially assumed to capture substrates in the cytoplasm and directly export them across both membranes. However, recent functional (2, 6, 12) and structural (4, 8, 13) data favor the view that substrate capture may actually occur in the periplasm, thus accounting for the fact that these systems may confer resistance against beta-lactam antibiotics. Nevertheless, since AcrAB also mediates resistance to cytoplasmically acting and highly membrane-permeative drugs, such as chloramphenicol and tetracyclines (10), the possibility remains that the latter might be captured from the cytoplasm, thus providing a dual route of substrate efflux.
A second type of efflux transporter is represented by single-component pumps located in the inner membrane, such as TetA (7) and CmlA (1). In a very thorough study on the effects of coexpressing various combinations of efflux transporters (3), Lomovskaya and colleagues found that the combination of a multicomponent efflux transporter with a single-component pump (with overlapping drug specificity) resulted in multiplicative enhancements of the levels of drug resistance. In contrast, combinations of like types of transporters resulted in additive levels of drug resistance only. This pattern was consistently observed with several combinations and expression levels, with both E. coli and Pseudomonas aeruginosa, and with both chloramphenicol and tetracycline.
To explain the observed multiplicative enhancement of drug resistance, the authors presented a model based on the different target compartments of pump action; they did not explicitly address the compartment of substrate capture by the multicomponent pumps. Here, it is shown that the observed multiplicative level of resistance can be accounted for only if substrate capture by the multicomponent pump occurs in the periplasm but not in the cytosol. This conclusion is based on the following assumptions. (i) Drug diffusion and active efflux are in dynamic equilibrium across both the inner and the outer membranes. (ii) The rates of efflux are proportional to the substrate concentration in the cell, i.e., [S] ≪ Km.
We will use the following definitions: Ccyt, Cpp, Cex, concentration of the antibiotic in the cytoplasm, periplasm, exterior; Di, Do, diffusion rates across the inner, outer membrane; di, do, diffusion rate constants for the inner, outer membrane; Pi, P2M, Po, efflux pumping rates across the inner membrane, both membranes, the outer membrane; pi, p2M, po, efflux rate constants across the inner membrane, both membranes, the outer membrane; these constants comprise both the number of the efflux pump molecules and their specific activity for the antibiotic in question; p1i, p2i, etc., efflux rate constants for individual pumps expressed in combinations.
Note that both diffusion and efflux will be treated in a simplified manner, since the area across which mass transfer occurs is the same for both processes and thus cancels out.
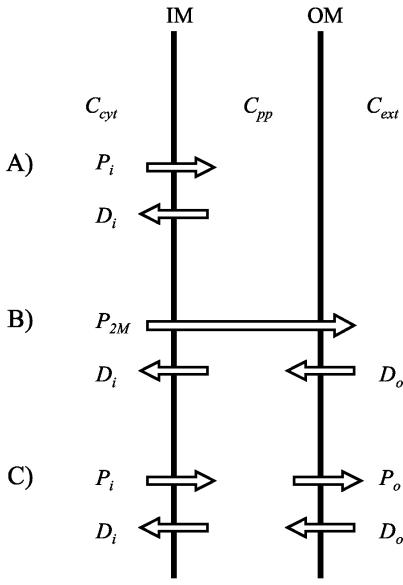
Let us first consider the simple case of a single-component efflux pump in the cytoplasmic membrane (cf. Fig. 1A). Here, the periplasmic substrate concentration will equal the exterior concentration:
 |
(1) |
FIG. 1.
Schematic of drug influx and efflux across the two membranes of the gram-negative cell. (A) A single-component efflux pump in the inner membrane. The symbols Pi and Dj represent efflux and diffusion, respectively, which will be equal at equilibrium. (B) A multicomponent efflux pump, with (hypothetical) substrate capture from within the cytoplasm. At equilibrium, the rate of efflux (P2M) equals diffusion across both the inner (Di) and the outer (Do) membrane. (C) A combination of a single-component pump with a multicomponent pump that captures its substrate from the periplasm. Equilibrium of pumping and diffusion rates exists separately for the two membranes; the pumps are working in series.
The rate of diffusion across the inner membrane will be proportional to the concentration gradient:
 |
(2) |
The rate of efflux will be given by
 |
(3) |
From equations 1 to 3 and the equilibrium condition (Di = Pi) we obtain
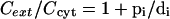 |
(4) |
For cytoplasmically acting antibiotics, Cext/Ccyt will be the factor of resistance conferred by the pump. If we combine two simple pumps (which work in parallel across the cytoplasmic membrane), it is easy to see that equation 4 will become
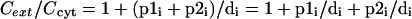 |
(4a) |
This is in line with the experimentally observed additive level of resistance conferred by two simultaneously expressed single-component pumps (3).
Next, let's consider a multicomponent pump, assuming substrate capture to occur exclusively in the cytosol (cf. Fig. 1B). At equilibrium, diffusion across the outer membrane will equal that across the inner membrane, and both will equal pumping:
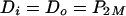 |
(5) |
Diffusion across the outer membrane will again depend on the concentration gradient:
 |
(6) |
Diffusion across the inner membrane will still conform to equation 2. With a P2M value of >0, this means that equation 1 will no longer apply; instead,
 |
(7) |
Since substrate capture is supposed to occur in the cytosol, the rate of efflux pumping will again be proportional to the cytoplasmic concentration:
 |
(8) |
From equations 2, 3, 5, 6, and 8 we obtain
 |
(9) |
For the case of combining two multicomponent pumps, we obtain
 |
(9a) |
Again, this agrees with the experimental finding of additive action of two different multicomponent efflux transporters combined (3).
