Abstract
To explore the function of adult hippocampal neurogenesis, we ablated cell proliferation by using two independent and complementary methods: (i) a focal hippocampal irradiation and (ii) an inducible and reversible genetic elimination of neural progenitor cells. Previous studies using these methods found a weakening of contextual fear conditioning but no change in spatial reference memory, suggesting a supportive role for neurogenesis in some, but not all, hippocampal-dependent memory tasks. In the present study, we examined hippocampal-dependent and -independent working memory using different radial maze tasks. Surprisingly, ablating neurogenesis caused an improvement of hippocampal-dependent working memory when repetitive information was presented in a single day. These findings suggest that adult-born cells in the dentate gyrus have different, and in some cases, opposite roles in distinct types of memory.
Keywords: hippocampus, irradiation, radial maze, interference
The fact that most mammals, including humans, continue to produce new neurons in the hippocampus throughout adulthood has led to the suggestion that neurogenesis may serve an important role in hippocampal-dependent memory processes (1–3). Consistent with this possibility, studies of adult rodents in which neurogenesis has been reduced, by systemic or whole-brain treatments or as a result of aging, suggest the involvement of these new neurons in some hippocampal-dependent tasks (4–7). Suppression of neurogenesis with the antimitotic agent MAM was shown to impair trace eyeblink and fear conditioning, whereas more restricted ablation strategies using irradiation or genetically targeted blockade of neurogenesis resulted in a weakening of contextual fear conditioning and had no effect in spatial learning (5, 8). Notably, all reported changes in hippocampal-dependent memory tasks after loss of adult neurogenesis have indicated either no role or a supportive role for this process in memory function. However, it is still unknown whether all types of hippocampal-dependent memory are similarly affected by the addition or presence of new neurons. This question is particularly relevant because the hippocampus is involved in a wide range of memory tasks.
For example, only a limited number of studies have begun to examine the role of adult hippocampal neurogenesis in tests of working memory, a form of short-term memory that involves both the hippocampus and the prefrontal cortex (9, 10). In adult rats, performance in a water maze task that utilizes short-term non-spatial memory was impaired after disruption of neurogenesis by whole brain irradiation (7). In the present study, we sought to clarify the contribution of neurogenesis to working memory by using two different strategies that have been shown to eliminate new neurons from the adult hippocampus. The first utilizes a focal x-irradiation procedure that results in a permanent loss of neurogenesis within the hippocampus of adult mice but spares neurogenesis in the subventricular zone and the olfactory bulb (11). The second is a complementary genetic approach in which dividing glial acidic fibrillary protein (GFAP)-positive cells, known to be progenitors of new neurons, are selectively eliminated by administering the drug ganciclovir (GCV) (12).
After both ablation procedures, we found a surprising improvement of working memory performance, but only in tasks where mice were required to discriminate highly similar cues presented closely in time (within a single session). Also, this effect was limited to trials in which a long temporal delay (30+ sec) was presented, consistent with previous observations that, in rodents, the hippocampus becomes crucial to working memory only when the delay is >10 sec (13, 14). Because we have previously found that contextual fear conditioning is reduced and that spatial reference memory is unaffected after ablation of neurogenesis using these same methods (8), our present study suggests that the new neurons may have more than one function and that their specific role in tests of hippocampal-dependent memory may differ depending on the nature and cognitive demands of the task.
Results
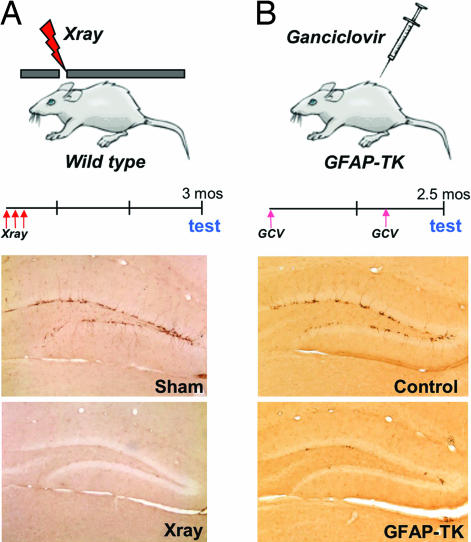
Our experimental design used two independent and complementary strategies to suppress neurogenesis in the hippocampus of adult mice. The first method targets the hippocampus with low-dose x-irradiation using a small window in a protective lead shield (Fig. 1A Top) that blocks exposure to the body and remaining brain regions. This procedure produces a complete and lasting ablation of neurogenesis in the dentate gyrus, as assessed by doublecortin immunoreactivity (Fig. 1A Middle and Bottom). Mice were allowed to recover for 3 months before behavioral testing to avoid the transient inflammatory effects of irradiation, such as microglial activation. We have previously shown that the number of activated microglia returns to basal levels by this time (15).
