Abstract
Event-related functional magnetic resonance imaging was used to study the effects of healthy aging on hippocampal and rhinal memory functions. Memory for past events can be based on retrieval accompanied by specific contextual details (recollection) or on the feeling that an event is old or new without the recovery of contextual details (familiarity). There is evidence that recollection is more dependent on hippocampus, whereas familiarity is more dependent on the rhinal cortex, and that healthy aging has greater effects on recollection than on familiarity. However, little evidence is available about the neural correlates of these effects. Here, we isolated activity associated with recollection and familiarity by distinguishing between linear and quasi-exponential “perceived oldness” functions derived from recognition confidence levels. The main finding was a double dissociation within the medial temporal lobes between recollection-related activity in hippocampus, which was reduced by aging, and familiarity-related activity in rhinal cortex, which was increased by aging. In addition, age dissociations were found within parietal and posterior midline regions. Finally, aging reduced functional connectivity within a hippocampal-retrosplenial/parietotemporal network but increased connectivity within a rhinal-frontal network. These findings indicate that older adults compensate for hippocampal deficits by relying more on rhinal cortex, possibly through a top-down frontal modulation. This finding has important clinical implications because early Alzheimer's disease impairs both hippocampus and rhinal cortex.
Keywords: aging, familiarity, fMRI, medial temporal lobe, recollection
Introduction
The early diagnosis of Alzheimer's dementia (AD) is complicated by the fact that most diagnostic features in noninvasive behavioral and imaging measures can also be observed in healthy aging. For example, AD patients show deficits in episodic memory performance (Welsh and others 1992) and a deterioration of medial temporal lobe (MTL) anatomy and function (Nestor and others 2004). However, episodic memory impairment (Craik and Simon 1980) and MTL deterioration (Raz 2005) are also present in healthy older adults. Beyond the initial stages of AD, quantitative differences in the severity of these memory and MTL deficits become so pronounced that diagnosis is less problematic. Still, early diagnosis would be dramatically improved if it relied not only on “quantitative” but also on “qualitative” differences. For instance, there is evidence that different MTL subregions are differentially affected by early AD and healthy aging. Whereas early AD seems to impair the rhinal cortex to a greater extent than the hippocampus (Killiany and others 2002), healthy aging impairs the hippocampus to a greater extent than the rhinal cortex (Raz and others 2005). Moreover, there is evidence that the hippocampus and the rhinal cortex support different episodic memory processes and that healthy aging tends to impair processes dependent on the hippocampus (recollection) but not processes dependent on the rhinal cortex (familiarity). In the present study, we used event-related functional magnetic resonance imaging (fMRI) to clarify the contributions of hippocampal and rhinal regions to the effects of aging on recollection versus familiarity.
“Recollection” refers to the recovery of specific contextual information associated with a study item and “familiarity” to the feeling that the item is old in the absence of contextual details (Yonelinas 2002). There is considerable behavioral evidence that recollection processes are markedly impaired by aging, whereas familiarity shows little or no age-related decline. In a paradigm in which participants indicate whether their recognition responses are based on recollection (remember or “R” responses) or familiarity (know or “K” responses), several studies have found an age-related reduction in recollection but not in familiarity (Parkin and Walter 1992; Mantyla 1993; Maylor 1995; Java 1996; Davidson and Glisky 2002; Bastin and Van der Linden 2003). At the same time, there is almost no evidence available regarding the effects of aging on the neural correlates of recollection and familiarity.
Lesion, electrophysiological, and functional neuroimaging studies have associated recollection and familiarity with different subregions of the MTLs, the left parietal cortex, and the posterior midline cortex. Within MTL, recollection has been associated with the hippocampus and familiarity with cortical MTL regions, such as parahippocampal and rhinal cortices (Eichenbaum and others 1992; Brown and Aggleton 2001). In lesion studies, damage specific to the hippocampus may impair recollection without affecting familiarity (Holdstock and others 2002; Yonelinas and others 2002), whereas damage extending into the cortical MTL regions tends to impair both recollection and familiarity (Yonelinas 2002). A recent study, which measured the contribution of recollection to recognition performance on the basis of the shape of receiver operating characteristic (ROC) curves, found that selective hippocampal lesions in rodents affected recollection estimates but not familiarity estimates (Fortin and others 2004). In functional neuroimaging studies, recollection has been associated with “increases” in hippocampal activity (Eldridge and others 2000; Wheeler and Buckner 2004; Yonelinas and others 2005). In contrast, intracranial recording (Brown and Aggleton 2001) and functional neuroimaging (Henson and others 2003; Daselaar and others 2005; Gonsalves and others 2005) studies have associated familiarity with “decreases” in rhinal activity. In sum, whereas recollection has been associated with increases in hippocampal activity, familiarity has been associated with decreases in rhinal activity.
Beyond MTL, recollection and familiarity have been associated with different subregions within left parietal (Wheeler and Buckner 2004; Daselaar and others 2005; Yonelinas and others 2005) and posterior midline (retrosplenial, posterior cingulate) regions (Daselaar and others 2005; Yonelinas and others 2005). Whereas in rhinal cortex, familiarity has been associated with decreases in activity, in left parietal and posterior midline regions, it typically involves increases in activity.
Very little is known about the effects of aging on the neural correlates of recollection and familiarity. To our knowledge, only 1 functional neuroimaging study has provided evidence relevant to this issue (Cabeza and others 2004). In 1 condition of this study, young and older adults performed a verbal recognition task with R/K responses. Within MTL, older adults showed weaker activity in the hippocampus but greater activity in a cortical MTL region. Given aforementioned evidence linking the hippocampus to recollection and the cortical MTL regions to familiarity (Eichenbaum and others 1992; Brown and Aggleton 2001), this finding suggests a differential effect of aging on the neural correlates of recollection and familiarity. Consistent with this interpretation, older adults showed fewer R but more K responses than young adults, and the latter were correlated with the age-related increase in cortical MTL activation. Yet, this study did not clearly distinguish between recollection-related versus familiarity-related activity, and hence, the link between the MTL dissociation and the recollection/familiarity distinction is indirect. To address this issue, we conducted an fMRI study specifically designed to investigate the effects of aging on recollection-related and familiarity-related activity within MTL and other brain regions.
The task we investigated was a word recognition test with confidence ratings. In each trial, participants made first an old/new decision and then rated their confidence from low to high. These responses were combined into a 3-point “perceived oldness” scale (level 1, “new”; level 2, “probably old”; level 3, “definitely old”). There is a considerable amount of evidence that familiarity-based responses increase gradually as a function of perceived oldness, whereas recollection-based responses are primarily associated with the highest level of perceived oldness (Yonelinas 2001, 2002; Wixted and Stretch 2004). For example, the probability of K responses increases monotonically from new to definitely old trials, whereas most R responses are clustered on definitely old trials (Yonelinas 2001; Wixted and Stretch 2004). Thus, in parametric fMRI analyses with perceived oldness as a covariate, we defined “recollection-related activity” as a quasi-exponential function in which activity remains relatively low for levels 1 and 2 and increases sharply for level 3 (i.e., definitely old trials). In contrast, we defined familiarity as a linear change as a function of perceived oldness. Given the aforementioned evidence that familiarity may involve increases as well as decreases in activation (Henson and others 2003; Daselaar and others 2005; Gonsalves and others 2005), we defined “familiarity-related activity” as a continuous linear increase or decrease in activity from level 1 to level 3. Further, we investigated the effect of aging on the extent to which quasi-exponential and linear functions account for observed activations. Thus, the expressions “age-related increases/decreases in recollection/familiarity-related activity” refer to changes in the “shape” of activity functions rather than changes in overall activity, which are eliminated by our mean-centered parametric analyses.
