Abstract
Spiral ganglion neurons (SGNs), the primary afferent neurons of the cochlea, degenerate following a sensorineural hearing loss (SNHL) due to lack of trophic support normally received from hair cells. Cell transplantation is emerging as a potential strategy for inner ear rehabilitation, as injected cells may be able to replace damaged SGNs in the deafened cochlea. An increase in the number of surviving SGNs may result in improved efficacy of cochlear implants (CIs). We examined the survival of partially differentiated mouse embryonic stem cells (MESCs), following xenograft transplantation into the deafened guinea pig cochlea (n=15). Cells were delivered directly into the left scala tympani, via micro-injection through the round window. Small numbers of MESCs were detected in the scala tympani for up to 4 weeks following transplantation and a proportion of these cells retained expression of neurofilament protein 68kDa in vivo. While this delivery method requires refinement for effective long-term replacement of damaged SGNs, small numbers of MESCs were capable of survival in the deafened mammalian cochlea for up to 4 weeks, without causing an inflammatory tissue response.
Keywords: cell-based therapy, cochlea, deafness, embryonic stem cell, transplantation
INTRODUCTION
In mammals, damage to sensory receptor cells (hair cells) of the inner ear results in a permanent sensorineural hearing loss (SNHL). Inner hair cells normally provide trophic support to spiral ganglion neurons (SGNs), the primary afferent neurons of the auditory nerve, in the form of neurotrophins (38). Loss of these inner hair cells initiates a number of morphological and physiological changes which ultimately result in the degeneration of SGNs. An initial rapid loss of peripheral processes (36) is followed by a more gradual degeneration of cell bodies within Rosenthal’s canal (16, 32, 33). Degeneration of auditory neurons is an ongoing process which eventually results in very small numbers of surviving SGNs in long-term deafened animals (8, 16). This process and related terminology is illustrated in Figure 1.
Figure 1. Anatomy of the deafened guinea pig cochlea and schematic of scala tympani cell delivery technique.
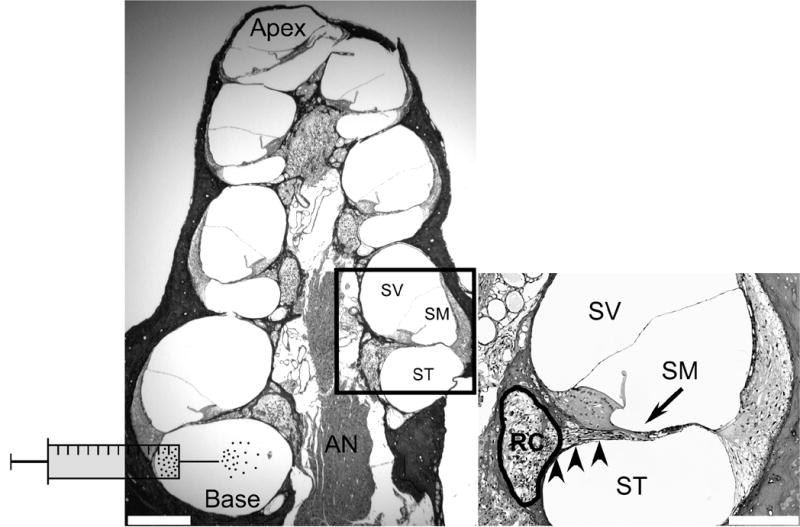
Transverse section of a guinea pig cochlea 4 weeks following ototoxic deafening, labelled with H & E. The auditory nerve (AN) is located in the middle of the structure and comprises central processes, which originate from spiral ganglion neurons (SGNs) in Rosenthal’s canal (RC; refer to inset). The AN and RC are collectively referred to as the modiolus. Encircling the modiolus are 4 cochlear turns, each comprising 3 fluid-filled compartments; scala vestibuli (SV), scala media (SM) and scala tympani (ST). The ST and SV are joined at the apex and are filled with the same fluid, perilymph. The SM is filled with endolymph, and is isolated from the ST and SV. MESCs were transplanted into the ST, at the base of the cochlea, as illustrated schematically. Scale bar = 300μm.
Inset: Higher magnification photomicrograph illustrating more comprehensively the anatomy of a single cochlear turn. The SV and SM are separated by Reissner’s membrane while the basilar membrane separates the SM and ST. Note the lack of hair cells in deafened animals (arrow), which are normally present at this location on the basilar membrane. SGNs are located within RC (circled) and are degenerating. Peripheral processes extend from SGNs toward the normal site of hair cells (arrow). RC and the ST are separated by a thin bony wall called the osseous spiral lamina (arrowheads). This wall contains numerous bony pores or canaliculae perforantes, which are thought to provide fluid communication channels between the SGNs housed within RC and the ST. Scale bar = 100μm.
Currently, the only clinical treatment for a severe-profound SNHL is a cochlear implant (CI), a neural prosthesis capable of directly stimulating residual SGNs following the loss of hair cells (30). The clinical success of CIs is due, at least in part, to the survival of a critical number of SGNs. Greater numbers of SGNs is likely to result in improved clinical outcomes for CI subjects (6, 20). Previous studies have reported that SGNs can be protected from degeneration following a SNHL by the exogenous supply of neurotrophins (5, 7, 15, 18, 19, 34, 39). While significant SGN survival is observed during the delivery of neurotrophins, this survival effect is lost immediately following the cessation of treatment (7). An effective therapy for hearing loss may therefore employ techniques capable of replacing degenerating SGNs. In order to provide beneficial rehabilitation, such an approach would need to include delivery of replacement cells to the target site, Rosenthal’s canal (Figure 1). Cell-based therapy offers such alternatives.
Recent studies investigating the viability of cell-based therapy in the cochlea are summarized in Table 1. While these studies utilize different cell types and methods for transplantation, they can be grouped into three categories based on the primary aim of the research: 1) to regenerate hair cells thereby restoring auditory function; 2) to provide neurotrophic support to degenerating SGNs; and 3) to replace degenerating SGN populations in an attempt to increase the efficacy of CIs. The present study explores the last of these goals using a clinically relevant mammalian model of deafness and mouse embryonic stem cells (MESCs).
Table 1.