Now, let's turn to the case of combining a single-component pump with a multicomponent pump, again assuming substrate capture by the multicomponent pump to occur exclusively in the cytoplasm. Direct export across both membranes will equal diffusion across the outer membrane:
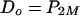 |
(10) |
The combined efflux from the cytoplasm mediated by the two pumps will equal diffusion from the periplasm into the cytoplasm:
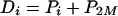 |
(11) |
The individual components of mass transfer will still obey the above equations 2, 3, 6, and 8. In conjunction with equations 10 and 11, we obtain
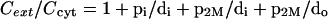 |
(12) |
By comparison with equations 4 and 4a and 9 and 9a above and from the example data in Fig. 2A, we see that this is just the additive effect of the two individual pumps. This is because both pumps, despite their different target compartments, capture the substrate from the same source compartment (the cytosol) and thus still will work in parallel rather than in series. For working in series with the simple efflux transporter, the multicomponent pump must capture its substrate from the periplasm. In that case, the relation expressed in equation 4 will hold for both the inner and outer membranes, respectively (cf. Fig. 1A and C):
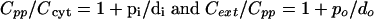 |
(13) |
from which we obtain
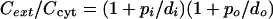 |
(14) |
FIG. 2.
Modeled antibiotic resistance levels for the combination of a multicomponent efflux pump with a single-component pump. The axis next to the solid arrow represents the level of resistance conferred by the multicomponent pump alone, while the dashed arrow indicates the resistance due to the single-component pump; the ranges have been selected arbitrarily. The axes are graded in units of fold increase of inhibitory drug concentrations conferred by the pumps. The surfaces represent the levels of resistance conferred by the two pumps combined. In panel A, substrate capture by the multicomponent pump is assumed to occur in the cytosol, and the area has been calculated using equation 12. In panel B, periplasmic substrate capture is assumed, and equation 14 has been used. Only periplasmic substrate capture results in a multiplicative action of the two pumps.
Figure 2B illustrates that this final formula indeed accounts for the experimentally documented multiplicative effect of coexpressing a single-component efflux pump with a multisubunit efflux pump (3). It thus turns out that only periplasmic substrate capture enables multisubunit-type efflux pumps to cooperate in a multiplicative manner with single-component transporters located in the inner membrane. Moreover, coexpression with a well-characterized pump should provide a useful experimental strategy for assessing the compartment of substrate capture for an uncharacterized extrusion system.
Acknowledgments
This work was supported by an Operating Research Grant from NSERC to M.P. (no. 250265-2003-GSC-032).
REFERENCES
- 1.Bissonnette, L., S. Champetier, J. P. Buisson, and P. H. Roy. 1991. Characterization of the nonenzymatic chloramphenicol resistance (cmlA) gene of the In4 integron of Tn1696: similarity of the product to transmembrane transport proteins. J. Bacteriol. 173:4493-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkins, C. A., and H. Nikaido. 2002. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J. Bacteriol. 184:6490-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, A., W. Mao, M. S. Warren, A. Mistry, K. Hoshino, R. Okumura, H. Ishida, and O. Lomovskaya. 2000. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J. Bacteriol. 182:3142-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lomovskaya, O., H. I. Zgurskaya, and H. Nikaido. 2002. It takes three to tango. Nat. Biotechnol. 20:1210-1212. [DOI] [PubMed] [Google Scholar]
- 5.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 6.Mao, W., M. S. Warren, D. S. Black, T. Satou, T. Murata, T. Nishino, N. Gotoh, and O. Lomovskaya. 2002. On the mechanism of substrate specificity by resistance nodulation division (RND)-type multidrug resistance pumps: the large periplasmic loops of MexD from Pseudomonas aeruginosa are involved in substrate recognition. Mol. Microbiol. 46:889-901. [DOI] [PubMed] [Google Scholar]
- 7.McMurry, L., R. E. Petrucci, Jr., and S. B. Levy. 1980. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc. Natl. Acad. Sci. USA 77:3974-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami, S., R. Nakashima, E. Yamashita, and A. Yamaguchi. 2002. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 419:587-593. [DOI] [PubMed] [Google Scholar]
- 9.Nikaido, H. 2001. Preventing drug access to targets: cell surface permeability barriers and active efflux in bacteria. Semin. Cell Dev. Biol. 12:215-223. [DOI] [PubMed] [Google Scholar]
- 10.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 12.Tikhonova, E. B., Q. Wang, and H. I. Zgurskaya. 2002. Chimeric analysis of the multicomponent multidrug efflux transporters from gram-negative bacteria. J. Bacteriol. 184:6499-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]
- 14.Zgurskaya, H. I., and H. Nikaido. 2000. Multidrug resistance mechanisms: drug efflux across two membranes. Mol. Microbiol. 37:219-225. [DOI] [PubMed] [Google Scholar]