We also addressed an important confound inherent to functional neuroimaging of cognitive aging, namely, age differences in performance. If older adults perform more poorly than young adults, it is unclear whether group effects reflect differences in age or differences in performance. In contrast, when cognitive performance is similar in young and old groups, group differences in brain activity can be safely attributed to aging. Previous functional neuroimaging studies have used 2 different approaches to deal with this problem: matching performance in young and old groups by manipulating tasks (e.g., Cabeza and others 2000; Morcom and others 2003) and selecting high-functioning elderly who naturally perform as well as young adults (e.g., Cabeza and others 2002). In the present study, we incorporated both approaches. First, during encoding, words were presented once to young adults but twice to older adults. Second, participants were selected in rank order based on memory accuracy in the scanner to carefully match performance between the young and old groups.
On the basis of behavioral evidence that older adults are impaired in recollection but not in familiarity, we predicted that older adults would show reduced recollection-related activity but similar familiarity-related activity. Within MTL, we predicted that older adults would show reduced recollection-related activity in the hippocampus but preserved—or even enhanced—familiarity-related activity in the rhinal cortex. Likewise, in left parietal and posterior midline regions, we expected age-related reductions in recollection-related but not in familiarity-related activations.
Methods
Participants
The subjects were 14 young and 15 older adults that were paid for participation. All participants were healthy, right-handed English native speakers, with no history of neurological or psychiatric episodes. Written informed consent was obtained from each participant, and the study met all criteria for approval from the Duke University Institutional Review Board. Younger adults were recruited from the Duke University community. Older adults were community-dwelling high-functioning individuals that were selected from a pool of senior research volunteers at Duke University and screened for health problems and conditions that could affect blood flow (e.g., hypertension, certain medications) using a questionnaire. In order to match performance in the 2 groups, we selected 12 younger (mean age = 22.2 years) and 12 older adults (mean age = 69.2 years) from these participants in rank order based on recognition accuracy (d-prime) in the scanner (Fig. 1A).
Figure 1.
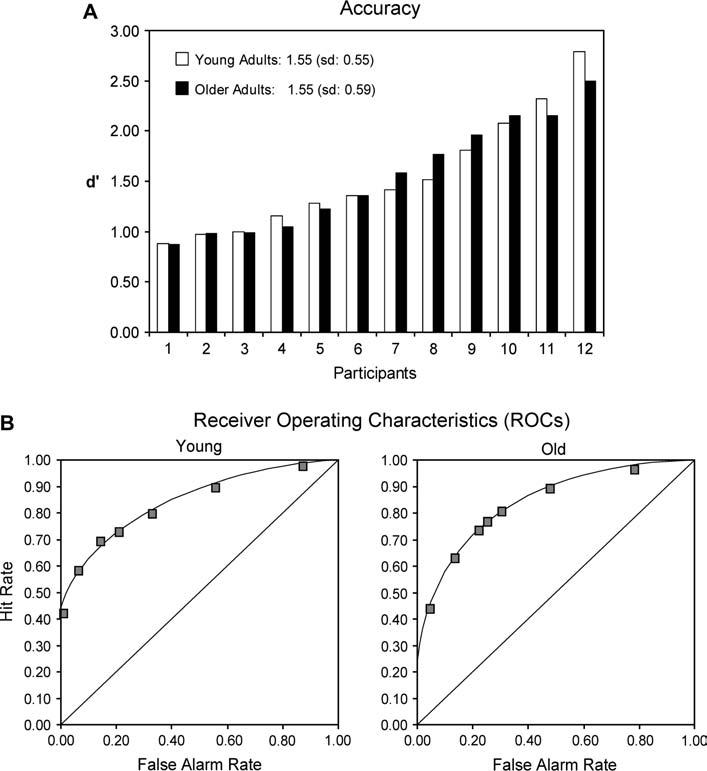
(A) Young and older adults were selected in rank order based on recognition accuracy in the scanner (d-prime). (B) ROCs in young and older adults. The flatter shape and greater asymmetry of the ROC in young compared with older adults indicate a greater contribution of recollection in the former group.
Prior to the scan session, several psychometric tests were administered in a separate testing session. The battery included the Brief Visuospatial Memory Test (BVMT)—Revised (Benedict and others 1996), the digit span and digit symbol subtests of the Wechsler Adult Intelligence Scale-Revised (Wechsler 1987), the Shipley vocabulary test (Shipley 1946), and the Raven Progressive Matrices (Raven 1941). Table 1 lists participant characteristics and neuropsychological data for the 2 groups. Older adults performed poorer on the BVMT (word memory: trial 2, P = 0.015; trial 3, P = 0.0015; delayed recall: P = 0.015), digit symbol (P = 0.004), and the Raven (Set C, P = 0.0027; Set E, P = 0.006), but, in keeping with the cognitive aging literature (Verhaeghen 2003), they outperformed the young adults on the Shipley vocabulary test (P = 0.0002).
Table 1.
Participant characteristics and neuropsychological data
| Young (N = 12) |
Old (N = 12) |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age | 22.2 | 2.5 | 69.2 | 7.6 |
| Educational years | UG | UG | 18.1 | 1.01 |
| MMSE | — | — | 29.8 | 0.4 |
| BVMT (picture memory) | ||||
| Trial 1 | 9.17 | 1.95 | 8.33 | 1.23 |
| Trial 2 | 10.83 | 0.94 | 11.25 | 0.62 |
| Trial 3 | 11.67 | 0.49 | 11.50 | 0.67 |
| Delayed recall | 11.33 | 0.78 | 11.58 | 0.67 |
| BVMT (word memory) | ||||
| Trial 1 | 8.58 | 1.93 | 6.67 | 2.57 |
| Trial 2* | 11.25 | 1.42 | 9.42 | 1.93 |
| Trial 3** | 11.83 | 0.58 | 10.50 | 1.17 |
| Delayed recall* | 11.75 | 0.87 | 10.17 | 1.90 |
| Digit symbol (speed)** | 38.08 | 1.56 | 34.17 | 2.66 |
| Shipley vocabulary** | 55.67 | 9.32 | 70.17 | 12.54 |
| Digit span | ||||
| Forward | 10.67 | 1.61 | 11.42 | 1.88 |
| Backward | 9.83 | 2.44 | 11.25 | 1.66 |
| Ascending | 12.33 | 1.30 | 12.00 | 1.28 |
| Raven (fluid intelligence) | ||||
| Test A | 12 | 0 | 12 | 0 |
| Test B | 11.92 | 0.29 | 11.75 | 0.62 |
| Test C** | 12.00 | 0.00 | 10.83 | 1.19 |
| Test D | 11.67 | 0.49 | 10.83 | 1.59 |
| Test E** | 10.92 | 1.00 | 8.75 | 2.26 |
Note: Mean, M; MMSE, minimental status examination; UG, undergraduate.