Summary of cell based therapy in the inner ear
| Author and experimental aim | Cell type transplanted | Animal model (recipient) | Deafened (✓ or X) | Maximum cell survival time (weeks) | Transplantation site | |
|---|---|---|---|---|---|---|
| 1. | Hair cell regeneration | |||||
| Ito et al., 2001 | Rat adult neural stem cell | Neonatal rat (P0–P2) | X | 4 | Cochlear cavity | |
| Tateya et al., 2003 | Mouse fetal neural stem cell | Mouse | ✓ | 3.3 | Cochlea (turn 2) | |
| XHildebrand et al., 2005 | Mouse embryonic stem cells | Guinea pig | ✓ | 9 | Scala media | |
| 2. | Trophic support to SGNs | |||||
| Iguchi et al., 2003 | Mouse fetal neural stem cell | Mouse | X | 4 | Semicircular canal (adjacent to cochlea) | |
| 3. | Replacement of SGNs | |||||
| Olivius et al., 2003 | Guinea pig fetal dorsal root ganglion explant | Guinea pig | ✓ | 3 | Scala tympani | |
| XHu et al., 2003 | Mouse fetal dorsal root ganglion explant | Rat | ✓ | 3 | Scala tympani | |
| Naito et al., 2004 | Chinchilla bone marrow stromal cell | Chinchilla | ✓ | 3 | Auditory nerve | |
| XRegala et al., 2005 | Mouse fetal dorsal root ganglion explant, Mouse embryonic stem cells, Mouse adult neural stem cells | Guinea pig Rat | X | 4 | Auditory nerve | |
| XHu et al., 2005 A | Mouse adult neural stem cells | Guinea pig | ✓, X | 4 | Scala tympani | |
| XHu et al., 2005 B | Mouse fetal dorsal root ganglion explant, Mouse embryonic stem cells | Guinea pig | ✓ | 4 | Scala tympani | |
| XColeman et al., 2005 | Mouse embryonic stem cell | Guinea pig | ✓ | 4 | Scala tympani |
indicates xenotransplant.
SGN: spiral ganglion neuron.
The ability of stem cells to divide and differentiate into specialized cell types makes them ideal candidates for cell replacement therapy and tissue repair (25). Embryonic stem cells hold significant potential for regenerative medicine, due primarily to their capacity to differentiate into numerous cell and tissue types. In this way embryonic stem cells could be induced to differentiate into SGNs, which may provide a source of replacement cells to the degenerating auditory nerve and ultimately improve CI function. Previous attempts to transplant adult stem cells (12, 14, 21, 35), dorsal root ganglion neurons (DRGNs; 11, 23, 24), and a combination of DRGNs and embryonic stem cells (10) into the auditory system have been reported. Some of these studies were conducted with a view to replacing damaged SGNs in the deafened mammalian cochlea (11, 21, 23, 24). Of these studies, Hu et al., (11) and Olivius et al., (23, 24) delivered whole explants of DRGNs into the base of the cochlea via the scala tympani. Others have examined cell transplantation directly into the auditory nerve (21, 27). In all cases, the authors report survival and migration of transplanted cells within the cochlea for periods of up to 4 weeks. A recent paper by Hu et al. (10), reported survival and differentiation of MESCs delivered to the cochlea within a DRGN explant. However, there are presently no published studies describing direct delivery of embryonic stem cell suspensions into the deafened mammalian cochlea, to address the replacement of SGNs and improvement of CI function.
A variety of methodologies have been utilized to deliver cells to the cochlea (Table 1). These include injection into semicircular canals, adjacent to the cochlea (12), direct transplantation into the cochlea (14, 35), delivery into the scala media (9), or scala tympani (10, 11, 12, 23, 24), and direct injection into the auditory nerve (21, 27). We chose a scala tympani approach for cell transplantation, which enabled the delivery of MESCs directly into the cochlea, proximal to the target site. This clinically relevant technique minimized mechanical damage to the cochlea. Aspects of this work have appeared in abstract form (2, 3).
MATERIALS AND METHODS
Experimental groups
Twenty pigmented adult guinea pigs (400–600g) were taken from a mixed gender and gene pool and used for the current study (Table 2). Guinea pigs were systemically deafened, allowed to recover for 2 weeks and then transplanted with either 9-day differentiated MESCs (n = 15) or Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, Victoria, Australia) (n = 5). Animals were sacrificed at 1, 2 or 4 week time points (Table 2). All animal handling and technical procedures were conducted in accordance with the guidelines set by the Royal Victorian Eye and Ear Hospital Animal Research and Ethics Committee (approval numbers 02/083 and 02/090).
Table 2.
Summary of experimental animals
| Transplantation | Number of animals | Duration of deafness prior to transplantation (weeks) | Survival time following cell transplantation (weeks) |
|---|---|---|---|
| MESC | 5 | 2 | 1 |
| MESC | 5 | 2 | 2 |
| MESC | 5 | 2 | 4 |
| DMEM (control) | 5 | 2 | 2 |
MESC: mouse embryonic stem cells, DMEM: Dulbecco’s Modified Eagle Medium.
Deafening procedure
The hearing status of all guinea pigs was evaluated using standard click-evoked auditory brainstem responses (ABRs; 8). Normal hearing guinea pigs were bilaterally deafened by co-administration of the ototoxic aminoglycoside kanamycin monophosphate (Sigma, St Louis, MO, USA) and the loop diuretic frusemide (Troy Laboratories, Sydney, Australia). Briefly, guinea pigs were anaesthetized using ketamine (60 mg/mL, Parnell Laboratories, Sydney, Australia) and xylazil (4 mg/mL; Troy Laboratories), and 2% Lignocaine (Troy Laboratories) administered subcutaneously to the incision site. The right jugular vein was exposed and cannulated using sterile surgical techniques and then slowly infused with frusemide (110mg/kg), tied-off with silk and the wound sealed with cyanoacrylate. Kanamycin monophosphate (440mg/kg), was administered subcutaneously immediately post-surgery. The deafening technique described mimics the clinical scenario by selectively damaging hair cells, subsequently causing a SNHL and gradual degeneration of SGNs (Figure 1). All animals used in this study were confirmed profoundly deaf via ABRs, two weeks post-deafening (click-evoked ABR thresholds ≥ 98dB peak equivalent sound pressure level; 8).
MESCs
Maintenance of MESCs
MESCs (R1 B5-EGFP (Tg(GFPU)5 Nagy/J) were obtained from Dr A. Nagy (Mt Sinai Hospital, Toronto) and utilized in this study. These cells were genetically modified to express green fluorescent protein (GFP) so that they could be detected in vivo using direct fluorescent microscopy. Undifferentiated MESCs were grown in standard embryonic stem cell media (ESCM) comprising DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen), 1% Penicillin/Streptomycin (Invitrogen), 1% nucleosides (Sigma), 1% nonessential amino acids (Invitrogen), 1% L-glutamine (Sigma), 1mL/L 1000X ß-mercaptoethanol (BME; GIBCO/BRL, Melbourne, Australia) and 1ml/L (1000 units) leukemia inhibitory factor (LIF; Chemicon; Temecula, California). MESCs were passaged every 2–3 days using 0.025% trypsin (Invitrogen) in phosphate buffered saline (PBS) and grown at 37°C, 5% CO2.
