Abstract
IncI1 plasmid R64 encodes a type IV pilus called a thin pilus, which includes PilV adhesins. Seven different sequences for the C-terminal segments of PilV adhesins can be produced by shufflon DNA rearrangement. The expression of the seven PilV adhesins determines the recipient specificity in liquid matings of plasmid R64. Salmonella enterica serovar Typhimurium LT2 was recognized by the PilVA′ and PilVB′ adhesins, while Escherichia coli K-12 was recognized by the PilVA′, PilVC, and PilVC′ adhesins. Lipopolysaccharide (LPS) on the surfaces of recipient cells was previously shown to be the specific receptor for the seven PilV adhesins. To identify the specific receptor structures of LPS for various PilV adhesins, R64 liquid matings were carried out with recipient cells consisting of various S. enterica serovar Typhimurium LT2 and E. coli K-12 waa mutants and their derivatives carrying various waa genes of different origins. From the mating experiments, including inhibition experiments, we propose that the GlcNAc(α1-2)Glc and Glc(α1-2)Gal structures of the LPS core of S. enterica serovar Typhimurium LT2 function as receptors for the PilVB′ and PilVC′ adhesins, respectively, while the PilVC′ receptor in the wild-type LT2 LPS core may be masked. We further propose that the GlcNAc(β1-7)Hep and Glc(α1-2)Glc structures of the LPS core of E. coli K-12 function as receptors for the PilVC and PilVC′ adhesins, respectively.
Bacterial adhesins are usually located at the tip of pili in gram-negative bacteria (7). Adhesins play key roles in the attachment of bacterial pathogens to host eukaryotic cells. They recognize and bind to specific receptors present on the host cells. For example, the PapG adhesin of P pili specifically binds to the Gal(α1-4)Gal moiety of glycolipids on the host cells.
Plasmid R64 encodes type IV pili, called thin flexible pili (9, 30). The PilV adhesins are thought to be located at the tips of the thin pili. They are unusual among bacterial adhesins, since the C-terminal segments of the PilV adhesins are exchanged by multiple DNA inversions of the shufflon (3, 11, 15) (Fig. 1A). The R64 shufflon consists of four DNA segments, which are separated and flanked by seven sfx sequences. Site-specific recombination is mediated by the rci product and occurs between any two inverted sfx sequences, resulting in the inversion of the four DNA segments independently or in groups. The sequence of the pilV gene encoding the seven C-terminal segments is joined in frame to the constant region of the pilV gene. Thus, the C-terminal segments of the PilV adhesins interconvert among seven types as a result of the shufflon DNA inversions. The constant region of a PilV adhesin consists of 361 amino acids and bears a structure similar to that of a type IV prepilin at the N terminus. The variable regions range between 69 and 113 amino acids, and no remarkable homology is found among them. To identify the biological significance of the shufflon, R64 derivatives in which the pilV gene encoding the seven C-terminal segments was fixed were constructed by inactivation of the rci gene (12). The seven PilV adhesins in donor cells were shown to determine the recipient specificity in R64 liquid matings (13, 14). The recipient Escherichia coli K-12 strain was recognized by the PilVA′, -C, and -C′ adhesins in liquid matings, while E. coli B was recognized only by the PilVA′ adhesin. Salmonella enterica serovar Typhimurium LT2, E. coli C, and Shigella flexneri were recognized by the PilVA′ and -B′, PilVA and -A′, and PilVA′ and -D′ adhesins, respectively.
FIG. 1.
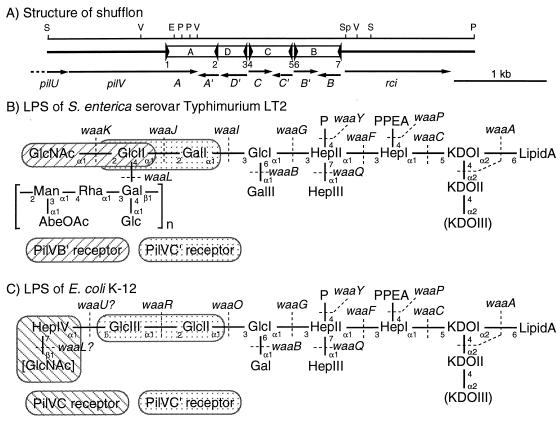
(A) Structure of the shufflon. The gene organization of pilU, pilV, the shufflon, and the rci region of plasmid R64 is shown below a restriction map. E, EcoRI; P, PstI; S, SspI; Sp, SphI; V, EcoRV. The panel represents an arrangement (corresponding to pKK010-85) of many isomers of the shufflon subjected to multiple DNA inversions. Open bars A, B, C, and D, shufflon segments; filled triangles, seven sfx sequences; arrows, coding sequences for pilU, pilV and the variable C-terminal segments encoded by it, and rci. (B and C) LPS molecules of S. enterica serovar Typhimurium LT2 (B) and E. coli K-12 (C) (2, 5, 22). Shown are the proposed LPS structures together with the genes encoding the enzymes thought to be responsible for the generation of each linkage. Brackets, O-antigen repeating units. In E. coli K-12 LPS, the terminal GlcNAc residue in brackets was shown to be an incomplete O-antigen moiety (2). The structure of the LPS core shows heterogeneity, as indicated by parentheses. Abbreviations: AbeOAc, O-acetylabecose; Gal, galactose; Glc, glucose; Hep, heptose; KDO, 3-deoxy-d-manno-2-octulosonic acid; Man, mannose; P, phosphate; PPEA, pyrophosphoethanolamine; Rha, rhamnose. The specific receptor structures for the PilVB′, PilVC, and PilVC′ adhesins in the LPS molecules are indicated.