P < 0.05;
P < 0.01.
Experimental Protocol
Prior to scanning, participants studied an intermixed list of 120 English words and 80 pronounceable nonwords at a rate of 2 s per item within the context of a lexical decision task. Participants responded whether the target was an English word or not, and they were told that there would be a memory test later for the English words. In order to match recognition performance in the 2 groups, the older adults performed the same lexical decision task twice. We chose this particular performance manipulation because behavioral studies have shown that spaced stimulus repetition has greater beneficial effects on recollection than on familiarity, leading to an increase in the recollection/familiarity ratio (Parkin and Russo 1993; Parkin and others 1995; Benjamin and Craik 2001). At the same time, we predicted that aging would have detrimental effects on recollection but preserved familiarity, leading to a decrease in the recollection/familiarity ratio. In other words, we chose a specific manipulation that would lead to similar recognition accuracy in young and older adults but, at the same time, would not be expected to drive the predicted age-related shift from recollection to familiarity.
Functional scanning occurred approximately 30 min following this study phase. During the scan session, participants performed 2 kinds of tasks, a recognition memory task and a nonmnemonic perceptual judgment task. These tasks were presented across 6 experimental runs (4 recognition and 2 perceptual, counterbalanced in order across participants), each lasting 442 s. During the recognition memory blocks, participants saw an equal mix of old words shown during the earlier lexical decision task and completely new words (60 total words per block). Each trial consisted of 2 phases. Participants first made an old/new judgment on the presented words (3.4 s) and were then prompted to report their confidence (1.7 s) for their answer from 1 (lowest confidence) to 4 (highest confidence), followed by an intertrial interval between 0 and 5.4 s. The perceptual judgment task involved participants viewing rectangles unevenly divided into 2 colored sections, blue and orange, by a random jagged line. Participants determined which color had the greater surface area. Again, this judgment was followed by a confidence rating. Data from the perceptual task are not reported here.
Stimulus Materials
The critical stimuli consisted of 240 5-letter words that were selected from the machine readable dictionary (MRC) psycholinguistic database (http:/www.psy.uwa.edu.au/MRCDataBase/mrc2.html). The words were of moderate frequency (mean 39), concreteness (mean 504), and imageability (mean 510).
fMRI Scanning
Images were collected using a 4-T GE scanner. High-resolution T1-weighted structural images (256 × 256 matrix, time repetition [TR] = 12 ms, echo time [TE] = 5 ms, field of view [FOV] = 24 cm, 68 slices, 1.9-mm slice thickness, 0-mm spacing) were collected first. Coplanar functional images were subsequently acquired utilizing an inverse spiral sequence (64 × 64 image matrix, TR = 1700 ms, TE = 31 ms, FOV = 24 cm, 34 slices, 3.8-mm slice thickness). Scanner noise was reduced with earplugs, and head motion was minimized with foam pads. Stimuli were presented with liquid crystal display goggles (Resonance Technology, Inc.). Behavioral responses were recorded with a 4-key fiber-optic response box (Resonance Technology, Inc.).
Data Analysis
Preprocessing and data analysis were performed using statistical parametric mapping software implemented in Matlab (SPM2; Wellcome Department of Cognitive Neurology, London, UK). After discarding initial volumes to allow for scanner stabilization, images were slice-timing corrected and motion corrected, spatially normalized to the Montreal Neurological Institute template, and smoothed using a Gaussian kernel of 8-mm full width half maximum. For each subject, trial-related activity was modeled by convolving a vector of trial onsets with a canonical hemodynamic response function within the context of the general linear model (GLM).
Perceived Oldness Function Analyses
To examine age-related changes in the neural correlates of recollection and familiarity, we employed a parametric approach with 4 steps. First, we created a 3-point oldness scale combining the old/new judgments with the confidence ratings (1, new—all new responses; 2, probably old—old responses with confidence levels 1-3; 3, definitely old—old responses with confidence level 4). The rationale for collapsing some confidence levels was to minimize group differences in response frequency and to ensure a sufficient number of trials in all 3 perceived oldness levels. The number of trials for perceived oldness levels 1, 2, and 3 was, respectively, 127, 59, and 51 for the young and 109, 63, and 56 for the old. Confirming the absence of group differences in response frequency, a 2 (group: young, old) × 3 (oldness: 1-3) analysis of variance (ANOVA) yielded no significant effects of group (P = 0.5) or group × oldness interaction (P = 0.6).
Second, to identify regions of interest (ROIs) showing familiarity-related “activity decreases” as a function of oldness, we created a GLM in which trial onsets were collapsed across accuracy and confidence and modulated by the oldness scale in reverse fashion (i.e., 3, 2, 1) using the first-order parametric modulation option integrated in SPM2. Subsequently, a random-effects analysis (P < 0.001, uncorrected, cluster size = 4) was performed on the parameter estimates of the parametric regressor using the combined data from the young and older adults (N = 24). The reason for collapsing data across age groups was to define general ROIs for the direct group comparisons at a later stage.
Third, to identify ROIs showing “activity increases” as a function of oldness, we created another GLM in which we only compared the 2 extremes of the oldness scale, that is, level 3 (definitely old) versus level 1 (new), again using random-effects analyses on the combined data from the 2 groups (N = 24, P < 0.001, cluster size = 4). The rationale for not including the middle level (oldness level 2) in this case was to remain neutral regarding the shape of the function. Then, to distinguish between “recollection-related increases” and “familiarity-related increases,” we created GLMs collapsing across age groups in which trials were modulated either by a linear oldness function (i.e., 1, 2, 3) or by a quasi-exponential oldness function with a sharp activity increase for the highest confidence level (i.e., 1, 2, 9). Subsequently, the parametric regressors for linear and quasi-exponential increases were directly compared using paired t-tests at P < 0.05, uncorrected, and masked with the regions showing significant increases in the previous analysis.
Finally, within the ROIs identified by the earlier analyses, age differences in recollection and familiarity were assessed by directly comparing the relevant perceived oldness functions (recollection: 1, 2, 9; familiarity-related increases: 1, 2, 3; familiarity-related decreases: 3, 2, 1) using 2-sample t-tests at P < 0.05, uncorrected.
Individual Trial Analyses
To test the hypothesis that young adults rely more on the hippocampus during recognition memory performance, whereas older adults rely more on rhinal regions, we conducted a 2nd analysis based on “individual trial activity.” As a 1st step, we created a GLM, in which each individual trial was modeled by a separate covariate, yielding different parameter estimates for each individual trial and for each individual subject. An issue with this design is that it does not systematically account for overlap of individual trial-evoked hemodynamic responses (Rissman and others 2004). However, because the stimulus onset asynchrony (SOA) was fairly long in the present study (mean SOA = 7.13 s), the hemodynamic overlap across trials was relatively minor. The validity of this design was confirmed by the fact that we obtained highly comparable results based on linear contrasts of the single-trial parameter estimates compared with those obtained with a standard GLM (data not shown). Moreover, a similar procedure has been successfully applied in a previous fMRI study with a similar design (Daselaar and others 2005). As a 2nd step, mean activity (i.e., mean parameter estimates) was extracted for each individual trial from the hippocampal and rhinal regions identified by the previous oldness function analyses. For each group, the resulting values were then entered into a mixed-effect multiple regression model with activity in the 2 MTL regions as independent variables, the 3-point oldness scale as the dependent variable, and subject as a random factor.