Differentiation
MESCs underwent a 9-day period of induction in vitro to form neurectoderm (26). Briefly, MESCs were induced to form free-floating embryoid bodies (EBs) by transfer to non-adherent bacterial culture plates containing 50% ESCM (without LIF) and 50% Med II media (from cultured HepG2 cells). Media was changed after 2 days and every day thereafter for a further 6 days. On day 7, the media was replaced by neurectoderm media (NM) comprising 50% serum-free DMEM, 50% serum-free Ham’s F-12 (GIBCO), 1mL/L insulin-transferrin-sodium selenite (ITSS; Roche; Indianapolis, IN) and 10ng/mL basic fibroblast growth factor (bFGF; Roche). NM was changed again at day 8 and EBs prepared for surgery after 9 days in vitro, as detailed in Figure 2. The stem cell differentiation protocol was timed so that cells would be ready for transplantation exactly 14 days post-deafening (Figure 2).
Figure 2. Timeline of differentiation and transplantation methodology for MESCs.
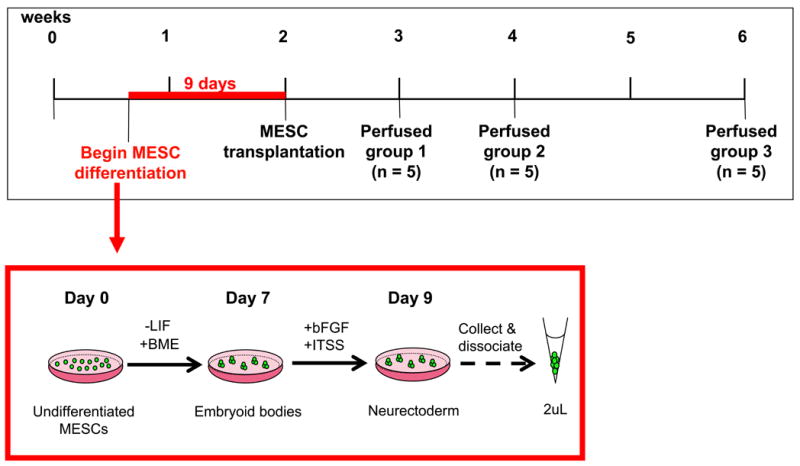
Twenty guinea pigs were systemically deafened and allowed to recuperate for 2 weeks. Four days after the deafening surgery, undifferentiated MESCs underwent a 9-day induction protocol to sequentially generate embryoid bodies (EBs) and neurectoderm (early neuronal tissue), as described in methods. Two weeks post-deafening, 15 guinea pigs received a 2μL suspension of MESCs, delivered into the left cochlea (Figure 1). A further 5 animals received a transplant of DMEM alone into the left cochlea. The right cochleae of all animals served as a deafened, untreated control. Animals receiving a transplant of MESCs (n=15) were placed randomly into 3 groups (5 animals per group) and perfused 1, 2 or 4 weeks post-stem cell transplantation (group 1, 2 or 3 respectively). Animals receiving a transplant of DMEM (n=5) were perfused 2 weeks post-delivery.
Preparation for surgery
EBs were collected after 9 days differentiation in vitro and washed twice in unsupplemented DMEM. EBs were then resuspended in 1mL DMEM, transferred to a sterile Eppendorf™ tube and gently dissociated using a 25-gauge needle. The resulting single-cell suspension was centrifuged for 10 seconds at 1000rpm, the supernatant discarded and the cells resuspended in unsupplemented DMEM to give a final cell concentration of ~ 1 x 106 viable cells/mL.
Agar embedding and processing of MESCs in vitro
A further 100μL of 9 day differentiated EBs was transferred into a sterile Eppendorf™ tube, excess media removed and cells washed twice in 1 mL of sterile PBS. After each wash, EBs were allowed to settle on the bottom of the tube and any excess PBS removed carefully without disturbing the pellet. EBs were then fixed for 30 minutes in 4% paraformaldehyde (PFA; BDH Laboratories, Leicestershire, UK) and rinsed 3 times in sterile PBS, as described previously. EBs were transferred quickly to pre-heated liquid agar (4%). After cooling and hardening at room temperature, agar blocks were embedded in paraffin wax and sectioned at 5μm. These sections were used for immunohistochemical comparison with in vivo sections.
Transplantation surgery
Two weeks post-deafening, guinea pigs were anaesthetized as previously described and the round window of the left cochlea exposed via a dorsal approach using sterile surgical techniques. MESCs were prepared for transplantation concomitant to exposure of the round window. The round window was perforated using a 30 gauge needle and a small volume of perilymph was aspirated. MESCs or DMEM (control) was delivered through the round window into the scala tympani of the left cochlea (Figure 1) via a sterile polyurethane and polyimide cannula attached to a 10μL Hamilton’s syringe (Coherent Scientific, Hilton, Australia). The syringe was operated by an electronic micro delivery pump (UltraMicroPump II, World Precision Instruments, Florida), which allowed for the consistent delivery of cells and media into the cochlea. A total volume of 2μL (half the volume of the guinea pig scala tympani; 37) was delivered at a rate of 0.5μL/minute. This technique was developed in our laboratory by Andrew, 2003 (1). Following transplantation the perforated round window was sealed with muscle and the wound sutured in two layers. The patency of the delivery system was tested before and after cell transplantation by delivering 0.5μL of cell suspension at a rate of 0.5μL/minute into a sterile dish. These samples were then used to estimate the number and viability of cells delivered into the cochlea (~2000 viable cells per cochlea).
Histology and Immunohistochemistry
Guinea pigs that received MESC transplants were euthanased via intraperitoneal injection of sodium pentobarbitone, (160mg/kg; Troy Laboratories) and transcardially perfused with 4% PFA at 1 (n=5), 2 (n=5) or 4 (n=5) weeks post-transplantation. Control animals that received DMEM were euthanased in the same way, 2 weeks following surgery. The left and right cochleae were removed and post-fixed for a further 24 hours and then decalcified in 10% ethylenediamine tetra-acetic acid (EDTA; Applichem, Darmstadt, Germany) in PBS for approximately 2 weeks. Decalcification was confirmed via radiography and the cochleae trimmed and orientated in 4% agar. Specimens were embedded in paraffin wax, sectioned at 5μm in the mid-modiolar plane and placed onto super-frost slides (Menzel-Glaser, Braunschweig, Germany).