To identify the specific PilV receptors in recipient bacterial cells, we previously performed R64 liquid matings with various E. coli K-12 waa mutants and E. coli B derivatives carrying the waa genes of E. coli K-12 as recipient cells (8). The E. coli K-12 waa mutants had lost the receptors for specific PilV adhesins. E. coli B cells carrying the waaR gene or waaRUL genes of E. coli K-12 were recognized by the PilVC′ adhesin or the PilVC and PilVC′ adhesins, respectively, in addition to the PilVA′ adhesin. Addition of E. coli K-12 or B lipopolysaccharide (LPS) to R64 mating mixtures specifically inhibited liquid matings with E. coli K-12 or B recipients, respectively. Thus, it is suggested that the thin pilus PilV adhesins of the R64 donor cells recognize LPS moieties located on the surfaces of various recipient bacterial cells.
The LPS structures of S. enterica serovar Typhimurium LT2 and E. coli K-12 are illustrated in Fig. 1B and C, respectively (2, 5, 22). LPSs consist of three parts: the hydrophobic lipid A region, the core oligosaccharide region, and the O-antigen polysaccharide region. The LPS of bacterial strains forming rough colonies lacks the O-antigen region. The LPS core oligosaccharides are synthesized through the successive addition of component sugars to lipid A by the products of the waa genes (5, 23). Then O-antigen is attached to the LPS core by the waaL product. Hence, waa mutants produce incomplete LPS molecules. The steps in the formation of LPS involving actions by these waa genes are indicated in Fig. 1B and C (5). Recently, the LPS structure of E. coli K-12 was finally established (2) (Fig. 1C). The terminal sugar moiety of the E. coli K-12 LPS is GlcNAc, which is considered to be an incomplete O-antigen subunit (2). E. coli K-12 carries the rml-wbb genes, which are involved in the synthesis of the O-antigen O16, but the wbbL gene, encoding rhamnose transferase, has been inactivated by IS5 insertion (17). It has been demonstrated that an incomplete O-antigen subunit (even a single sugar) can be attached to the LPS core by O-antigen ligase encoded by the waaL gene (2).
In this study, genetic analyses were performed to determine the specific receptor structures in LPSs for each PilV adhesin encoded by plasmid R64. Various S. enterica serovar Typhimurium LT2 waa mutants, producing defined chemotypes of mutant LPS, were used as recipient strains in liquid matings. Various serovar Typhimurium LT2 and E. coli K-12 derivatives which produced chimeric LPS molecules were constructed by introducing the waa+ genes from different origins and were used as the recipient cells in liquid matings.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Nalidixic acid- or rifampin-resistant derivatives of the listed S. enterica serovar Typhimurium LT2 strains were used as the recipients of conjugation.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Source or reference |
|---|---|---|
| Bacterial strains | ||
| S. enterica serovar Typhimurium LT2 | ||
| SL4213 | galE496 metA22 metE551 xyl-404 rpsL 120 hsdL6 hsdS29 | B. A. D. Stocker |
| SL3770 | waa+ | SGSCa |
| SA1355 | waa+ | SGSC |
| SL428 | wzy458 | SGSC |
| SA1627 | Δrml-his (wba) | SGSC |
| SL3749 | waaL446 | SGSC |
| SL733 | waaK953 | SGSC |
| RF733 | waaK::Tc | This study |
| SL3750 | waaJ417 | SGSC |
| SL3748 | waaI432 | SGSC |
| SL3769 | waaG471 | SGSC |
| E. coli K-12 | ||
| JM83 | ara Δ(lac-proAB) rpsL φ80d lacZΔM15 | 29 |
| W3110 | Prototoroph | Laboratory stock |
| TN102 | W3110 Nalr | 12 |
| CS2198 | waaR19::Tnlac Δlac | 20 |
| CS2334 | waaL1::TnphoA | 10 |
| CS2529 | waaU2::Km | 10 |
| Plasmids | ||
| pUC119 | Apr; lacZ′ | 27 |
| pCL1921 | Spr; lacZ′ | 16 |
| pHSG415 | Apr Kmr Cmr; pSC101 rep (Ts) | 4 |
| pKK661 | pHSG576 derivative carrying 35.6-kb R64 tra segment | 12 |
| pKK641A | pKK641 derivative carrying a fixed pilVA gene | 12 |
| pKK641A′ | pKK641 derivative carrying a fixed pilVA′ gene | 12 |
| pKK641B | pKK641 derivative carrying a fixed pilVB gene | 12 |
| pKK641B′ | pKK641 derivative carrying a fixed pilVB′ gene | 12 |
| pKK641C | pKK641 derivative carrying a fixed pilVC gene | 12 |
| pKK641C′ | pKK641 derivative carrying a fixed pilVC′ gene | 12 |
| pKK641D′ | pKK641 derivative carrying a fixed pilVD′ gene | 12 |
| pRF101 | 3.0-kb LT2 waaK+ fragment in pBR322 | This study |
| pRF102 | 2.1-kb LT2 waaL+ fragment in pBR322 | This study |
| pRF103 | 1.8-kb LT2 waaJ+ fragment in pUC119 | This study |
| pRF005 | 2.3-kb K-12 waaU+ fragment in pCL1921 | 8 |
| pRF006 | 2.7-kb K-12 waaL+ fragment in pCL1921 | 8 |
| pRF009 | 4.6-kb K-12 waaU+waaL+ fragment in pCL1921 | 8 |
SGSC, Salmonella Genetic Stock Center.
Media.
Luria-Bertani (LB) medium was prepared as previously described (24). Solid medium contained 1.5% agar. Antibiotics were added to the liquid and solid media at the following concentrations when necessary: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 50 μg/ml; nalidixic acid, 20 μg/ml; rifampin, 50 μg/ml; spectinomycin, 50 μg/ml; tetracycline, 15 μg/ml.