Results
Behavioral Results
Table 2 lists behavioral data in the young and older groups. As can be seen in the top part of Table 1, our efforts to equate memory performance in young and older adults paid off: there were no significant group differences in d-prime (P = 1.00) and corrected recognition (P = 0.93) scores. However, we did see significant differences in reaction times. A 2 (group: young, older) × 3 (oldness scale: new, probably old, definitely old) repeated-measures ANOVA yielded significant main effect of oldness (F2,44 = 79.6; P < 0.0001), reflecting faster response times for definitely old than for the other responses, and a reliable main effect of group (F1,22 = 5.7; P = 0.03), reflecting slower responses in older adults. There was also a significant group × oldness interaction (F2,44 =; P = 0.04s), reflecting greater age-related slowing for new and probably old responses than for definitely old responses.
Table 2.
Behavioral results
| Young |
Old |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Recognition accuracy | ||||
| d-prime | 1.55 | 0.55 | 1.55 | 0.59 |
| Corrected recognition (hit--false alarm) | 0.53 | 0.16 | 0.52 | 0.16 |
| Reaction times | ||||
| New | 1.54 | 0.25 | 1.87 | 0.26 |
| Probably old | 1.64 | 0.32 | 1.90 | 0.30 |
| Definitely old | 1.27 | 0.25 | 1.40 | 0.24 |
| Familiarity estimates | 1.05 | 0.53 | 1.21 | 0.39 |
| Recollection estimates | 0.41 | 0.17 | 0.28 | 0.19 |
Figure 1B displays ROC curves for the young and older adults. ROCs relate the proportion of hits to false alarms as a function of recognition confidence and are constructed by cumulating from the least to the most confident responses on a scale from definitely old (old responses with confidence level 4) to definitely new (new responses with confidence level 4). The 1st point on the ROC (the leftmost point on the graph in Fig. 1B) identifies the most confident hit and false alarm proportions. The next point cumulates the initial and the next most confident proportions, and the process is continued until the confidence levels are exhausted. This cumulation process results in a maximum (the rightmost point on the graph in Fig. 1B) for which the proportions of both hits and false alarms are always one.
The importance of the ROC is that its shape is predicted to change based on the relative contributions of recollection and familiarity, given key assumptions about the signal distributions underlying each process (MacMillan and Creelman 1991; Yonelinas 1994). Increasing the contribution of recollection increases the asymmetry of the ROC and flattens its surface. In contrast, increasing the relative contribution of familiarity introduces more curvilinearity and symmetry to the ROC. Several reports indicate that damage to the hippocampus affects the shape of the expressed ROC in both humans and rodents in a manner consistent with a largely selective deficit in recollection (Yonelinas and others 1998; Yonelinas and others 2002; Fortin and others 2004). Here, using the same method that demonstrated recollection deficits due to selective hippocampal lesions in rodents (Fortin and others 2004), we fitted the ROC of each young and elderly participant in order to obtain estimates of recollection and familiarity processes (MacMillan and Creelman 1991; Yonelinas 1994). The bottom part of Table 2 shows the recollection estimates for the 2 groups that were derived from the ROCs. Direct comparisons of the process estimates in young and older adults with a 1-tailed t-test showed significantly greater recollection estimates in young compared with older adults (P = 0.05). Thus, although recognition accuracy was similar between the 2 groups, the difference in shape of the ROCs in the 2 groups indicates a greater contribution of recollection in the young adults.
fMRI Results
Perceived Oldness Function Analyses
Table 3 lists regions showing similarities and differences in recollection- and familiarity-related activity between the young and older adults. As noted in the Introduction, we predicted that older adults would show weaker activity in regions associated with recollection but similar activity in regions associated with familiarity. Within MTL, we predicted that older adults would show a reduction in the recollection-related hippocampal activity but a preserved, or even enhanced, familiarity-related rhinal activity. Likewise, in left parietal and posterior midline regions, we expected age-related reductions in recollection-related but not in familiarity-related activations. The results generally confirmed our predictions.
Table 3.
Brain regions showing recollection-and familiarity-related activity
| Regions | Brodmann area | Side | Talairach |
Oldness-related increase/decrease |
Exponential > linear | Young > old | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Recollection | ||||||||
| Hippocampus | — | L | −23 | −26 | −9 | 3.88 | 2.95 | 4.24 |
| Partietotemporal cortex | 39/40 | L | −53 | −57 | 38 | 3.65 | 2.07 | 4.01 |
| Retrosplenial cortex | 30/23 | L | −4 | −55 | 17 | 5.27 | 1.94 | 5.70 |
| Cingulate gyrus | 24 | — | 0 | −2 | 35 | 3.92 | 2.31 | 5.09 |
| Medial PFC | 10 | L | 0 | 49 | 19 | 4.33 | 1.96 | 6.32 |
| Primary motor cortex | 4 | L | −34 | −23 | 57 | 3.50 | 1.89 | 2.90 |
| Visual cortex | 18/19 | R | 4 | −85 | −2 | 6.54 | 2.07 | 5.01 |
| Cerebellum | — | R | 30 | −49 | −20 | 4.90 | 2.08 | 3.90 |
| Familiarity-related increase | ||||||||
| Parietooccipital cortex | 19/39 | L | −38 | −76 | 28 | 6.27 | −2.30 | — |
| Dorsal posterior cingulate | 31 | R | 4 | −35 | 44 | 3.75 | −1.72 | — |
| Familiarity-related decrease | ||||||||
| Rhinal cortex | 28/34 | L | −26 | −1 | −26 | −4.09 | — | −1.97 |
| Fusiform gyrus | 37 | R | 49 | −41 | −17 | −4.54 | — | −2.53 |
| Lateral temporal cortex | 20 | L | −49 | −19 | −18 | −3.95 | — | −2.59 |
| PFC | 44 | R | 45 | 12 | 10 | −3.71 | — | — |
| Caudate nucleus | — | L | −19 | 12 | 13 | −3.81 | — | — |
| Thalamus | — | L | −19 | −27 | 4 | −5.43 | — | — |
| Occipital cortex | 18 | L | −30 | −80 | 8 | −4.33 | — | — |
| Cerebellum | — | R | 34 | −57 | −35 | −6.33 | — | — |
| Premotor cortex | 6 | L | −45 | −6 | 35 | −4.20 | — | 2.25 |
| 6 | R | 53 | 5 | 28 | −5.54 | — | 2.42 | |
| Frontal pole | 10 | L | −30 | 52 | 8 | −3.70 | — | 2.79 |
| Anterior cingulate | 32 | R | 4 | 21 | 41 | −5.75 | — | 1.94 |
| Superior parietal cortex | 7 | R | 15 | −67 | 56 | −3.58 | — | 2.84 |
| Insula | — | L | −38 | −29 | 15 | −3.92 | — | 2.35 |
Note: Italics were used to stress that the values are negative and should be interpreted in the opposite direction (that is, a negative value for young > old means a significantly greater value for old).