Detection of MESCs
All sections were de-waxed in Histoclear and rehydrated using descending concentrations of alcohol. To examine cochlear morphology, representative slides were stained using Haemotoxylin and Eosin (H & E). Cell identification and survival was indicated by direct fluorescent microscopy for endogenous GFP in combination with the nuclear marker 4'-6-Diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). In addition, a selection of slides were labeled for neuronal marker neurofilament 68kDa (NF-L; Chemicon), using standard antigen retrieval and immunohistochemical techniques. Briefly, sections were heated to 100°C in 1mM EDTA, pH 8.0, allowed to cool to room temperature, then submerged into H2O2 for 30 minutes to block endogenous peroxidase activity. After permeabilisation in 0.1% Triton-X (Sigma) tissues were incubated in rabbit anti-NF-L (1:400) overnight, thoroughly rinsed in blocking solution, then incubated sequentially in biotinylated goat anti-rabbit (1:200; Vector Laboratories) and avidin-biotin complex (1:100; Vector Laboratories). Sections were rinsed in PBS and di-amino benzadine tetrahydrochloride (DAB; Vector Laboratories) applied until a colour change was observed. DAB-labeled sections were dehydrated in 100% alcohol and histoclear, then mounted in DPX (ProSciTech, Queensland, Australia).
All sections were evaluated under a Zeiss Axioplan Microscope (Zeiss, Victoria, Australia) using both transmitted light and a fluorescent lamp with appropriate filters (Zeiss filter set 00; 488000-0000, Zeiss filter set 02; 488002-0000 and Zeiss filter set 13; 488013-0000). Photomicrographs were taken using a Zeiss, AxioCam 12V monochrome digital camera.
Quantification of MESCs in vivo
The presence of MESCs in the deafened cochlea was examined qualitatively in the scala tympani of all turns. In order to quantify the number of surviving MESCs in the cochlea, we randomly selected 9 sections between the injection site (round window) and the mid-modiolar plane from left (MESC treated) cochleae. All sections were labeled with DAPI and visualized using direct fluorescent microscopy (previously described). Surviving MESCs were identified by size (between 5–15 μm in diameter), and by co-expression of endogenous GFP and the nuclear DAPI. In each section we counted the number of MESCs in the lower basal turn scala tympani, and within Rosenthal’s canal. A two-way analysis of variance was used to detect whether there was a significant difference between the number of surviving cells in the left cochleae at each time point. All statistical analyses were performed using Sigma Stat 3 software.
RESULTS
Detection of MESCs in the deafened guinea pig cochlea
H & E staining was employed to detect the presence of transplanted cells in the scala tympani of MESC treated and DMEM treated cochleae, for periods up to 4 weeks post-transplantation (Figure 3). Transplanted cells were identified in the scala tympani of MESC treated cochleae (Figure 3A), but not in DMEM treated (control) cochleae (Figure 3B). Interestingly, there was no inflammatory tissue response detected in any cochleae treated with MESCs (n=15) or DMEM (n=5), at any of the time points examined (Figures 3A and 3B respectively).
Figure 3. Detection of MESCs in the deafened guinea pig cochlea.
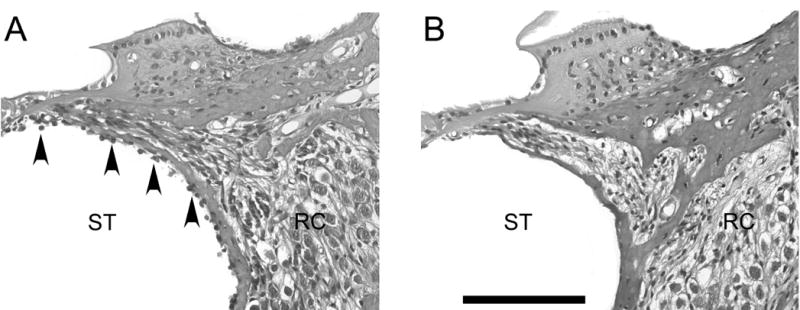
Transverse sections of left guinea pig cochleae stained with H & E. MESCs were observed in the scala tympani of transplanted animals for up to 4 weeks post-delivery (arrowheads, A), but not in DMEM treated controls (B). There was no inflammatory tissue response observed in any animals receiving MESC (n=15) or DMEM (n=5) transplants. ST = scala tympani. RC = Rosenthal’s canal. Scale bar = 100μm for both photomicrographs.
Identification of endogenous GFP in MESCs in vitro
To confirm endogenous GFP expression in vitro, 9-day differentiated MESCs were labeled with the nuclear marker DAPI and examined using direct fluorescent microscopy (Figure 4). MESCs were identified by co-localisation of the nuclear marker DAPI (Figure 4A) and endogenous GFP (Figure 4B). Overlay shows co-localisation of DAPI and GFP (Figure 4C).
Figure 4. Identification of endogenous GFP in MESCs in vitro.
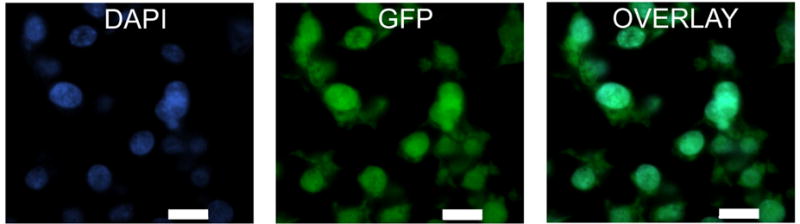
High magnification fluorescent photomicrographs showing expression of the nuclear marker DAPI (blue; A), and endogenous GFP (green; B) in MESCs grown in vitro. Co-localisation of DAPI and GFP is illustrated in C. Scale bars = 10μm.
Identification of GFP positive MESCs in the deafened guinea pig cochlea
To confirm cell identification, transplanted MESCs were visualized via direct fluorescent microscopy for endogenous GFP and DAPI (Figure 5). Small numbers of MESCs were identified in the scala tympani of left (MESC treated) cochleae, both in the basal turns (arrowheads) and in more apical turns. When quantified, there was a significant decline (p=0.01) in the number of transplanted MESCs in the left scala tympani (lower basal turn) between 2 and 4 weeks in vivo (Figure 6). There were no MESCs detected in the scala tympani of DMEM treated cochleae (Figure 3B).
Figure 5. Survival of GFP positive MESCs in vivo.