Recombinant DNA techniques.
Recombinant DNA techniques were performed as previously described (24). Plasmids containing the serovar Typhimurium LT2 waaL or waaK genes were constructed by modifying pKZ38 waaL+ waaK + (18). To remove the 0.8-kb MluI segment within waaL, pKZ38 DNA was digested with MluI and self-ligated, giving pRF101 (LT2 waaK+). To remove the 1.75-kb SnaBI-EcoRV segment within waaK, pKZ38 DNA was digested with SnaBI and EcoRV and self-ligated, giving pRF102 (LT2 waaL+). To clone the waaJ gene of serovar Typhimurium LT2, a 1.8-kb DNA segment was amplified by PCR using appropriate primers and chromosomal DNA as the template and inserted into pUC119, giving pRF103 (LT2 waaJ+).
To construct a waaK-null mutant, the LT2 waaK gene was first cloned into temperature-sensitive suicide vector pHSG415 (4). A tetracycline resistance gene was then inserted into the SnaBI site within waaK, and the resultant waaK::Tc allele was introduced into the SL3770 chromosome by a gene replacement method, giving RF733.
Conjugal transfer.
Liquid matings were performed as previously described (12). Two sets of donor cells were used: S. enterica serovar Typhimurium LT2 SL4213 cells harboring pKK661 and one of the seven pKK641 series plasmids were used in the experiments using serovar Typhimurium LT2 recipients; E. coli K-12 W3110 cells harboring pKK661 and one of the seven pKK641 series plasmids were used in the experiments using E. coli K-12 recipients. A log-phase culture (200 μl) of donor cells was mixed with an overnight culture (50 μl) of recipient cells. The mating mixture was incubated for 90 min at 37°C and plated at various dilutions on selective media. The transfer frequencies were calculated as the ratio of the number of transconjugants to the number of donor cells.
As specific inhibitors for liquid matings, Glc(α1-2)Glc (kojibiose) and Glc(α1-2)Gal were purchased from Wako (Osaka, Japan) and Toronto Research Chemicals (Toronto, Canada), respectively. LPS molecules were prepared from E. coli K-12 TN102 cells by the phenol-chloroform-petroleum ether method as described previously (8). To examine their effects on liquid matings, both the log-phase donor culture and overnight recipient culture were diluted 100-fold with LB broth before mixing and then inhibitors were added (8). At time intervals, aliquots of mating mixture were plated onto selective media.
Phage sensitivity analysis.
Phage sensitivity was analyzed by spotting phage stocks (P22, Felix O [FO], and Ffm) on lawns of various bacterial strains on LB agar plates.
Preparation and gel analysis of LPS.
Outer membrane fractions containing LPS molecules from various bacterial strains were prepared by the method of Schnaitman and McDonald (25). After treatment of the outer membranes with proteinase K, the residual fractions were used as LPS samples (21). The LPS samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining (6).
RESULTS AND DISCUSSION
Dependency of the frequency of transfer to various S. enterica serovar Typhimurium LT2 waa recipient strains on the seven PilV adhesins.
Since various E. coli K-12 waa mutants have been shown to lack receptors for one or two PilV adhesins (8), the effects of various mutations of S. enterica serovar Typhimurium LT2 waa on PilV recognition were examined. LT2 strains with waa, wzy, and wba mutations were used as the recipient strains for liquid matings of R64 derivatives expressing each of the seven PilV adhesins (Table 2).
TABLE 2.
Dependency of the frequency of transfer to various recipient S. enterica serovar Typhimurium LT2 waa strains on the seven PilV adhesins in the donor cells
| Recipient strain | Transfer frequency ratioa for donor strain expressing:
|
||||||
|---|---|---|---|---|---|---|---|
| PilVA | PilVA′ | PilVB | PilVB′ | PilVC | PilVC′ | PilVD′ | |
| SL3770 waa+ | 0.003 | 0.35 | 0.003 | 1 | 0.004 | 0.009 | 0.004 |
| SA1355 waa+ | 0.002 | 0.42 | 0.002 | 1 | 0.002 | 0.004 | >0.001 |
| SL428 wzy | >0.001 | 1 | 0.008 | 0.86 | 0.002 | 0.001 | 0.001 |
| SA1627 wba | 0.009 | 1 | 0.068 | 0.43 | 0.005 | 0.009 | 0.007 |
| SL3749 waaL | 0.010 | 0.78 | 0.009 | 1 | 0.010 | 0.022 | 0.009 |
| SL733 waaK | 0.003 | 0.85 | 0.001 | 0.064 | 0.002 | 1 | 0.002 |
| RF733 waaK | 0.001 | 0.95 | 0.001 | 0.001 | 0.001 | 1 | 0.001 |
| SL3750 waaJ | 0.005 | 1 | 0.004 | 0.015 | 0.004 | 0.005 | 0.010 |
| SL3748 waaI | 0.001 | 1 | 0.002 | 0.025 | 0.001 | 0.001 | 0.002 |
| SL3769 waaG | 0.009 | 1 | 0.013 | 0.005 | 0.008 | 0.010 | 0.008 |
| SL3749/pRF102 | 0.002 | 0.80 | 0.030 | 1 | 0.002 | 0.010 | 0.001 |
| SL733/pRF101 | 0.001 | 0.67 | 0.010 | 1 | 0.001 | 0.16 | 0.002 |
| RF733/pRF101 | >0.001 | 1 | 0.001 | 0.88 | 0.001 | 0.20 | 0.001 |
| SL3750/pRF103 | 0.001 | 1 | 0.001 | 0.30 | 0.001 | 0.007 | 0.001 |
S. enterica serovar Typhimurium LT2 SL4213 cells harboring pKK661 and one of seven pKK641 series plasmids were used as donors. S. enterica serovar Typhimurium LT2 strains with various waa mutations were used as recipient strains. Liquid matings were carried out at 37°C for 1.5 h. For each recipient strain, the ratio of the frequency of transfer of pKK661 from S. enterica serovar Typhimurium LT2 donor cells expressing various PilV adhesins to the highest transfer frequency (1.0) is estimated. High ratios are indicated by boldface type.