Fig. 1.
Two different methods eliminate hippocampal neurogenesis in adult mice. (A) Targeted exposure of the hippocampal region of the brain to x-rays using stereotaxic positioning of a lead shield. Behavioral testing began 3 months after treatment with three 5-gray x-ray doses. Representative images show doublecortin-positive cells in the dentate gyrus of sham-treated mice and a nearly complete ablation of neurogenesis after hippocampal irradiation. (B) GFAP-TK transgenic mice were treated with GCV through sequential implantation of two s.c. osmotic minipumps, 6 weeks apart. Doublecortin immunoreactivity was significantly reduced in transgenic mice, and the few remaining cells had almost no dendritic processes.
The second strategy used a transgenic mouse line in which GFAP promoter drives the expression of herpes virus thymidine kinase (TK). Exogenous delivery of the pro-drug GCV induces the selective death of dividing GFAP-positive cells, whereas nondividing astrocytes are spared (see supporting information (SI) Fig. 5). Because this system has previously been shown to cause gastrointestinal toxicity at high doses (16), we delivered a constant low dose of GCV for several weeks via s.c. osmotic minipumps. This modification achieved a nearly complete reduction of hippocampal neurogenesis in GFAP-TK transgenic mice, as revealed by BrdU (SI Fig. 5) and doublecortin immunoreactivity (Fig. 1B Middle and Bottom), but resulted in no effects on activity, body weight, food consumption, or gastrointestinal pathology (data not shown). Together, x-irradiation and GCV-induced ablation of progenitor cells provide a means for avoiding the potential confounds of using only a single method and enables independent validation of behavioral effects associated with a loss of hippocampal neurogenesis.
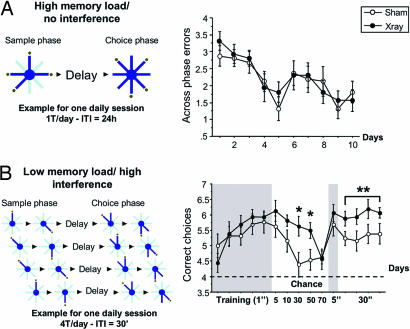
Mice that received either hippocampal x-ray or sham treatment were first trained in a high-memory load (HML)/no intertrial interference (NI) radial maze task using one trial per day and a fixed delay of 60 sec between sample and choice phases (Fig. 2A; see also ref. 17). In the sample phase, mice were allowed to retrieve a food reward from four baited arms of an eight-armed maze. In the subsequent choice phase, all eight arms were opened, and visits to arms presented in the sample phase were scored as working memory errors. Both groups performed comparably in this task. Weight, start latency, and number of errors were unaltered in irradiated mice, suggesting equivalent motivation, locomotion, and cognitive abilities. We next tested these animals in a low-memory load (LML)/high interference (HI) task where highly repetitive trials were presented. In the sample phase, two different baited arms were presented sequentially. After a variable delay, mice had to choose first between the first sample arm and an adjacent arm, followed by a choice between the second sample arm and an adjacent arm. Correct choices (visits to the novel arms) from four trials each day were added to provide an index of working memory performance. With short delays (<5 sec), both groups of mice performed equivalently. However, the performance of irradiated mice was significantly better than shams when the delay increased to 30 and 50 sec (P = 0.01 and 0.02; Fig. 2B). This difference was delay-specific and not due to a change of motivation in the control mice, because the performance of both groups was similar when the delay was returned to 5 sec but diverged again when the 30-sec delay was used continuously for 4 days (ANOVA, P = 0.015).
Fig. 2.
Performance of irradiated mice in two versions of the radial arm maze. (A) Number of errors in a HML/NI working memory task (n = 16 x-ray, 16 sham). Across-phase errors were scored, and no significant effect of irradiation was observed. (B) Score in an LML/HI task (n = 16 x-ray, 13 sham). The same two pairs of arms are used every day for every trial (HI). The number of correct choices was scored. No difference was found between groups during training, but x-ray mice showed enhanced performance when the delay increased to 30 and 50 sec (P = 0.01 and 0.02, respectively).