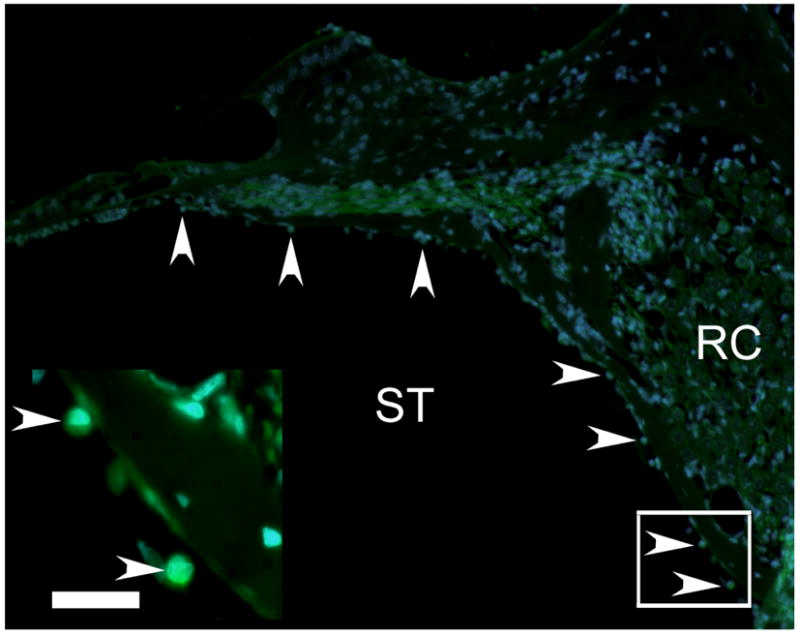
MESCs were detected in the left cochleae of treated animals, using direct fluorescent microscopy for endogenous GFP (green) in combination with the nuclear marker DAPI (blue). Fluorescent photomicrographs illustrate MESCs in the left (treated) cochlea (arrowheads). Inset illustrates higher magnification photomicrograph of the boxed region in the image. ST = scala tympani. RC = Rosenthal’s canal. Scale bar = 20μm.
Figure 6. Mean number of MESCs in the left scala tympani (lower basal turn) after 1, 2 and 4 weeks in vivo.
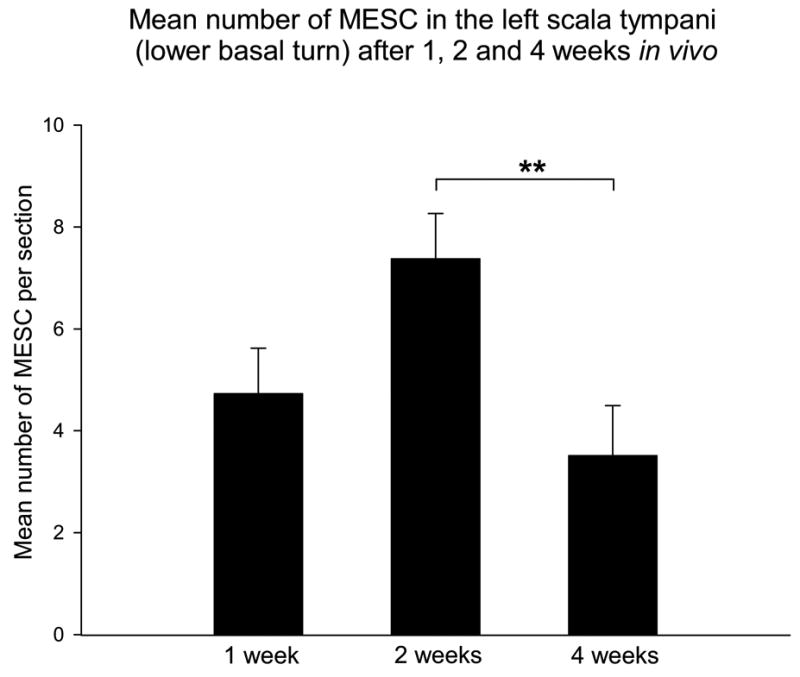
A two-way analysis of variance was used to determine whether there was a statistical difference between the mean number of MESCs in the lower basal turn scala tympani of the left cochleae at 1, 2 and 4 weeks in vivo. This graph illustrates survival of MESCs in the deafened mammalian cochlea for periods up to 4 weeks in vivo, and significantly fewer MESCs in the left cochleae after 4 weeks in vivo. Corresponding p value; ** p=0.01. ST = scala tympani.
Detection of transplanted MESCs within the target site, Rosenthal’s canal
Using direct fluorescent microscopy for endogenous GFP and DAPI, we detected MESCs within the target site, Rosenthal’s canal, in the left cochleae (Figure 7). When quantified using the described methodology, there was no significant difference observed between the number of stem cells detected within Rosenthal’s canal at any time point (Figure 8). At all 3 time-points, the mean number of stem cells observed within Rosenthal’s canal was less than 1 cell per section.
Figure 7. Detection of transplanted MESCs within Rosenthal’s canal.
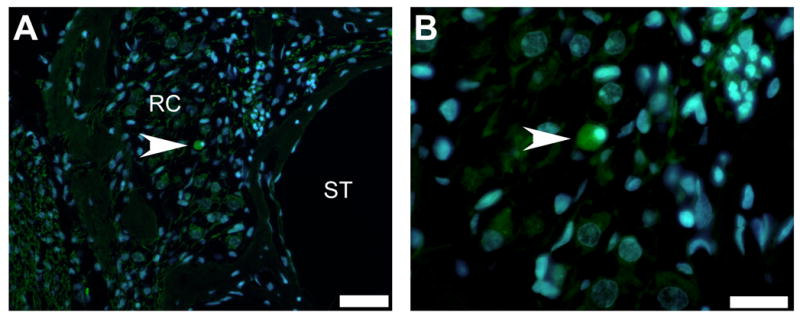
A small number of MESCs were detected within Rosenthal’s canal (RC) in the left cochleae (arrowhead, A), using direct fluorescent microscopy for endogenous GFP (green) in combination with the nuclear marker DAPI (blue). Under high magnification these cells were observed to be similar in size and morphology to MESCs in vitro (arrowhead, B). ST = scala tympani. RC = Rosenthal’s canal. Scale bar = 50μm (A) and 20μm (B).
Figure 8. Mean number of MESCs within Rosenthal’s canal after 1, 2 and 4 weeks in vivo.