S. enterica serovar Typhimurium LT2 wild-type strains SL3770 and SA1355 were recognized by the PilVA′ and PilVB′ adhesins, as was wild-type SL4213 (13, 14). The wild-type S. enterica serovar Typhimurium LT2 strain has LPS with O-antigen (Fig. 1B). Mutants SL428 wzy, SA1627 wba, and SL3749 waaL, lacking O-antigen, were recognized by the PilVA′ and PilVB′ adhesins, as were the wild-type strains (Table 2). These results indicate that the O-antigen of S. enterica serovar Typhimurium LT2 is not required for recognition by the PilVA′ and PilVB′ adhesins in R64 liquid matings and does not block recognition.
On the other hand, a series of waa mutants producing incomplete LPS cores exhibited different mating patterns (Table 2). The SL733 waaK mutant showed very low recognition by the PilVB′ adhesin, suggesting that the PilVB′ adhesin may recognize the GlcNAc(α1-2)Glc moiety, the terminal structure of the S. enterica serovar Typhimurium LPS core. Residual recognition by the PilVB′ adhesin was observed in SL733, suggesting that SL733 is a leaky mutant and that a small number of LPS molecules carrying the PilVB′ receptor structure are still produced in this strain. This postulation was confirmed by using the RF733 waaK-null mutant, which was not recognized by the PilVB′ adhesin. To our surprise, SL733 and RF733 waaK mutants were recognized by the PilVC′ adhesin, which did not recognize any wild-type serovar Typhimurium LT2 strains (Table 2). The SL3750 waaJ, SL3748 waaI, and SL3769 waaG mutants were recognized only by the PilVA′ adhesin. These results suggest that the PilVC′ adhesin recognizes the Glc(α1-2)Gal moiety in the LPS of serovar Typhimurium LT2, the formation of which requires the waaJ, waaI, and waaG genes. In the previous study, the PilVC′ adhesin was shown to recognize the Glc(α1-2)Glc structure in the LPS of E. coli K-12 (8).
The internal Glc(α1-2)Gal structure in the wild-type serovar Typhimurium LT2 cells was not recognized by the PilVC′ adhesin, while the internal Glc(α1-2)Glc structure in wild-type E. coli K-12 cells was recognized. This apparent discrepancy may be explained as follows. In the wild-type LT2 LPS, the PilVC′ receptor is masked by the addition of GlcNAc, while, in the wild-type K-12 LPS, the neighboring sugars attached to the PilVC′ receptor do not affect PilVC′ recognition. The difference in the positions of the modification may be responsible for this, since the C-2 position of GlcII in the LT2 LPS is modified by GlcNAc, while the C-6 position of GlcIII in the K-12 LPS is modified by heptose (Fig. 1B and C). Alternatively, it is possible that not all of the E. coli K-12 LPS core has the terminal disaccharide and that PilVC′ may recognize the incomplete LPS core of E. coli K-12, in which the PilVC′ receptor is exposed.
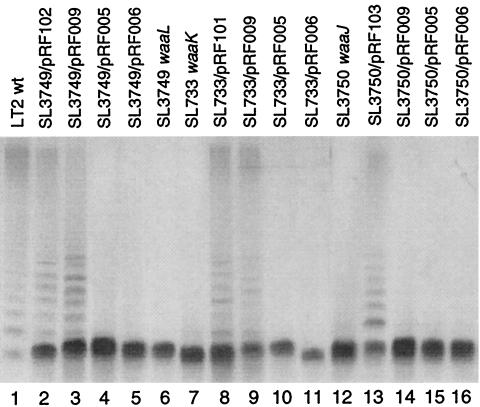
Complementation experiments were performed by introducing plasmids carrying the corresponding wild-type waa+ genes into LT2 waa mutants (Table 2). The waaK and waaJ mutants were complemented by pRF101 and pRF103, respectively, while the complementation of the waaK mutant by pRF101 was incomplete (recognition by PilVC′). The complementation of waaL by pRF102 was confirmed by SDS-PAGE analysis of the LPS (Fig. 2, lane 2) and by the sensitivity of the mutants to phage P22 (data not shown).
FIG. 2.
Gel electrophoretic analysis of chimeric LPSs. The LPSs from S. enterica serovar Typhimurium LT2 strains harboring the indicated plasmids were analyzed by SDS-PAGE and visualized by silver staining. wt, wild type.
These results strongly suggest that the PilVB′ and PilVC′ adhesins recognize the GlcNAc(α1-2)Glc and Glc(α1-2)Gal structures, respectively, of the LPS core of S. enterica serovar Typhimurium cells in R64 liquid matings, while the PilVC′ receptor in the wild-type LT2 LPS may be masked by GlcNAc.
Various chimeric LPSs as PilV receptors.
The results described above, together with previous results (8), suggest that specific moieties of LPSs function as receptors for various PilV adhesins. To confirm this, we tried to construct the PilVC and PilVB′ receptors on the LPSs of serovar Typhimurium LT2 and E. coli K-12, respectively. Various chimeric LPSs were constructed by introducing waa+ genes from different bacterial strains on multicopy plasmids into wild-type or waa strains of serovar Typhimurium LT2 or E. coli K-12, which were used as recipient strains (Table 3).