Medial Temporal Lobe
Within MTL, there was a dissociation with age between the hippocampus and the rhinal cortex. As shown in the line graphs in Figure 2A, there was an age-related reduction in the recollection-related quasi-exponential function in the hippo-campus: whereas young adults showed a distinct exponential increase in hippocampal activity as indicated by a sharp rise in activity for the highest oldness level (definitely old), the older adults showed a less pronounced exponential increase as a function of the 3-point perceived oldness scale. The rhinal cortex, in contrast, showed an age-related enhancement in the familiarity-related continuous linear function (activity decrease). As shown in the line graphs in Figure 2B, both groups showed a linear decrease in rhinal activity with increasing levels of oldness, but the function showed a steeper decline in the older adults.
Figure 2.
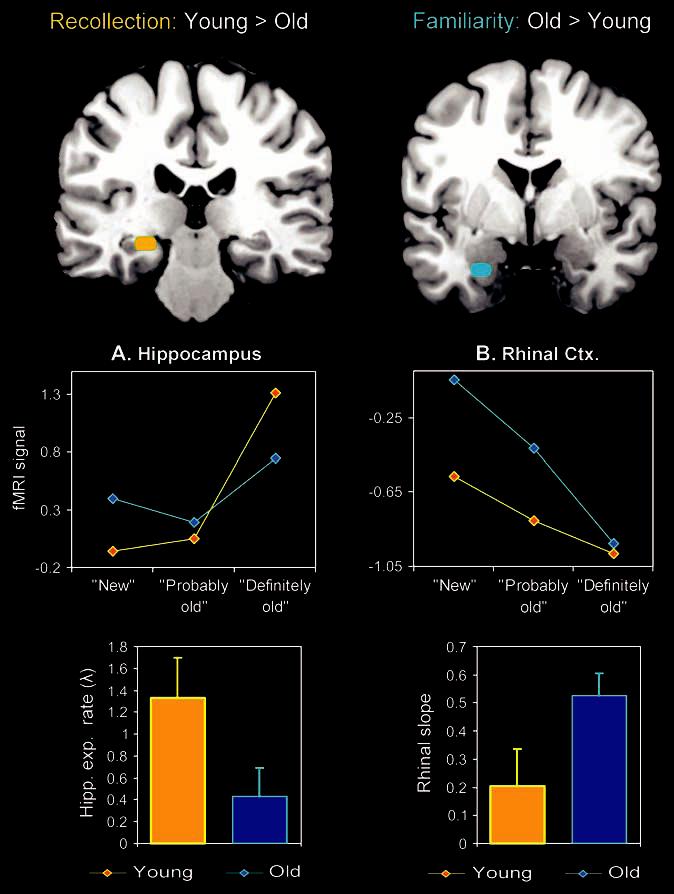
The effects of aging yielded a double dissociation between 2 MTL subregions: whereas recollection-related (exponential) activity in the hippocampus was attenuated by aging, familiarity-related (linear) activity in the rhinal cortex was enhanced by aging.
We further confirmed these observations by fitting exponential (i.e., y = a exp(λx), after normalizing the 1st point to zero) and linear (i.e., y = ax + b) functions, respectively, to each participant's perceived oldness-related activity in the hippocampus and rhinal cortex. Subsequently, the relevant parameters for these functions were directly contrasted using 1-tailed 2-sample t-tests. To capture the sharpness of the hippocampal perceived oldness function, we used the exponential rate parameter lambda (λ), which determines the rate of the exponential increase. To capture the steepness of the rhinal oldness function, we used the magnitude of the “negative” linear slope. As shown in the bar graphs in Figure 2A, young adults showed a significantly sharper exponential increase than older adults in the hippocampus (young adults: λ = 1.33, standard deviation [SD] = 1.28; older adults: λ = 0.43, SD = 0.91; P = 0.03). In contrast, as shown in the bar graphs in Figure 2B, older adults showed a significantly steeper decline than young adults in the rhinal cortex (young adults: negative slope = 0.20, SD = 0.45; older adults: negative slope = 0.53, SD = 0.27; P = 0.02). A 2 (group: young, old) × 2 (region/function: hippocampal exponential rate, rhinal slope) ANOVA confirmed these results by showing a significant age × region/function interaction (P = 0.01).
Other Brain Regions: Parietal and Posterior Midline Cortex
In addition to the dissociation in MTL, we also found differential age effects on the neural correlates of recollection and familiarity in 2 regions outside MTL, left parietal cortex, and the posterior midline region. Within left parietal cortex, there was a dissociation with age between parietotemporal and parietooccipital cortex. As shown in Figure 3A, older adults showed a reduction in the recollection-related exponential function in parietotemporal cortex: whereas young adults showed a clear exponential increase in parietotemporal activity as indicated by a sharp rise in activity for the highest perceived oldness level (definitely old), the older adults showed a less pronounced exponential increase as a function of perceived oldness. This finding was confirmed by direct comparison of the exponential rate parameters in young and older adults (young adults: λ = 0.73, SD = 1.05; older adults: λ = 0.44, SD = 0.42; P = 0.04). In contrast to the age-related reduction in parietotemporal activity, young and older adults showed a similar familiarity-related continuous linear function (activity decrease) in the parietooccipital cortex. As shown in Figure 3B, both groups showed a comparable continuous linear increase in parietooccipital activity as a function of oldness. The lack of group differences in this region was confirmed by a 2-sample t-test on the slope of the individual functions (young adults: mean slope = 0.23, SD = 0.25; older adults: mean slope = 0.27, SD = 0.18) indicating no significant group differences (P = 0.4).
Figure 3.
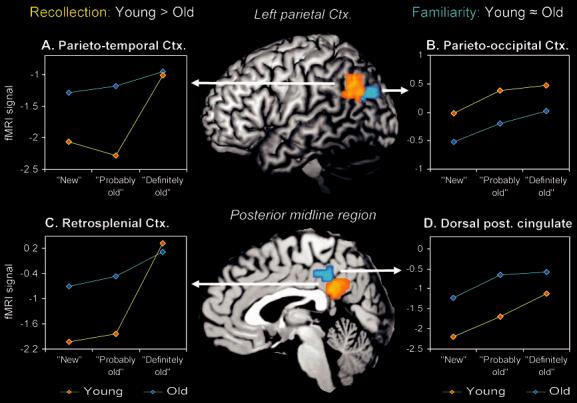
Differential effects of aging on recollection-related (exponential) and familiarity-related (linear) activity in different subregions within left parietal and the posterior midline regions. Within the left parietal region, aging attenuated recollection-related parietotemporal activity but not familiarity-related parietooccipital activity. Within the posterior midline region, aging attenuated recollection-related retrosplenial activity but not familiarity-related dorsal posterior cingulate activity.