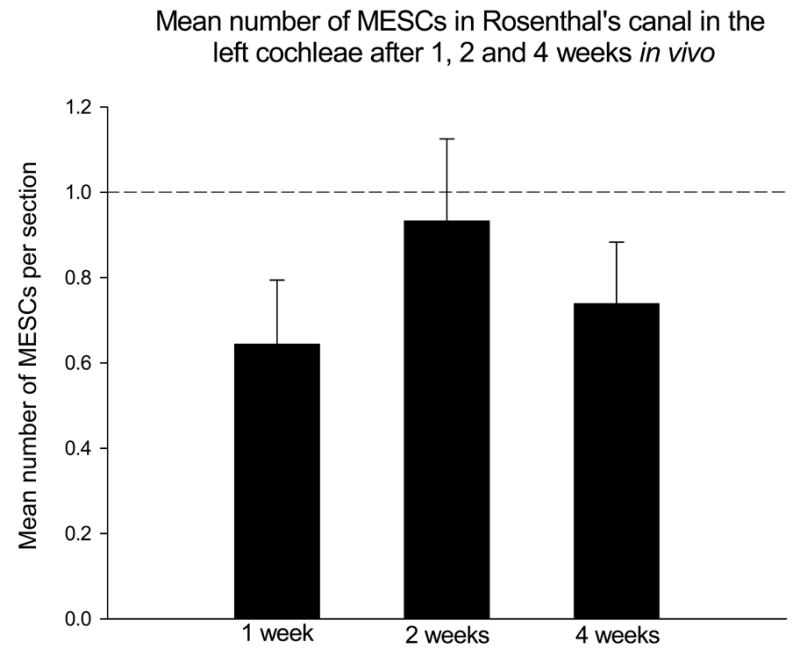
A two-way analysis of variance illustrated there was no significant difference detected between the mean number of MESCs within Rosenthal’s canal in the left cochleae after 1, 2 or 4 weeks in vivo. In addition, the mean number of MESCs detected within Rosenthal’s canal at any time point was never greater than 1 cell per section (perforated line). RC = Rosenthal’s canal.
Neurofilament labeling of MESCs in vitro and in vivo
Using immunohistochemical techniques and light microscopy, 9-day differentiated EBs and representative cochlear sections were labeled for neuron specific protein NF-L. A range of NF-L expression was observed in neurectodermal EBs in vitro, with the strongest labeling occurring on the periphery of the embryoid body, where the most differentiated cells are located (Figure 9A). NF-L expression was retained in a portion of transplanted MESCs for up to 4 weeks in vivo (Figure 9B). There were no NF-L positive cells observed in the scala tympani of control sections from DMEM treated cochleae (Figure 9C).
Figure 9. Neurofilament labeling of MESCs in vitro and in vivo.
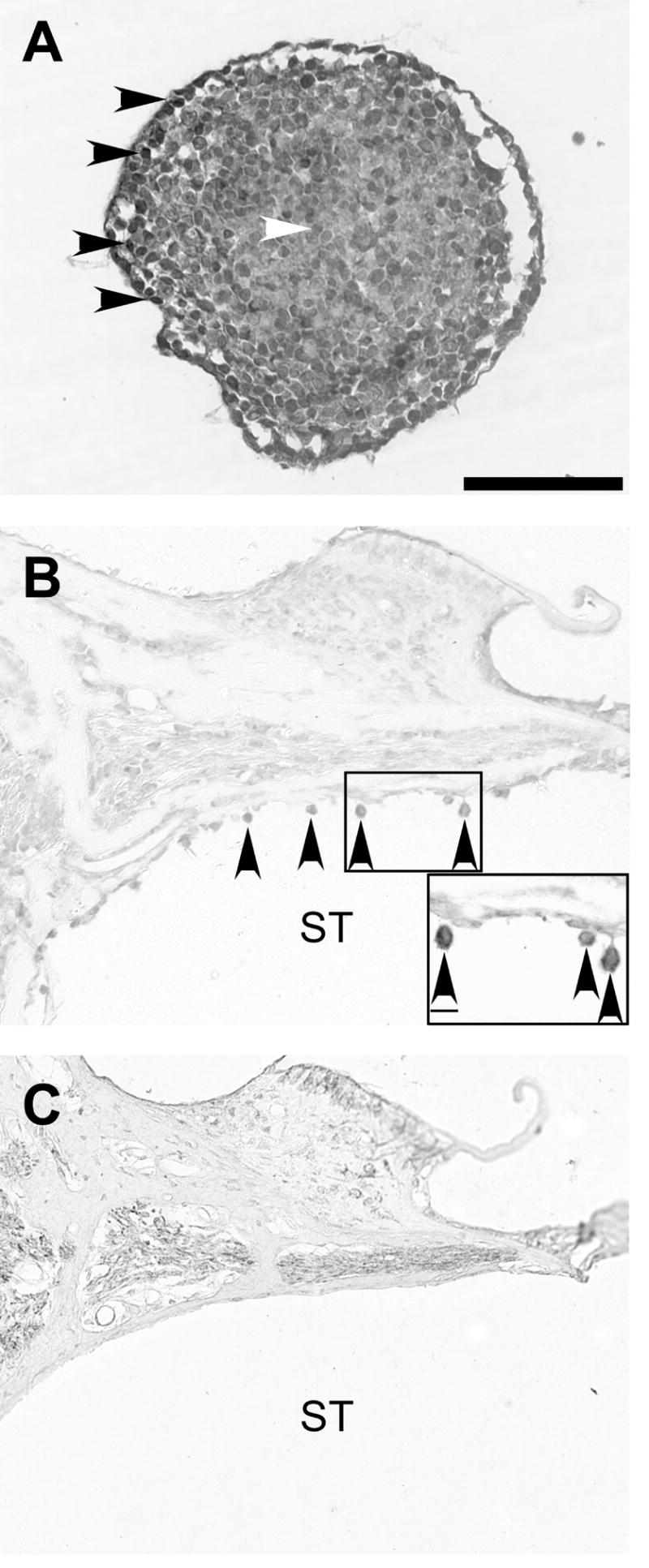
Light photomicrographs illustrating neurofilament (NF-L) labelling in vitro (A) and in vivo (B, C). NF-L expression in EBs in vitro was strongest around the periphery (black arrowheads, A) and weakest in the centre (white arrowheads, A). Transverse section from the left cochlea 4 weeks following transplantation, demonstrates that a portion of MESCs retained NF-L expression in vivo (arrowheads, B). Neurofilament positive cells were not observed in the scala tympani of DMEM-treated (control) cochleae (C). ST = scala tympani. Scale bar = 100μm (for all photomicrographs). Scale bar for inset (B) = 10μm.
DISCUSSION
Previous studies transplanting live cells into the mammalian auditory system have been published (9, 10, 11, 12, 13, 14, 21, 23, 24, 27, 35). Although cell type and transplantation methodology varied between laboratories, all report survival of transplanted cells for 3–9 weeks following delivery. Based on previous research using guinea pig animal models, we decided to deliver cells directly into the scala tympani. We anticipated that the chosen approach would enable the efficient delivery of cells proximal to the principle target site (SGNs within Rosenthal’s canal), while minimizing mechanical trauma to the cochlear cytoarchitecture. Furthermore, we considered this technique the most relevant from a clinical and surgical perspective.