TABLE 3.
PilV dependency of the frequency of transfer to various recipient S. enterica serovar Typhimurium LT2 and E. coli K-12 waa strains carrying various waa+ genes from different origins
| Recipient strain | Transfer frequency ratioa for donor strain expressing:
|
Sensitivityb to phage:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PilVA | PilVA′ | PilVB | PilVB′ | PilVC | PilVC′ | PilVD′ | P22 | FO | Ffm | |
| SL3770 waa+ | 0.003 | 0.35 | 0.003 | 1 | 0.004 | 0.009 | 0.004 | + | + | − |
| SL3770/pRF009 | 0.014 | 0.89 | 0.009 | 1 | 0.67 | 0.20 | 0.014 | + | ± | − |
| SL3770/pRF005 | 0.006 | 0.57 | 0.003 | 1 | 0.007 | 0.30 | 0.006 | + | ± | ± |
| SL3770/pRF006 | >0.001 | 0.18 | >0.001 | 1 | >0.001 | 0.004 | >0.001 | + | + | − |
| SL3749 waaL | 0.010 | 0.78 | 0.009 | 1 | 0.010 | 0.022 | 0.009 | − | + | + |
| SL3749/pRF009 | 0.003 | 1 | 0.004 | 0.002 | 0.27 | 0.03 | 0.002 | + | − | + |
| SL3749/pRF005 | >0.001 | 1 | 0.001 | 0.007 | 0.001 | 0.05 | 0.001 | − | ± | + |
| SL3749/pRF006 | 0.002 | 1 | 0.002 | 0.30 | 0.003 | 0.009 | 0.002 | − | + | + |
| SL733 waaK | 0.003 | 0.85 | 0.001 | 0.064 | 0.002 | 1 | 0.002 | − | − | + |
| SL733/pRF009 | 0.001 | 1 | 0.003 | 0.001 | 0.30 | 0.06 | 0.001 | + | − | ± |
| SL733/pRF005 | 0.003 | 1 | 0.002 | 0.002 | 0.003 | 0.04 | 0.002 | − | − | + |
| SL733/pRF006 | 0.002 | 1 | 0.001 | 0.027 | 0.001 | 0.42 | 0.002 | − | − | + |
| SL3750 waaJ | 0.005 | 1 | 0.004 | 0.015 | 0.004 | 0.005 | 0.010 | − | − | + |
| SL3750/pRF009 | 0.001 | 1 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | − | − | + |
| SL3750/pRF005 | 0.002 | 1 | 0.001 | 0.003 | 0.002 | 0.004 | 0.001 | − | − | + |
| SL3750/pRF006 | 0.002 | 1 | 0.002 | 0.006 | 0.005 | 0.007 | 0.004 | − | − | + |
| JM83 | 0.007 | 0.29 | 0.007 | 0.006 | 0.62 | 1 | 0.006 | − | − | + |
| JM83/pRF101 | 0.020 | 0.33 | 0.020 | 1 | 0.15 | 0.38 | 0.013 | − | + | + |
| JM83/pRF102 | 0.013 | 1 | 0.017 | 0.006 | 0.09 | 0.32 | 0.007 | − | − | + |
| CS2334 waaL | 0.016 | 0.68 | 0.021 | 0.019 | 0.015 | 1 | 0.016 | − | − | + |
| CS2334/pRF006 | 0.003 | 0.63 | 0.003 | >0.001 | 0.94 | 1 | 0.003 | − | − | + |
| CS2334/pRF102 | 0.001 | 1 | 0.002 | 0.001 | 0.002 | 0.52 | 0.001 | − | − | + |
| CS2529 waaU | 0.004 | 0.6 | 0.006 | 0.005 | 0.005 | 1 | 0.005 | − | − | + |
| CS2529/pRF005 | 0.039 | 0.48 | 0.030 | 0.017 | 0.38 | 1 | 0.017 | − | − | + |
| CS2529/pRF101 | 0.004 | 1 | 0.005 | 0.045 | 0.001 | 0.049 | >0.001 | − | + | + |
| CS2198 waaR | 0.007 | 1 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | − | − | + |
| CS2198/pRF101 | 0.007 | 1 | 0.007 | 0.007 | 0.007 | 0.005 | 0.007 | − | − | + |
S. enterica serovar Typhimurium LT2 SL4213 and E. coli K-12 W3110 harboring pKK661 and one of seven pKK641 series plasmids were used as donor strains. S. enterica serovar Typhimurium LT2 and E. coli K-12 with and without various waa+ plasmids were used as recipient strains. Liquid matings were carried out at 37°C for 1.5 h. For each recipient strain, the ratio of the frequency of transfer of pKK661 from E. coli K-12 or serovar Typhimurium LT2 donor cells expressing various PilV adhesins to the highest transfer frequency (1.0) is estimated. High ratios are indicated by boldface type.
+, sensitive; ±, partially sensitive; −, resistant.
S. enterica serovar Typhimurium LT2 SL3770 cells harboring pRF009 (carrying waaU+ and waaL+ of E. coli K-12) were recognized by the PilVC and PilVC′ adhesins in addition to the PilVA′ and PilVB′ adhesins (Table 3). SL3770 cells harboring pRF005 (K-12 waaU+) were recognized by the PilVC′ adhesin, while SL3770 cells harboring pRF006 (K-12 waaL+) were not. These results can be explained as follows. In SL3770 cells harboring pRF005, heptose may be attached to the C-6 position of GlcII of the LT2 LPS by WaaU of E. coli K-12, resulting in recognition of the cells by the PilVC′ adhesin. In SL3770 cells harboring pRF009, heptose and GlcNAc may be successively attached to the C-6 position of GlcII of the LT2 LPS by WaaU and WaaL of E. coli K-12, respectively, resulting in recognition of the cells by the PilVC′ and PilVC adhesins. In SL3770 cells harboring pRF006, modification of the LT2 LPS did not occur. Thus, the GlcNAc(β1-7)Hep structure (PilVC receptor) can be constructed on the LT2 LPS by K-12 WaaU and WaaL. However, in SL3770 cells harboring pRF009 or pRF005, the attachment of heptose to GlcII of the LT2 LPS might be partial. N-Acetylglucosamine and O-antigen were also bound to some portion of GlcII in these LPSs, since the cells were also recognized by the PilVB′ adhesin. Thus, these LPSs may be heterogeneous.