Within the posterior midline region, there was an age dissociation between a retrosplenial/ventral posterior cingulate region and a dorsal posterior cingulate region. As shown in Figure 3C, older adults again showed a reduction in the recollection-related exponential function in the retrosplenial/ventral posterior cingulate cortex: whereas young adults showed a marked exponential increase in retrosplenial/ventral posterior cingulate activity as indicated by a sharp rise in activity for the highest oldness level, the older adults showed a less pronounced exponential increase with oldness. Confirming this observation, a direct comparison of the exponential rate parameters revealed a sharper exponential increase in younger adults than in older adults (young adults: λ = 1.41, SD = 1.1; older adults: λ = 0.49, SD = 0.49; P < 0.0001). In contrast to the age-related reduction in retrosplenial/ventral posterior cingulate activity, young and older adults showed a similar familiarity-related continuous linear function (activity increase) in the dorsal posterior cingulate cortex. As shown in Figure 3D, the linear increase in dorsal posterior cingulate activity as a function of oldness showed a similar slope in both groups. Again, the lack of group differences in this region was confirmed by a direct comparison of the slopes of the linear functions, which indicated no significant group difference (young adults: mean slope = 0.53, SD = 0.61; older adults: mean slope = 0.35, SD = 0.52; P = 0.2).
Individual Trial Analyses
In order to examine if the hippocampus and rhinal cortex make different contributions to recognition performance in young and older adults, we performed mixed-effects multiple regression analyses with mean individual trial activity in the hippocampus and rhinal cortex as predictors, the perceived oldness scale as the variable of interest, and subjects as a random factor. As shown in Table 4, the only region that made a significant contribution in young adults was the hippocampus (P < 0.0001). In contrast, both MTL regions made significant contributions in older adults. However, the contribution of the rhinal cortex was much stronger (P = 0.001) than that of the hippocampus (P = 0.02). Hence, in line with the previous perceived oldness function analyses, the individual trial analyses also suggest an age-related shift from recollection-based (hippocampus) to familiarity-based (rhinal cortex) recognition.
Table 4.
Individual trial multiple regression results with 3-point perceived oldness scale as dependent variable
| Predictors (individual trial activity) |
Young |
Old |
||||
|---|---|---|---|---|---|---|
| Beta value | T value | P value | Beta value | T value | p value | |
| Hippocampus | 0.06 | 4.64 | <0.0001 | 0.03 | 2.28 | 0.02 |
| Rhinal cortex | −0.02 | −1.63 | 0.10 | −0.04 | −3.27 | 0.001 |
Discussion
The present study yielded 2 main findings regarding the effects of aging on the neural correlates of recollection and familiarity. First, we found a double dissociation between 2 MTL regions: whereas the hippocampus showed recollection-related activity that was attenuated by aging, the rhinal cortex showed familiarity-related activity that was enhanced by aging. Confirming the different role of these regions on memory performance in young and older adults, multiple regression models based on individual trial activity showed that recognition performance in young adults was primarily predicted by changes in hippocampal activity, whereas recognition performance in older adults was better predicted by changes in rhinal activity. Second, in different subregions of left parietal and posterior midline cortices, recollection-related activity was attenuated in older adults, whereas familiarity-related activity was preserved. Within the left parietal cortex, aging attenuated recollection-related activity in a parietotemporal region but not familiarity-related activity in a parietooccipital region. Within the posterior midline region, aging attenuated recollection-related activity in the retrosplenial cortex but not familiarity-related activity in dorsal posterior cingulate cortex. Below, we discuss the 2 main findings, and then we consider the implications of the findings regarding the diagnosis of AD.
Double Dissociation Within the MTLs
The finding that older adults showed reduced recollection-related activity in the hippocampus compared with young adults is consistent with previous anatomical, behavioral, and functional evidence and provides the first direct link between these 3 lines of evidence. Regarding anatomy, there is a large body of postmortem and structural magnetic resonance imaging (MRI) evidence showing marked age-related atrophy in the hippocampus (Raz 2005). Cross-sectional MRI studies have indicated that the hippocampus declines at a rate of 2-3% per decade (Raz 2005), and hippocampal shrinkage has been directly linked to age-related impairments in episodic memory function (De Leon and others 1997). Regarding behavior, there is a wealth of behavioral evidence of age-related deficits in recollection (Parkin and Walter 1992; Davidson and Glisky 2002; Bastin and Van der Linden 2003; Howard and others 2005). In keeping with this evidence, using a method that demonstrated recollection deficits in rodents with selective hippocampal lesions (Fortin and others 2004), we found significantly lower recollection estimates in older adults (0.28) than in younger adults (0.41). Regarding function, several fMRI studies have reported age-related decreases in hippocampal activity during working memory maintenance (Mitchell and others 2000; Park and others 2003; Cabeza and others 2004), episodic encoding (Grady and others 1995; Daselaar and others 2003a, 2003b; Gutchess and others 2005), and recognition memory (Cabeza and others 2004). Integrating these anatomical, behavioral, and functional findings, the present study provided a direct link between recollection deficits and hippocampal decline in older adults.
In contrast with the reduction in recollection-related activity in the hippocampus, in the rhinal cortex, older adults showed enhanced familiarity-related activity. This finding is in agreement with anatomical evidence of preserved rhinal volume and with behavioral evidence of preserved familiarity measures in healthy older adults. Regarding anatomy, a recent longitudinal MRI study examined volumetric changes in different brain regions over a period of 5 years in a sample of participants ranging from young to old adulthood (Raz and others 2005). In line with previous cross-sectional studies, the results indicated marked shrinkage of the hippocampus over the 5-year period, which increased exponentially beyond the age of 55 years. In sharp contrast, the same study found minimal age-related changes in the rhinal cortex, suggesting that rhinal anatomy is relatively preserved in healthy older adults. Moreover, given the presumed role of the rhinal cortex in familiarity (Brown and Aggleton 2001), the finding of preserved rhinal activity during recognition memory is also consistent with the aforementioned behavioral evidence of preserved familiarity-based recognition in older adults (e.g., Parkin and Walter 1992; Davidson and Glisky 2002; Bastin and Van der Linden 2003; Howard and others 2005). Linking these anatomical and behavioral findings, the present fMRI study provides the first evidence that memory-related activity in the rhinal cortex is preserved in older adults.
Moreover, the finding that familiarity-related rhinal activity was greater (steeper slope) in older than in younger adults suggests that the contribution of rhinal cortex to familiarity is not just preserved but it is actually augmented in older adults. In view of the age-related reduction in hippocampal activity, this finding suggests that, during recognition performance, older adults may compensate for deficits in hippocampus-based recollection by relying more on rhinal-based familiarity. Given that the hippocampus shows anatomical decline in healthy aging, whereas the rhinal cortex does not (Raz and others 2005), the present MTL dissociation suggests a shift from a memory process mediated by a declining brain region to a memory process mediated by a preserved brain region. Consistent with this idea, multiple regression analyses directly linking activity in individual trials to perceived oldness scores in the same trials showed that recognition performance in young adults was predicted mainly by changes in hippocampal activity, whereas performance in older adults was predicted mainly by changes in rhinal activity (see Table 4).