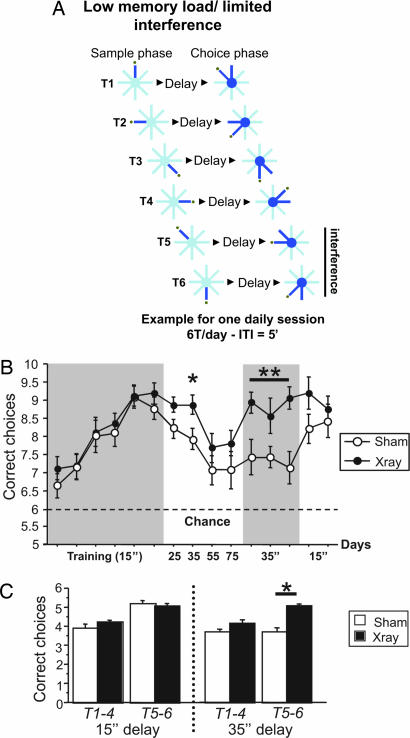
Our observations in this two-choice task with multiple daily trials suggest that loss of neurogenesis alters the response to either intratrial or intertrial memory interference. We then used a single-choice task [LML/limited interference (LI); see ref. 14] in which intratrial interference was eliminated and the contribution of intertrial interference was specifically examined (Fig. 3A). This task is similar to the LML/HI task, but mice choose between only a single pair of arms in each of the six trials per day. Again, there was no difference between groups when mice were trained by using a short delay of 15 sec (Fig. 3B); however, irradiated mice performed significantly better than controls when the delay increased to 35 sec (P = 0.02, marginal effect with 25-sec delay). This result was consistent when replicated over three additional blocks of trials (P = 0.001). A posthoc comparison revealed an effect of irradiation during trials 5 and 6 (P < 0.0001), but not during trials 1–4 (Fig. 3C). Thus, the improved performance in irradiated mice was specific to trials in which the baited arms had previously been visited that day.
Fig. 3.
Performance of irradiated mice in the LML/LI version of the radial arm maze. (A) Schematic diagram of the testing procedure for a single day. (B) Score in LML/LI task (n = 16 x-ray, 14 sham). Only one pair of arms is presented per trial, with six trials/day. Score is plotted as blocks (2 days). Irradiated mice again showed a delay-specific improvement in their performance compared with control subjects (P = 0.05 and 0.02 for 25 and 35 sec, respectively). The improvement was confirmed by using the same delay (35 sec) over 6 consecutive days (3 blocks; P = 0.001). (C) The significant difference in performance between x-ray and sham mice was due to delay-dependent differential processing of intertrial interference. Bar graph represents the average number of correct choices per trial with a delay of 15 (3 last blocks of training) or 35 sec. Each day, six different pairs of arms were presented pseudorandomly. For trials 1–4, four different pairs of arms were used. However, because the maze only has eight arms, the repetition of previously presented arms (interference) occurred during trials 5 and 6. X-ray mice showed a specific enhancement in performance as compared with sham during trials 5 and 6 (with interference; P < 0.0001), but not during trials 1–4 (without interference; P = 0.07) with a delay of 35 sec.
Because our experiments using x-ray treated mice suggested an improvement of performance in working memory tasks that included both a long delay (>30 sec) and HI within each daily session, we sought to verify these results by using both an alternative strain of mice and an independent method of ablating neurogenesis. To this end, we tested GFAP-TK transgenic mice in the same radial-arm task (LML/LI) 10 weeks after GCV treatment, a time when neurogenesis has been virtually eliminated from the hippocampus. These same animals were then retested after a 10-week period during which GCV delivery was withdrawn (Fig. 4A). At this time, we found that neurogenesis had recovered to a level that is ≈25% of that in control mice, as revealed by both BrdU and doublecortin immunohistochemistry (data not shown). The results we obtained in the GFAP-TK mice on GCV were similar to those observed after hippocampal irradiation (Fig. 4B). We observed no difference at short delays up to 35 sec in duration; however, mice without neurogenesis performed significantly better than controls when the delay increased to 55, 75, and 135 sec (P = 0.039, 0.03, and 0.016, respectively). Again, the improvement observed was restricted to trials 5 and 6 where interference occurred (Fig. 4C; P = 0.011). Transgenic mice that were retested after a 10-week recovery period displayed normal working memory performance at all delays (Fig. 4B Right), suggesting that a partial recovery of neurogenesis is sufficient for a complete recovery of working memory. However, it is also possible that the partial recovery of doublecortin immunoreactivity is accompanied by an increase in survival of these cells and may provide an alternative explanation for the behavioral recovery. Together, results from these two series of experiments strongly suggest that new neurons can inhibit specific forms of working memory.