Introduction of pRF005, pRF006, or pRF009 had similar effects on PilV dependency in SL3749 waaL cells and SL3770 cells (Table 3). In the case of SL3749, however, modification by heptose of GlcII in the LT2 LPS appears to be nearly complete, since the cells were not recognized by the PilVB′ adhesin. The reason for the differences in the levels of heptose modification between SL3770 harboring pRF009 or pRF005 and SL3749 harboring pRF009 or pRF005 is not known at present. Introduction of pRF009, pRF005, and pRF006 into SL733 waaK cells affected the PilV dependency similarly to their introduction into SL3749. In contrast, introduction of the same plasmids into SL3750 waaJ exhibited no effect on PilV dependency. These results indicate that GlcII of the LT2 LPS is essential for the construction of the PilVC receptor [GlcNAc(β1-7)Hep] on it.
E. coli K-12 JM83 cells harboring pRF101(waaK+ of serovar Typhimurium LT2) were recognized by the PilVB′ adhesin in addition to the PilVA′, PilVC, and PilVC′ adhesins (Table 3). Introduction of pRF102 (LT2 waaL+) into JM83 cells slightly affected the PilV recognition pattern of JM83 cells. It is likely that GlcNAc was added to the C-2 position of GlcIII in the LPS of E. coli K-12 cells harboring pRF101 by LT2 WaaK, forming the GlcNAc(α1-2)Glc moiety as the PilVB′ receptor. However, the addition of GlcNAc to GlcIII might be partial, since E. coli K-12 cells harboring pRF101 were still recognized by the PilVC and PilVC′ adhesins, suggesting heterogeneity of their LPSs.
E. coli K-12 CS2334 waaL could not be complemented by pRF102 (LT2 waaL+), while it was complemented by pRF006 (K-12 waaL+) (Table 3). Although the WaaL proteins of the two strains carry O-antigen ligase activity, their substrate specificities may be different since E. coli K-12 and serovar Typhimurium LT2 carry distinct O-antigen and LPS core structures. In fact, only limited amino acid sequence similarity between the two WaaL proteins is found.
E. coli K-12 CS2529 waaU cells harboring pRF101 (LT2 waaK+) were recognized weakly by PilVB′ but not by PilVC, while those harboring pRF005 (K-12 waaU+) were recognized by PilVC (Table 3). These results confirm that both the WaaU and WaaL proteins of E. coli K-12 are essential for the formation of the PilVC receptor and that WaaK of serovar Typhimurium LT2 is responsible for the formation of the PilVB′ receptor. E. coli K-12 CS2198 waaR cells harboring pRF101 (LT2 waaK+) were not recognized by PilVB′, suggesting that WaaK of serovar Typhimurium LT2 requires GlcIII of the K-12 LPS for the attachment of GlcNAc.
From the construction of the chimeric LPSs together with the results of the experiments using waa mutant versions of various bacteria, we conclude that the products of various waa genes are involved in the formation of the specific PilV receptor structures within the LPS.
SDS-PAGE analysis of chimeric LPSs.
The formation of the postulated chimeric LPSs in the strains carrying the waa+ genes from different bacterial strains was confirmed by SDS-PAGE analysis of the chimeric LPSs. Typical results are shown in Fig. 2. The LPS samples were prepared from the serovar Typhimurium LT2 waaL, waaK, and waaJ mutants harboring various plasmids and analyzed by SDS-PAGE followed by silver staining. The LT2 LPSs to which heptose and GlcNAc were attached by E. coli K-12 WaaU and WaaL, respectively, exhibited slower mobilities than unmodified LPSs (Fig. 2, compare lanes 3 and 4 to 6 and lanes 9 and 10 to 7). Attachment of O-antigen to the LPSs in SL3749 and SL733 cells harboring pRF009 was unexpected (Fig. 2, lanes 3 and 9). In these cells, the O-antigen of LT2 is likely to be attached to the HepIV-modified LT2 LPSs by WaaL of E. coli K-12. Attachment of O-antigen to LPSs in these cells was confirmed by determining the sensitivity to phage P22 (Table 3; see below).
Phage sensitivity analysis.
The formation of the postulated chimeric LPS was further confirmed by phage sensitivity (Table 3). Phage P22 recognizes the O-antigen unit structure of the LPS molecule of S. enterica serovar Typhimurium LT2 (1, 26). Phage FO recognizes the terminal structure of the E. coli R2-type LPS core, including that of S. enterica serovar Typhimurium LT2 (28). Phage Ffm recognizes the rough-type LPS (28). S. enterica serovar Typhimurium LT2 wild-type SL3770 strains harboring pRF009, pRF005, or pRF006 were sensitive to phage P22 (Table 3), indicating that all three strains produce LPS molecules carrying O-antigen. SL3770 cells harboring pRF009 and pRF005 exhibited weak sensitivity to FO, and SL3770 cells harboring pRF005 were partially sensitive to Ffm. All of SL3749 waaL, SL733 waaK, and SL3750 waaJ derivatives were sensitive to Ffm, indicating a rough-type character, while SL3749 and SL733 cells harboring pRF009 were sensitive to P22. All of the E. coli K-12 derivatives were sensitive to Ffm. E. coli JM83 harboring pRF101 and CS2529 harboring pRF101, which carried the PilVB′ receptor, were sensitive to FO. These results suggest that the PilVB′ receptor is similar to the FO receptor.