The notion that older adults rely more on rhinal novelty signals than young adults assumes that rhinal activity can be modulated in a top-down fashion, for instance, by selectively attending to the perceptual salience of study items. One possible candidate for such a control mechanism is the prefrontal cortex (PFC). There is much evidence that PFC regions can enhance novelty processes by exerting top-down control over sensory areas in order to bias the salience of perceptual signals (Corbetta and Shulman 2002; Ranganath and Rainer 2003). Moreover, the strong anatomical and functional connections between PFC and MTL (Simons and Spiers 2003) suggest that PFC could have modulated rhinal activity in older adults. To investigate this hypothesis, we conducted a functional connectivity analysis based on individual trial data. For each individual subject, we correlated activity in the rhinal cortex with activity in all other brain voxels across individual trials (Rissman and others 2004). As shown in Figure 4, consistent with this hypothesis, older adults showed significantly greater correlations than younger adults between rhinal cortex and both left (Fig. 4A: x, y, z = −34, 18, −10; T = 4.82) and right (Fig. 4B: x, y, z = 41, 22, −4; T = 4.91) PFC regions. Thus, the present study suggests that older adults shifted from recollection-related hippocampal signals to familiarity-related novelty rhinal signals via the modulatory control of PFC.
Figure 4.
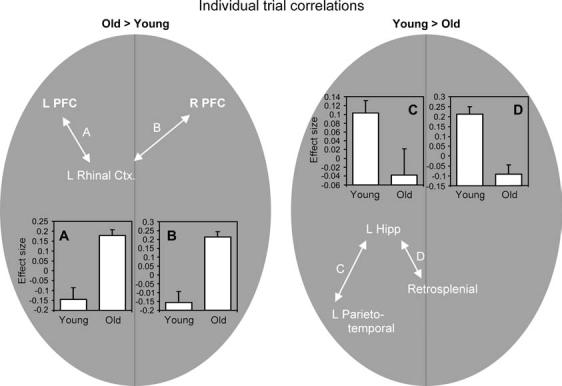
Correlation analyses using individual trial activity showed an age-related increase in connectivity within a rhinal-frontal network (A, B) coupled with an age-related decrease in connectivity within a hippocampal-retrosplenial/parietotemporal network (C, D). Group comparisons were based on 2-sample tests at P < 0.05 on the individual correlation maps, inclusively masked with the relevant main effects at P < 0.001 (e.g., young > old, inclusively masked with young). The bar graphs indicate effect sizes of the individual trial correlations on the group level.
Other Brain Regions: Parietal and Posterior Midline Cortex
In addition to the double dissociation within MTL, we also found differential age effects on the neural correlates of recollection and familiarity in left parietal and posterior midline regions. Within the left parietal cortex, older adults showed a reduced recollection-related parietotemporal activity (Fig. 3A) but preserved familiarity-related parietooccipital activity (Fig. 3B). Within the posterior midline, older adults showed a reduced recollection-related activity in the retrosplenial cortex (Fig. 3C) but preserved familiarity-related activity in dorsal posterior cingulate cortex (Fig. 3D). These findings are consistent with the aforementioned evidence that older adults are impaired in recollection but not in familiarity.
The dissociation between different parietal and posterior midline subregions could be explained by the divergent anatomical connections of these regions. In the case of lateral-parietal regions, the recollection-related parietotemporal area approximates the location of region 7a in macaques, which is a region with strong reciprocal projections to the hippocampus (Suzuki and Amaral 1994; Clower and others 2001). In contrast, the proximity of the familiarity-related parietooccipital region to secondary visual cortex suggests that this region may have a greater role in visuoperceptual processes. Likewise, in the case of posterior midline regions, the recollection-related retrosplenial region has strong connections with posterior MTL regions, including the hippocampus, whereas the familiarity-related dorsal posterior cingulate region has dense connections with visuospatial areas (Kobayashi and Amaral 2003). In sum, the strong connections of parietotemporal and retrosplenial regions with MTL suggest that these areas have a more direct role in episodic memory functions, whereas the connections of parietooccipital and dorsal posterior cingu-late regions with sensory regions fit well with the evidence that, compared with recollection, familiarity is more dependent on perceptual processes (Yonelinas 2002).
In line with their anatomical connectivity, the parietotemporal and retrosplenial cortex also show strong functional connectivity with the hippocampus. All 3 regions are part of a network of brain areas that tend to show functional deactivation during task performance as compared with a passive baseline (Stark and Squire 2001; Greicius and others 2003, 2004). According to a recent theory, these regions form a “default” network involved in specific cognitive processes that occur during passive rest and that are suspended during demanding cognitive tasks (Gusnard and Raichle 2001; Raichle and others 2001). Recent studies have demonstrated high functional connectivity between the default regions in young adults (Greicius and others 2003, 2004; Damoiseaux and others 2005). At the same time, there are indications that aging disrupts default network connectivity (Lustig and others 2003; Greicius and others 2004). For instance, a recent study examined default connectivity in young adults, healthy older adults, and Alzheimer's dementia (AD) patients (Greicius and others 2004). Although the study specifically focused on the comparison between healthy older adults and the AD patients, there were also indications of reduced connectivity in the healthy older adults (M. Greicius, personal communication).
In view of these findings, we examined whether age differences in functional connectivity between these regions also existed in the present study by conducting a correlation analysis based on individual trial activity, similar to the one we used to assess rhinal connectivity. As shown in Figure 4, the results showed significantly greater correlations in young compared with older adults between individual trial activity in hippocampus and both left parietotemporal (Fig. 4C: x, y, z = −41, −58, 27; T = 2.13) and retrosplenial cortex (Fig. 4D: x, y, z = −4, −51, 20; T = 5.04). Taken together with the rhinal connectivity findings, these results suggest that older adults compensated for deficits in a recollection-related hippocampal-restroplenial/parietotemporal network by shifting to a familiarity-related rhinal-frontal network. Further research is required in order to characterize the operation of this compensatory mechanism in greater detail.
Clinical Implications
The present fMRI study showed age differences in 3 brain regions that have also been implicated in the early diagnosis of AD: the hippocampus, the retrosplenial cortex, and the rhinal cortex (Nestor and others 2004). Regarding hippocampus, postmortem studies indicate that this region is one of the 1st areas to be affected by AD as indicated by neurofibrillary pathology (Arriagada and others 1992), and the extent of hippocampal decline has been shown to be a reliable indicator of the progression of AD (Laakso and others 2000). Yet, as noted, structural decline of the hippocampus is also present in healthy aging (De Leon and others 1997), and several fMRI studies have found age-related reductions in hippocampal activity using a variety of cognitive tasks (Daselaar and others 2003a, 2003b; Cabeza and others 2004; Gutchess and others 2005). Our findings confirm the presence of hippocampal decline in healthy older adults and relate it specifically to deficits in recollection-based recognition.