Fig. 4.
Difference in performance of GFAP-TK transgenic mice during and after GCV treatment in the LML/LI version of the radial arm maze. (A) Experimental timeline. Both control and transgenic mice were treated with GCV for 10 weeks before behavioral testing in the radial maze and retested after a 10-week recovery period. (B) The performance of transgenic mice on GCV (n = 14 GFAP-TK, 12 control) was normal at delays of 35 sec and below but was improved relative to control mice when the delay was 55, 75, and 135 sec (P = 0.039, 0.030, and 0.016, respectively). This effect was confirmed by using the same delay (75 sec) over 6 consecutive days (3 blocks; P = 0.021). After 10 weeks of recovery (GCV Off), performance in both groups of mice was equivalent at all delays. (C) In GCV On mice, the significant difference in performance between GFAP-TK and control mice was due to delay-dependent differential processing of intertrial interference. Bar graph represents the average number of correct choices per trial with a delay of 15 (3 last blocks of training) or 75 sec. Transgenic mice showed a specific enhancement in performance as compared with controls during trials 5 and 6 (with interference; P < 0.011) but not during trials 1–4 (without interference; P = 0.067) with a delay of 75 sec.
Discussion
We have shown that, in specific conditions, ablation of neurogenesis in the adult dentate gyrus appears to relieve an inhibitory constraint on working memory. These results are surprising for the following reasons. First, lesions of the dentate gyrus have been shown to impair rather than to enhance performance in delay-dependent working memory tasks (13). Second, our previous study showed that disruption of neurogenesis using the same ablation techniques (x-ray and genetic ablation) had no effect on spatial reference memory and caused a weakening of contextual fear conditioning (8), two different types of long-term memory that require the hippocampus. Similar results have been reported after exposure of adult rats to gamma irradiation (7). This same study also reported an impairment of performance in a different working memory task (delayed-nonmatch-to-sample), contrasting with our results. However, it is unclear whether the impairment observed was due specifically to inhibition of hippocampal neurogenesis as the irradiation procedure affected the whole brain. In addition, there is no evidence for hippocampal dependency in this task. Thus, neurogenesis does not appear to have a unitary function in memory. Instead, the removal of this small cell population appears to differentially influence diverse hippocampal-dependent behaviors.
Ablation of neurogenesis improved performance in working memory tasks, but only in situations where animals needed to ignore or forget conflicting nonrelevant information from previous trials (18). The altered performance in neurogenesis-deficient animals appears to indicate a lack of sensitivity to memory interference. During multiple-trial radial maze tasks, interference-dependent reduction of performance was evident in control animals, but only when the same pair of arms was repeated within a session and a long delay was used. At least two different explanations for the observed enhancement of working memory performance in neurogenesis-deficient animals are possible. The first is that blockade of neurogenesis reduces short-term memory capacity by eliminating cells that are involved in the rapid encoding of memory traces, such as those formed during the sample phase of the radial arm maze task, that require only a brief exposure to spatial configurations. Because working memory performance was normal in the HML/NI task, where memory load is high but must only be stored for a period of 60 sec, this interpretation suggests that defective retention is observed only when memory traces are maintained for several minutes. Indeed, an effect of ablation in the additional two tasks was only found when there was memory interference from trials that took place ≈30 min earlier. Thus, the enhancement of performance in later trials may be due to a lack of memory for earlier trials. However, in opposition to this argument are previous findings that short-term memory in irradiated mice was normal in the Y-maze, a spatial task that used a 30-min intertrial delay (8). An alternative explanation is that removal of neurogenesis does not alter short-term memory in the radial maze but reduces the effects of interference by reducing the amount of overlap between the sets of neurons that represent spatial information during distinct trials. Central to this interpretation is the notion that the hippocampus, and the dentate gyrus in particular, provides a means for separately encoding information that is highly similar but distinct in temporal or associative relevance, a function referred to as pattern separation or orthogonalization (19–21).
The fact that opposite effects on working memory were found after eliminating neurogenesis than after more complete lesions of the dentate gyrus suggests that young dentate granule cells have a different function than mature granule cells (19, 22). Recent computational models have suggested that the addition of new neurons to the stable network of the adult hippocampus may impact memory processes such as information encoding, storage, or retrieval adversely. This could result from the replacement of existing synapses by new ones (21, 23). Moreover, because young neurons are more excitable than mature granule cells (8, 24, 25), their response to related but distinct stimuli, such as repetitive spatial representations, may contribute to memory interference or reduced discrimination but, at the same time, enhance encoding of highly distinct spatial information (21, 26). Indeed, our previous finding that ablation of neurogenesis impairs contextual fear conditioning, where distinct and nonoverlapping contextual cues must be discriminated, is consistent with this hypothesis (8).