Specific inhibition of PilVC′-mediated liquid matings by Glc(α1-2)Glc and Glc(α1-2)Gal.
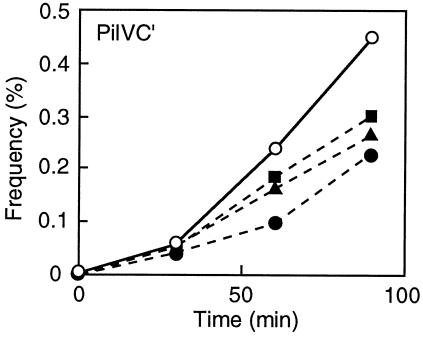
We have previously shown that addition of E. coli K-12 or B LPS specifically inhibits liquid matings (8). The effects of the addition of Glc(α1-2)Glc and Glc(α1-2)Gal as well as LPS from E. coli K-12 on the transfer frequency were examined in liquid matings with E. coli K-12 W3110 cells harboring pKK661 together with pKK641C′ as the donor strain (Fig. 3). Addition of LPS decreased the transfer frequency, as was found previously (8). Glc(α1-2)Glc and Glc(α1-2)Gal also decreased the transfer frequencies, although the effects were not so marked as that of LPS, even in the presence of higher concentrations. The inhibition was dependent on the concentrations of Glc(α1-2)Glc and Glc(α1-2)Gal (data not shown). In contrast, addition of maltose [Glc(α1-4)Glc] did not affect liquid matings (data not shown). These results further support the notion that the PilVC′ protein recognizes Glc(α1-2)Glc and Glc(α1-2)Gal moieties of LPS molecules in recipient cells.
FIG. 3.
Effects of Glc(α1-2)Glc, Glc(α1-2)Gal, and LPS on PilVC′-mediated liquid matings. E. coli K-12 W3110 harboring pKK661 together with pKK641C′ and TN102 were used as donor and recipient strains, respectively. Donor and recipient cultures were diluted 100-fold with LB broth before mixing. As inhibitor molecules, 1 mM Glc(α1-2)Glc (filled triangles), 1 mM Glc(α1-2)Gal (filled squares), and 0.1 mM LPS from E. coli K-12 TN102 (filled circles) were added to the mating mixture. The control mating mixture (open circles) did not contain any inhibitor molecule. The transfer frequency is expressed as a percentage of the number of donor cells and was determined at time intervals.
Receptor structures within LPSs for PilVB′, PilVC, and PilVC′ adhesins.
It is proposed that the PilVB′ receptor is GlcNAc(α1-2)Glc, since (i) the S. enterica serovar Typhimurium LT2 waaK mutant was not recognized by PilVB′ and (ii) the E. coli K-12 strain carrying the LT2 waaK gene was recognized by PilVB′. In addition, the PilVB′ adhesin also recognized E. coli R2 F632, in which the LPS contained the GlcNAc(α1-2)Glc structure (data not shown).
It is proposed that the PilVC receptor is GlcNAc(β1-7)Hep, since (i) the E. coli K-12 waaU or waaL mutants were not recognized by PilVC and (ii) the S. enterica serovar Typhimurium LT2 strain carrying the K-12 waaU and waaL genes was recognized by PilVC. In addition, it was previously shown that the E. coli B strain carrying the K-12 waaRUL genes was recognized by PilVC and PilVC′ (8).
The PilVC′ receptor of the LT2 LPS was shown to be Glc(α1-2)Gal, since the S. enterica serovar Typhimurium LT2 waaJ mutant was not recognized by PilVC′. The PilVC′ receptor of the K-12 LPS was previously shown to be Glc(α1-2)Glc, since the E. coli K-12 waaR mutant was not recognized by PilVC′ (8). An E. coli B strain carrying the K-12 waaR gene was recognized by PilVC′. In addition, Glc(α1-2)Glc and Glc(α1-2)Gal were shown to inhibit PilVC′-mediated liquid matings (Fig. 3).
From these results, we propose that the GlcNAc(α1-2)Glc and Glc(α1-2)Gal structures of the LPS core of S. enterica serovar Typhimurium LT2 function as receptors for the PilVB′ and PilVC′ adhesins, respectively, while the PilVC′ receptor in the wild-type LT2 LPS core is masked. We further propose that the GlcNAc(β1-7)Hep and Glc(α1-2)Glc structures of the LPS core of E. coli K-12 function as receptors for the PilVC and PilVC′ adhesins, respectively. These receptor structures are indicated in Fig. 1B and C. It is important that the disaccharide structures of the LPSs presented in Fig. 1B and C as specific receptors for the three PilV adhesins represent only the minimal essential structures. The results indicating that addition of LPS to the mating mixtures decreased the transfer frequency more efficiently than addition of disaccharides (Fig. 3) suggest the involvement of other structures of the respective LPSs in binding to the PilV adhesins.
Many bacteriophages use the LPS molecules of the host bacterial cells as receptors (19). Since gram-negative bacteria produce specific LPSs, the recognition of LPS by bacteriophages results in their host specificity. The LPS binding of the bacteriophage P22 tailspike proteins has been extensively studied (1, 26). To directly demonstrate LPS-PilV adhesin interaction, in vitro binding experiments may be helpful. Such work is in progress in our laboratory.
Acknowledgments
We are grateful to C. Whitfield for his helpful advice and to K. E. Sanderson for providing S. enterica serovar Typhimurium LT2 strains and plasmid pKZ38 used in this study. We thank N. Furuya for critical reading of the manuscript.