Survival and dispersal of MESCs in the mammalian cochlea
The primary indicators of the efficacy of our approach were the survival and dispersal of MESCs in the deafened guinea pig cochlea. Examination of cochlear sections revealed that transplanted MESCs could be detected in small numbers for up to 4 weeks in vivo, and were capable of widespread dispersal throughout the cochlea. Surviving cells were observed attached to the cytoarchitechture and free-floating within the perilymph. While previous studies have reported the survival of exogenous cells in the mammalian cochlea for short periods (9, 10, 11, 12, 13, 14, 21, 23, 24, 27, 35), few studies have quantified the number of surviving cells. Where counts have been performed, there appears to be a decrease in the number of surviving cells after 4 weeks in vivo. According to Iguchi and colleagues (2003), approximately 10% of neural stem cells delivered to the mouse cochlea were detected 4 weeks following transplantation (13). Similar findings have been reported more recently in guinea pigs (10, 12). Specifically, these studies report a decline in the number stem cells transplanted into the guinea pig scala tympani between 2 and 4 weeks in vivo. Quantitative data from the current study also shows a significant decline in the number of cells in the scala tympani between 2 and 4 weeks in vivo (Figure 6). The reason for this decline in surviving cells after 4 weeks in vivo is unclear, however, we suggest that this may be related to both the delivery site, and to the presence of a cochlear aqueduct; a canal which connects the fluid-filled inner ear to the cerebrospinal fluid (CSF) of the CNS.
While some authors have suggested that transplanted cells migrate within the cochlea, it seems more likely that the primary mechanism behind intra-cochlear cell dispersal, at least through the perilymphatic space, is via the movement of perilymph, which flows at a slow rate of 1.6nL/minute in an apical direction (22). While the flow of perilymph may provide an innate dispersal mechanism for transplanted cells, it has the potential disadvantage of transporting the cells to regions distal to their target site. Given that guinea pigs have a patent cochlear aqueduct, it is feasible that a large proportion of cells in suspension could disperse from the cochlea into the CSF via this route. This may explain the observed decrease in the number of transplanted cells between 2 and 4 weeks in vivo, both in this study and previous studies (10, 12). Notably, in previous studies delivering live cells into the scala tympani, there has been no attempt to confirm whether transplanted cells could be detected in the cochlear aqueduct or CSF. Examination of these compartments in future studies will be necessary in order to confirm this hypothesis.
Detection of transplanted MESCs within Rosenthal’s canal
A small number of MESCs were detected in Rosenthal’s canal and observed within close proximity to surviving SGNs. Similar findings have been reported by other groups, following cell transplantation in guinea pigs (23) and rats (11). We hypothesize that transplanted cells are able to reach these otherwise confined areas via pores which are present in the osseous spiral lamina, lining the medial wall of the scala tympani (Figure 1). These pores or canaliculae perforantes, are reported to provide fluid communication channels between Rosenthal’s canal and the perilymphatic fluid in the scala tympani (4, 17, 28, 29). Previous research has demonstrated that canaliculae perforantes are present in guinea pigs (4, 28) and encouragingly, in humans where they are reported to be both numerous and large (31). While transplanted cells were detected within Rosenthal’s canal in the present study, they did not occur in significant numbers to be considered an efficient means by which to replace degenerating SGNs in the deafened cochlea (Figure 8). This finding implies the necessity for a more directed delivery technique.
Future directions and clinical implications for cell based therapy in the mammalian cochlea
Results from the present study suggest that the delivery of cell suspensions to the cochlea via the scala tympani is not the most appropriate surgical approach for long-term cell-replacement therapy for SGNs. Although MESCs were capable of survival for periods up to 4 weeks in the cochlea, the widespread dispersal of cells and low number detected within the target site is not ideal. Future experimentation will need to address ways in which cells can be delivered directly into Rosenthal’s canal and retained within this site. Although this approach is surgically difficult and causes mechanical damage to the modiolus, it has been used successfully by other groups (21, 27).
It is interesting to note that a portion of transplanted MESCs retained expression of early neuronal marker NF-L for up to 4 weeks post-transplantation, without causing an inflammatory tissue response. While further work is required to improve the numbers of implanted cells within the target site, this finding suggests that the deafened cochlea environment can support the survival of exogenous neural tissue.
For the delivery of cell suspensions directly into the scala tympani, the use of biocompatible matrices designed to prevent the movement of transplanted cells out of the cochlea, would significantly improve the efficacy of this approach. Previous studies using whole DRGN explants have been successful in terms of cell survival and migration of cells into Rosenthal’s canal (23, 24). More recently, this group reported that delivery of stem cells within this DRGN explant, showed improved survival and differentiation (10). This finding highlights both the importance of matrices to hold transplanted cells in place, and the co-administration of trophic support to promote their maintenance and survival in vivo. Such a model could also be manipulated to incorporate electrical stimulation via a CI. This may have the dual advantage of maintaining surviving SGNs and activating the transplanted population of neurons. The ultimate goal of this research is to test whether newly transplanted cells will form functional, tonotopic connections with second order neurons in the cochlear nucleus.
SUMMARY
This study aimed to evaluate the viability of cell-based therapy to replace SGNs in the deafened mammalian cochlea. MESCs were delivered to the guinea pig cochlea via the scala tympani and their survival and tissue compatibility assessed after 1, 2 and 4 weeks. Although small numbers of transplanted cells were capable of survival 4 weeks in vivo, few cells were detected in Rosenthal’s canal, suggesting that the described delivery method is not the most appropriate surgical approach for SGN replacement. Importantly, no inflammatory tissue response was observed in response to the xenotransplantation of MESCs, and transplanted cells retained expression of early neuronal marker NF-L. Future studies will be directed towards delivering these cells directly into their target site within Rosenthal’s canal, using biocompatible matrices to minimize their dispersal.
Acknowledgments
The authors extend their sincere thanks to the following people; Dr A. Nagy (Mt Sinai Medical School, Toronto) for providing the MESCs for this study; Ms Jacqueline Andrew for providing the DMEM-treated control tissue; Maria Clarke for her histological expertise, and to Dr Lisa Gillespie for her immunohistochemical contribution. This work was supported by the NIDCD of the National Institutes of Health (N01-DC-0-2109 and N01-DC-3-1005), the University of Melbourne (postgraduate research scholarship) and a Wagstaff Fellowship from the Royal Victorian Eye and Ear Hospital.