Irrespective of the mechanisms involved, our results suggest that young neurons can have a negative influence on specific forms of working memory. Therefore, strategies aimed at stimulating hippocampal neurogenesis to elicit antidepressant or procognitive effects will need to strike a fine balance between restoring function and avoiding the potential negative consequences of an excess of neurogenesis.
Materials and Methods
Animals.
For all irradiation experiments, 10-week-old adult male 129Sv/Ev mice were purchased from Taconic Farms (Germantown, NY). Animals were maintained on a 12/12-h light/dark cycle throughout the course of the experiment. Behavioral testing began 3 months after irradiation or sham treatment, and all tests were performed during the light phase. GFAP-TK transgenic mice (line 7.1) were generated as described (16, 27). We transferred the GFAP-TK transgene onto a C57/BL6-BALB/c mixed background and used 12- to 20-week-old male littermates derived from heterozygote crossings. Mice were housed four or five per cage in a 12-h (06:00–18:00) light–dark colony room at 22°C with freely available water. The procedures described herein were conducted in accordance with National Institutes of Health regulations and approved by the Institutional Animal Care and Use Committees of Columbia University and the New York State Psychiatric Institute.
Drugs.
GCV (Roche, Indianapolis, IN) was dissolved in sterile saline at a concentration of 25 mg/ml and delivered through osmotic minipumps (Alzet, Cupertino, CA) implanted s.c. under anesthesia. An average dose of 10 mg/kg per day was delivered over a period of 10 weeks. Two pumps were implanted sequentially, lasting 4 weeks each, with 2 weeks in between implantations. Control mice were also implanted with minipumps containing GCV.
Irradiation Procedure.
The irradiation procedure was performed as described (11).
Histology and Stereology.
To assess the effect of the irradiation or GCV treatments on the number of BrdU or doublecortin-positive cells, mice were deeply anesthetized with ketamine/xylazine (100 and 7 mg/kg, respectively), then transcardially perfused (cold saline, followed by 4% cold paraformaldehyde/0.1 M phosphate buffer), and brains were collected for immunohistochemistry. All brains were postfixed overnight in 4% paraformaldehyde at 4°C, then cryoprotected in 30% sucrose and stored at 4°C. Serial sections (35 μm) were cut through the entire hippocampus (corresponding to plates 41–61 of Franklin and Paxinos Atlas, 1997) on a cryostat, and stored in PBS with 0.1% NaN3.
Sections were slide-mounted, and procedures for doublecortin consisted of the following steps: 1 h incubation in 0.1 M TBS with 0.5% Triton X-100 (Tx) and 10% normal donkey serum (NDS), followed by goat anti-doublecortin (1:3,500; Santa Cruz Biotechnology, Santa Cruz, CA) primary antibody in TBS/Tx for 24 h at 4°C. The secondary antibody was biotinylated donkey anti-goat (1:500) in TBS/NDS, followed by amplification using an avidin–biotin complex, both for 1 h at room temperature. Sections were developed by using DAB, and bright field images were taken with an Axioplan-2 upright microscope (Zeiss, Thornwood, NY). The procedure for BrdU immunolabeling has been described (11).
Double-labeling of GFAP and TK was done as described (12). Stereological quantification of labeled cells was performed by using a Zeiss Axioplan-2 microscope and a CCD camera. Digitized images were collected and analyzed with Stereo Investigator software (Microbrightfield, Williston, VT). Using the optical fractionator, the total number of GFAP/TK double-labeled cells in the molecular layer of the dorsal dentate gyrus was estimated in transgenic animals given saline or GCV (n = 3 and 5, respectively) by an investigator blind to the treatment status. Both hemispheres were examined in every 12th section throughout the entire dentate gyru. The molecular layer was defined as the region directly above the granule cell layer of the dentate gyrus and bounded dorsally by the hippocampal fissure. Contours were traced at 10× and cells were counted at 40× by using DIC optics.
Eight-Arm Radial Maze.
Habituation.
Food-deprived males (85% of ad libitum weight) were habituated for 10 days to retrieve food pellets in wells at the end of the eight baited arms of a radial maze. The mice used distal visual cues located on the walls surrounding the maze for spatial orientation.