Functional changes in the retrosplenial cortex have also been highlighted in the search for early AD markers. A recent positron emission tomography (PET) study found that patients who had a high risk of developing AD in the next few years (mild cognitive impairment [MCI]) showed reduced metabolism in a region near the retrosplenial cortex, whereas changes in other regions were not yet apparent (Minoshima and others 1997). Similarly, another PET study found that all MCI patients that participated in the study showed signs of hypometabolism restricted to the retrosplenial cortex (Nestor and others 2003). These metabolic findings fit well with the aforementioned finding of reduced default connectivity in this region in AD patients (Greicius and others 2004). Yet, despite these promising findings, we also found evidence of reduced retrosplenial function and connectivity in our sample of healthy older adults.
The rhinal cortex also shows pronounced atrophy in the early stages of AD (Pennanen and others 2004), but, contrary to the hippocampus, this region shows only minimal decline in healthy older adults (Raz and others 2005). Consequently, structural changes in the rhinal cortex have been found to be much more diagnostic of early AD than changes in the hippocampus (Pennanen and others 2004). Importantly, the present findings indicate that the changes in rhinal function between early AD patients and healthy older adults do not just represent a quantitative difference (i.e., reduced vs. preserved rhinal function, respectively) but rather a qualitative difference (i.e., reduced vs. enhanced rhinal function, respectively). Hence, the present results indicate that future studies aimed at an earlier diagnosis of AD could benefit from focusing specifically on changes in rhinal function rather than hippocampus or retrosplenial cortex because the latter regions also show decline in healthy older adults. Although the practicality of the recollection/familiarity distinction in early diagnosis still has to be demonstrated, implementation of the current fMRI paradigm may be promising in that respect.
Conclusion
In sum, aging differentially affected recollection-related and familiarity-related activity within MTL and within left parietal and posterior midline regions. Within MTL, there was a double dissociation between the hippocampus, whose recollection-related activity was attenuated by aging, and the rhinal cortex, whose familiarity-related activity was enhanced by aging. Within left parietal and posterior midline cortices, aging attenuated recollection-related activations (in parietotemporal and retrosplenial regions) but not familiarity-related activations (in parietooccipital and dorsal posterior cingulate regions). Directly linking the MTL double dissociation to performance, multiple regression models showed that in young adults recognition performance was primarily predicted by changes in hippocampal activity, whereas in older adults recognition performance was primarily predicted by changes in rhinal activity. Functional neuroimaging studies have associated recollection with the hippocampus and familiarity with the rhinal cortex, and behavioral studies have shown that older adults tend to be impaired in recollection but not in familiarity. Integrating these 2 lines of research, the present study provides the first direct evidence that the differential effects of aging on recollection versus familiarity reflect differential effects of aging on hippocampal versus rhinal activity. Moreover, rhinal was actually enhanced by aging, suggesting that older adults compensate for reduced hippocampal-mediated recollection by relying more on rhinal-mediated familiarity. Furthermore, the present study provided evidence that this shift within MTL reflects changes in functional connectivity across the brain. Correlation analyses using individual trial activity showed an age-related reduction in connectivity within a hippocampal-retrosplenial/parietotemporal network coupled with an age-related increase in connectivity within a rhinal-frontal network. In sum, given that early AD is accompanied by deterioration of the rhinal cortex, the present finding of “enhanced” rhinal function in healthy older adults has direct implications for future clinical studies aimed at an earlier diagnosis of AD.
Footnotes
This was supported by a National Institutes of Health Grant (AG19731) to RC and by a National Institute of Aging Grant (AG 11622) to DJM. The authors would like to thank Amber Baptiste Tarter for assistance in subject recruitment and Rakesh Arya for analysis support.
References
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17:14–24. [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the brief visuospatial memory test: studies of normal performance, reliability, and validity. Psychol Assess. 1996;8:145–153. [Google Scholar]
- Benjamin AS, Craik FI. Parallel effects of aging and time pressure on memory for source: evidence from the spacing effect. Mem Cognit. 2001;29:691–697. doi: 10.3758/bf03200471. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: a positron emission tomography study. J Cogn Neurosci. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Simon D. Age differences in memory: the roles of attention and depth of processing. In: Poon LW, Fozard JL, Cermak LS, Arenberg D, editors. New directions in memory and aging. Proceedings of the George A. Talland Memorial Conference. Lawrence Erlbaum Associates; Hillsdale (NJ): 1980. [Google Scholar]
- Damoiseaux JS, Beckmann CF, Smith SM, Barkhof F, Stam CJ, Scheltens PH, Rombouts SARB. Consistent networks of resting state connectivity across healthy subjects measured with fMRI; Proceedings of the 11th Annual Meeting for the Organization for Human Brain Mapping Conference; 2005 June 12-16; Toronto: 2005. [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. A triple dissociation within the medial temporal lobes: recollection, familiarity, and novelty. 2005 doi: 10.1152/jn.01029.2005. Forthcoming. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiol Aging. 2003a;24:1005–1011. doi: 10.1016/s0197-4580(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003b;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci. 2002;2:174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- De Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. The hippocampus—what does it do? Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Grady CL, Mcintosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O'Reilly RC, Norman KA. Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus. 2002;12:341–351. doi: 10.1002/hipo.10011. [DOI] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and ROC curves. Psychol Aging. 2005 doi: 10.1037/0882-7974.21.1.96. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Java R. Effects of age on state of awareness following implicit and explicit word-association tasks. Psychol Aging. 1996;11:108–111. doi: 10.1037//0882-7974.11.1.108. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Lehtovirta M, Partanen K, Riekkinen PJ, Soininen H. Hippocampus in Alzheimer's disease: a 3-year follow-up MRI study. Biol Psychiatry. 2000;47:557–561. doi: 10.1016/s0006-3223(99)00167-5. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan NA, Creelman CD. Detection theory: a user's guide. Cambridge University Press; New York: 1991. [Google Scholar]
- Mantyla T. Knowing but not remembering: adult age differences in recollective experience. Mem Cognit. 1993;21:379–388. doi: 10.3758/bf03208271. [DOI] [PubMed] [Google Scholar]
- Maylor EA. Remembering versus knowing television themes in middle-aged and elderly adults. Br J Psychol. 1995;86:21–25. [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cogn Brain Res. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann Neurol. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer's disease. Nat Rev Neurosci. 2004;5:S34–S41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complex scenes: age differences in frontal and hippocampal activations. J Cogn Neurosci. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Gardiner JM, Rosser R. Functional aspects of recollective experience in face recognition. Conscious Cogn. 1995;4:387–398. doi: 10.1006/ccog.1995.1046. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Russo R. On the origin of functional differences in recollective experience. Memory. 1993;1:231–237. doi: 10.1080/09658219308258235. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychol Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso M, Hallikainen M, Vanhanen M, Nissinen A, Helkala E, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Raven JC. Standardization of progressive matrices, 1938. Br J Med Psychol. 1941;19:137–150. [Google Scholar]
- Raz N. The aging brain observed in vivo. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive neuroscience of aging. Oxford University Press; New York: 2005. pp. 19–57. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Institute of living scale. Western Psychological Services; Los Angeles, CA: 1946. [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Aging and vocabulary scores: a meta-analysis. Psychol Aging. 2003;18:332–339. doi: 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler adult intelligence scale—revised. The Psychological Corporation; New York: 1987. [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the consortium to establish a registry for Alzheimer's disease. Arch Neurol. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