References
- 1.Andrew JK. Department of Otolaryngology. Melbourne: University of Melbourne; 2003. Rehabilitation of the Deafened Auditory Nerve With Schwann Cell Transplantation; pp. 1–114. [Google Scholar]
- 2.Coleman B, Hardman J, Crook J, de Silva M, Epp S, Coco A, Shepherd RK. Frontiers in Otorhinolaryngology. Noosa; 2004. Survival and migration of partially differentiated stem cells in the adult guinea pig cochlea. [Google Scholar]
- 3.Coleman B, Hardman J, de Silva M, Crook J, Shepherd RK. International Society for Stem Cell Research. San Francisco; 2005. Strategies for stem cell-based therapy in the mammalian cochlea. [Google Scholar]
- 4.Duckert LG, Duvall AJ., 3rd Cochlear communication routes in the guinea pig-spiral ganglia and osseous spiral laminae: an electron microscope study using microsphere tracers. Otolaryngology. 1978;86:ORL434–446. doi: 10.1177/019459987808600312. [DOI] [PubMed] [Google Scholar]
- 5.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nature Medicine. 1996;2:463–7. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 6.Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–16. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: cessation of treatment leads to accelerated loss of survival effects. Journal of Neuroscience Research. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 8.Hardie NA, Shepherd RK. Sensorineural hearing loss during development:morphological and physiological response of the cochlear and auditory brainstem. Hearing Research. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 9.Hildebrand MS, Dahl H-HM, Hardman J, Coleman B, Shepherd RK, de Silva MG. Survival of partially differentiated mouse embryonic stem cells in the scala media of the guinea pig cochlea. JARO. 2005 doi: 10.1007/s10162-005-0012-9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005;1051(1–2):137–44. doi: 10.1016/j.brainres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Experimental Neurology. 2004;185:7–14. doi: 10.1016/j.expneurol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302(1):40–7. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, Naito Y, Ito J. Trophic support of mouse inner ear by neural stem cell transplantation. Neuro Report. 2003;14:77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- 14.Ito J, Kojima K, Kawaguchi S. Survival of neural stem cells in the cochlea. Acta Otolaryngology. 2001;121:140–142. doi: 10.1080/000164801300043226. [DOI] [PubMed] [Google Scholar]
- 15.Kanzaki S, Stover T, Kawamoto K. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–60. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 16.Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33:11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 17.Lim DJ. Surface ultrastructure of the cochlear perilymphatic space. J Laryngol Otol. 1970;84:413–28. doi: 10.1017/s0022215100072029. [DOI] [PubMed] [Google Scholar]
- 18.McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26(5):1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JM, Chi DH, O'Keefe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. International Journal of Developmental Neuroscience. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 20.Nadol JB, Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 21.Naito Y, Nakamura T, Nakagawa T, Iguchi F, Endo T, Fujino K, Kim TS, Hiratsuka Y, Tamura T, Kanemaru S, Shimizu Y, Ito J. Transplantation of bone marrow stromal cells into the cochlea of chinchillas. Neuroreport. 2004;15:1–4. doi: 10.1097/00001756-200401190-00001. [DOI] [PubMed] [Google Scholar]
- 22.Ohyama K, Salt AN, Thalmann R. Volume flow rate of perilymph in the guinea-pig cochlea. Hear Res. 1988;35:119–29. doi: 10.1016/0378-5955(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 23.Olivius P, Alexandrov L, Miller J, Ulfendahl M, Bagger-Sjoback D, Kozlova EN. Allografted fetal dorsal root ganglion neuronal survival in the guinea pig cochlea. Brain Research. 2003;979:1–6. doi: 10.1016/s0006-8993(03)02802-6. [DOI] [PubMed] [Google Scholar]
- 24.Olivius P, Alexandrov L, Miller JM, Ulfendahl M, Bagger-Sjoback D, Kozlova EN. A model for implanting neuronal tissue into the cochlea. Brain Res Brain Res Protoc. 2004;12 (3):152–6. doi: 10.1016/j.brainresprot.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Passier R, Mummery C. Origin and use of embryonic and adult stem cells in differentiation and tissue repair. Cardiovascular Research. 2003;1 doi: 10.1016/s0008-6363(02)00770-8. [DOI] [PubMed] [Google Scholar]
- 26.Rathjen J, Haines B, Hudson K, Nesci A, Dunn S, Rathjen PD. Directed differentiation of pluripotent cells to neural lineages: homogenous formation and differentiation of a neurectoderm population. Development. 2002;129:2649–2661. doi: 10.1242/dev.129.11.2649. [DOI] [PubMed] [Google Scholar]
- 27.Regala C, Duan M, Zou J, Salminen M, Olivius P. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp Neurol. 2005;193(2):326–33. doi: 10.1016/j.expneurol.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Sando I, Masuda Y, Wood RP, 2nd, Hemenway WG. Perilymphatic communication routes in guinea pig cochlea. Ann Otol Rhinol Laryngol. 1971;80:826–34. doi: 10.1177/000348947108000609. [DOI] [PubMed] [Google Scholar]
- 29.Schuknecht HF, Seifi AE. Experimental observations of the fluid physiology of the inner ear. Ann Otol Rhinol Laryngol. 1963;72:687–712. doi: 10.1177/000348946307200308. [DOI] [PubMed] [Google Scholar]
- 30.Seligman P, Shepherd RK. In: Neuroprosthetics: Theory and practice. Horch KWGD, editor. World Scientific Press; Singapore: 2004. pp. 878–904. [Google Scholar]
- 31.Shepherd RK, Colreavy MP. Surface microstructure of the perilymphatic space: Implications for cochlear implants and cell or drug based therapies. Arch Otolaryngology. 2004 doi: 10.1001/archotol.130.5.518. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiology and Neuro-Otology. 2001;6:305–318. doi: 10.1159/000046843. [DOI] [PubMed] [Google Scholar]
- 33.Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol Suppl. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- 34.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 35.Tateya I, Nakagawa T, Iguchi F, Kim TS, Endo T, Yamada S, Kageyama R, Naito Y, Ito J. Fate of neural stem cells grafted into injured inner ears of mice. Neuroreport. 2003;14:1677–81. doi: 10.1097/00001756-200309150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Terayama Y, Kaneko Y, Kawamoto K, Sakai N. Ultrastructural changes of the nerve elements following disruption of the organ of Corti. I. Nerve elements in the organ of Corti. Acta Otolaryngol. 1977;83:291–302. doi: 10.3109/00016487709128848. [DOI] [PubMed] [Google Scholar]
- 37.Thorne M, Salt AN, DeMott JE, Henson MM, Henson OW, Jr, Gewalt SL. Cochlear fluid space dimensions for six species derived from reconstructions of three-dimensional magnetic resonance images. Laryngoscope. 1999;109:1661–8. doi: 10.1097/00005537-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hearing Research. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 39.Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler RA, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hearing Research. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]