HML/NI task.
This task was performed as described (17). To limit intertrial interference, the mice performed one trial per day, consisting of a sample and a test phase. During the sample phase, mice were allowed to visit four baited arms (HML) chosen randomly each day. Mice were returned to the central platform for a 60-sec delay before the test phase, where all eight arms were open, but only the previously blocked arms contained food. A maximum of 5 min was allowed to retrieve the four remaining pellets. A visit to any arm from the sample phase was counted as a working memory error (Across phase error). Latency to first entry, time to perform the task, weight and food regimen, rank of the first error, and “within phase error” were also recorded.
LML/HI task.
Mice were submitted to four trials per day, each consisting of a sample and a choice phase. In the sample phase, mice were first allowed to enter one randomly chosen baited arm of a pair (pair-1), followed by a second baited arm from the opposite pair (pair-2). Mice then returned to the platform for a short delay of 1 sec (Training phase). During the choice phase, both pair-1 arms were opened and, after one arm was visited, both pair-2 arms were opened, and a second choice was made (two-choice task). Correct choices were visits to the two arms that were blocked during the sample phase (max score for one trial = 2; max score/day = 8). The same two pairs of arms were used each day and trial (HI). After training, the delay between sample and choice phase increased gradually to a maximum of 70 sec.
LML/LI task.
This procedure was performed as described [see ref. 13]. Mice were submitted to six trials per day. One pair of arms was used in each trial (one-choice task), but the procedure was similar to the LML/HI task (sample–delay–choice phase). To avoid a postural mediation strategy (18), mice were removed from the maze after the sample phase and placed in a box for a delay of 3 sec (during training), then returned to the maze for the choice phase (to remove and replace the mouse in the maze took ≈15 sec). After training, the time spent between the sample and choice phase increased gradually to 75 sec. During this task, a different pair of arms was used for each trial (LI). However, because the maze only has eight arms, the repetition of previously presented arms (interference) occurred during trials 5 and 6. The number of correct choices was averaged per day. This number was also totaled per trial across days for a posthoc analysis.
Data Analysis.
For all statistical analyses, ANOVAs were performed by using irradiation or genotype as a main factor. In the radial maze tasks, session, days, or trials were analyzed as main factors. Where ANOVA revealed a significant interaction between factors, a posthoc analysis was performed.
Supplementary Material
Abbreviations
- GCV
ganciclovir
- GFAP
glial acidic fibrillary protein
- HML
high-memory load
- LML
low-memory load
- HI
high intertrial interference
- LI
limited intertrial interference
- NI
no intertrial interference
- TK
thymidine kinase.
Footnotes
Conflict of interest statement: E.R.K. declares a conflict of interest (such as defined by PNAS policy). E.R.K. is one of four founders of Memory Pharmaceuticals and is Chairman of its Scientific Advisory Board. Memory Pharmaceuticals is concerned with developing drugs for age-related memory loss. Some of these drugs are also potentially useful in depression and schizophrenia. E.R.K.'s own laboratory is not involved in developing these drugs. E.R.K. is also a consultant for BrainCells, Inc., which works on neurogenesis, an area in which he is not directly involved.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611718104/DC1.
References
- 1.Kempermann G. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould E, Tanapat P, Hastings NB, Shors TJ. Trends Cognit Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 3.Leuner B, Gould E, Shors TJ. Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 4.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 5.Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 8.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones MW. Curr Mol Med. 2002;2:639–647. doi: 10.2174/1566524023361989. [DOI] [PubMed] [Google Scholar]
- 10.Wall PM, Messier C. Behav Brain Res. 2001;127:99–117. doi: 10.1016/s0166-4328(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 11.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 12.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 13.Lee I, Kesner RP. J Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee I, Kesner RP. Behav Neurosci. 2003;117:1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- 15.Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 16.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 17.Floresco SB, Seamans JK, Phillips AG. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudchenko PA. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Kesner RP, Lee I, Gilbert P. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- 20.McClelland JL, Goddard NH. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Becker S. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- 22.Jarrard LE. Behav Neurol Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer LA, Yabaluri R, Deisseroth K. Trends Neurosci. 2005;28:653–660. doi: 10.1016/j.tins.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Doetsch F, Hen R. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Snyder JS, Kee N, Wojtowicz JM. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 26.Manev R, Manev H. Med Hypotheses. 2005;64:114–117. doi: 10.1016/j.mehy.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Glia. 1995;13:174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.