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Baxa, U., S. Steinbacher, S. Miller, A. Weintraub, R. Huber, and R. Seckler. 1996. Interactions of phage P22 tails with their cellular receptor, Salmonella O-antigen polysaccharide. Biophys. J. 71:2040-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldman, M. F., C. L. Marolda, M. A. Monteiro, M. B. Perry, A. J. Parodi, and M. A. Valvano. 1999. The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J. Biol. Chem. 274:35129-35138. [DOI] [PubMed] [Google Scholar]
- 3.Gyohda, A., N. Furuya, N. Kogure, and T. Komano. 2002. Sequence-specific and non-specific binding of the Rci protein to the asymmetric recombination sites of the R64 shufflon. J. Mol. Biol. 318:975-983. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto-Gotoh, T., F. C. Franklin, A. Nordheim, and K. N. Timmis. 1981. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene 16:227-235. [DOI] [PubMed] [Google Scholar]
- 5.Heinrichs, D. E., J. A. Yethon, and C. Whitfield. 1998. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 30:221-232. [DOI] [PubMed] [Google Scholar]
- 6.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hultgren, S. J., C. H. Jones, and S. Normark. 1996. Bacterial adhesins and their assembly, p. 2730-2756. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 2. ASM Press, Washington, D.C.
- 8.Ishiwa, A., and T. Komano. 2000. The lipopolysaccharide of recipient cells is a specific receptor for PilV proteins, selected by shufflon DNA rearrangement, in liquid matings with donors bearing the R64 plasmid. Mol. Gen. Genet. 263:159-164. [DOI] [PubMed] [Google Scholar]
- 9.Kim, S.-R., and T. Komano. 1997. The plasmid R64 thin pilus identified as a type IV pilus. J. Bacteriol. 179:3594-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klena, J. D., R. S. Ashford II, and C. A. Schnaitman. 1992. Role of Escherichia coli K-12 rfa genes and the rfp gene of Shigella dysenteriae 1 in generation of lipopolysaccharide core heterogeneity and attachment of O antigen. J. Bacteriol. 174:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komano, T. 1999. Shufflons: multiple inversion systems and integrons. Annu. Rev. Genet. 33:171-191. [DOI] [PubMed] [Google Scholar]
- 12.Komano, T., N. Funayama, S.-R. Kim, and T. Nisioka. 1990. Transfer region of IncI1 plasmid R64 and role of shufflon in R64 transfer. J. Bacteriol. 172:2230-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komano, T., S.-R. Kim, and T. Yoshida. 1995. Mating variation by DNA inversions of shufflon in plasmid R64. Adv. Biophys. 31:181-193. [DOI] [PubMed] [Google Scholar]
- 14.Komano, T., S.-R. Kim, T. Yoshida, and T. Nisioka. 1994. DNA rearrangement of the shufflon determines recipient specificity in liquid mating of IncI1 plasmid R64. J. Mol. Biol. 243:6-9. [DOI] [PubMed] [Google Scholar]
- 15.Komano, T., A. Kubo, and T. Nisioka. 1987. Shufflon: multi-inversion of four contiguous DNA segments of plasmid R64 creates seven different open reading frames. Nucleic Acids Res. 15:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerner, C. G., and M. Inouye. 1990. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 18:4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, D., and R. Reeves. 1994. Escherichia coli regains its O antigen. Microbiology 140:49-57. [DOI] [PubMed] [Google Scholar]
- 18.MacLachlan, P. R., S. K. Kadam, and K. E. Sanderson. 1991. Cloning, characterization, and DNA sequence of the rfaLK region for lipopolysaccharide synthesis in Salmonella typhimurium LT2. J. Bacteriol. 173:7151-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picken, R. N., and I. R. Beacham. 1977. Bacteriophage-resistant mutants of Escherichia coli K-12. Location of receptors within the lipopolysaccharide. J. Gen. Microbiol. 102:305-318. [DOI] [PubMed] [Google Scholar]
- 20.Pradel, E., C. T. Parker, and C. A. Schnaitman. 1992. Structures of the rfaB, rfaI, rfaJ, and rfaS genes of Escherichia coli K-12 and their roles in assembly of the lipopolysaccharide core. J. Bacteriol. 174:4736-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradel, E., and C. A. Schnaitman. 1991. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J. Bacteriol. 173:6428-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raetz, C. R. H. 1996. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles, p. 1035-1063. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. ASM Press, Washington, D.C.
- 23.Reeves, P. R., M. Hobbs, M. A. Valvano, M. Skurnik, C. Whitfield, D. Coplin, N. Kido, J. Klena, D. Maskell, C. R. Raetz, and P. D. Rick. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 4:495-503. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Schnaitman, C. A., and G. A. McDonald. 1984. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J. Bacteriol. 159:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinbacher, S., S. Miller, U. Baxa, N. Budisa, A. Weintraub, R. Seckler, and R. Huber. 1997. Phage P22 tailspike protein: crystal structure of the head-binding domain at 2.3 Å, fully refined structure of the endorhamnosidase at 1.56 Å resolution, and the molecular basis of O-antigen recognition and cleavage. J. Mol. Biol. 267:865-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson, R. G., P. Gemski, Jr., and B. A. D. Stocker. 1972. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J. Gen. Microbiol. 70:527-554. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida, T., N. Furuya, M. Ishikura, T. Isobe, K. Haino-Fukushima, T. Ogawa, and T. Komano. 1998. Purification and characterization of thin pili of IncI1 plasmids ColIb-P9 and R64: formation of PilV-specific cell aggregates by type IV pili. J. Bacteriol. 180:2842-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]