Abstract
In adult rats, odor-evoked Fos protein expression is found in rostrocaudally-oriented bands of cells in anterior piriform cortex (APC), likely indicating functionally distinct subregions, while activated cells in posterior piriform cortex (PPC) lack apparent spatial organization. To determine whether these patterns are present during early postnatal life, and whether they change during development, Fos expression was assessed following acute exposure to single aliphatic acid odors in developing rats beginning at postnatal day 3 (P3). In the olfactory bulb, Fos-immunoreactive cells were present in the granule cell, mitral cell and glomerular layers at the earliest ages examined. Cells immunopositive for Fos were clustered in areas previously reported as active in response to these odors. In piriform cortex, activation in layers II/III shared some features with that seen in the adult; in APC, rostro-caudally oriented bands of Fos-positive cells alternated with bands relatively free of label, while labeled cells were found dispersed throughout PPC. However, in P3-P7 animals, Fos-positive cells in APC were found in a central rostrocaudally oriented band that was flanked by two bands relatively free of Fos-positive cells. This contrasted with the adult pattern, a central cell-poor band flanked by cell-rich bands, which was observed beginning at P10. These results suggest that subregions of APC visualized by odor-evoked Fos expression are active and functionally distinct shortly after birth. Changes in activity within these subregions during early postnatal development coincide with a shift toward adult-like olfactory learning behavior in the second postnatal week, and may play a role in this behavioral shift.
Olfactory information is processed by several higher-order brain areas, including the anterior (APC) and posterior piriform cortex (PPC). In adult animals, acute presentation of odorants evokes activity in cells that are spatially distributed throughout piriform cortex, as revealed by in situ hybridization and immunolabeling for immediate early genes (Illig and Haberly, 2003; Zou et al., 2005). One feature of odor-evoked activity is that the distribution of activated cells appears to have some order in the APC, where activity is concentrated in prominent rostrocaudally-oriented bands. This banding pattern is mirrored by regional differences in cytoarchitectural features and by connectivity patterns, suggesting the presence of subregions within APC (Luskin and Price, 1983; Haberly, 2001; Ekstrand et al., 2001a; Ekstrand et al., 2001b; Illig and Haberly, 2003; Illig, 2005). In PPC, however, activity appears more broadly distributed, without obvious odor-associated patterns, suggesting differences in the organization and function of APC and PPC.
The physiological responses of cells in piriform cortex and the maturation of mitral and tufted cell afferent fibers is incomplete until the second week of life or later (Schwob et al., 1984; Schwob and Price, 1984a; Schwob and Price, 1984b; Walz et al., 2006). However, early postnatal survival is critically dependent on the ability of rat pups to use olfactory cues for approach to the mother and nipple attachment (Shair et al., 1997; Polan and Hofer, 1999; Moriceau and Sullivan, 2005). The comparatively late arrival of afferent projections to the piriform cortex raises the question of whether piriform cortex participates in olfactory information processing during early postnatal life, and whether adult-like subregional differences in odor-evoked activity are present in neonatal animals. To examine these questions, we used immunocytochemical localization of Fos protein following acute exposure to aliphatic acid odors in rats during the first two weeks of life.
EXPERIMENTAL PROCEDURES
Subjects
All procedures were performed according to NIH guidelines for animal use under protocol approved by the University of Virginia Animal Care and Use Committee. For each test, two male juvenile littermate Long-Evans hooded rats (Harlan), were removed from their home cage and placed in individual odor-free cages to minimize levels of Fos in the olfactory system (see Illig and Haberly, 2003). Temperature within the cages was maintained at 30° C using a heat lamp, and all pups were ambulatory during testing. After 90 minutes, one rat was exposed to 30 seconds of odorized air, which was repeated every 90 seconds, while the other rat received only purified air. This intermittent odor delivery was performed to decrease cortical habituation to the odors (Illig and Haberly, 2003; Wilson, 1998).
Olfactory stimuli
Odors obtained were at the highest purity available (>99.5%; Fluka), and individually diluted to equivalent vapor pressure in light mineral oil (Sigma) in separate tall glass columns filled with nitrogen to prevent oxidation. Caproic acid and butyric acid were chosen because they have been shown to evoke activity in spatially discrete regions of the olfactory bulb in both adult and developing animals (e.g., Illig and Haberly, 2003; Guthrie and Gall, 2003). Using these odors in the present study served both as a control, to confirm that we could visualize such differences using the Fos method, and as a tool, to examine whether these differences in bulbar activation patterns are reflected in patterns of neuronal activation in the piriform cortex.
Odor delivery
The odors were presented to animals via a system of flowmeters and tubing constructed of Teflon and glass connected to the animal chambers (see Illig and Haberly, 2003 for details). High-purity nitrogen was bubbled through the odor glass columns, and the saturated vapor was further mixed 1:200 in the purified airstream just prior to presentation. Continuous airflow (200 liters/min) was achieved using a blower fan connected via tubing to the opposite end of the animal chambers. This setup allowed for a complete exchange of air in the animal chambers every few seconds. After 1 hour of odor exposure, animals were either deeply anesthetized with sodium pentobarbital and perfused through the aorta with 4% fresh formaldehyde, or decapitated. Brains from perfused animals were cryoprotected with a sucrose-glycerol mixture. Brains from decapitated animals were quickly removed from the skull and immersed in 2-methyl butane on dry ice. Six odor-exposed (three for each odor) and six age-matched control rats were tested at each age: P3, P5, P7, P10, P20 and P30 (72 animals total).
Immunocytochemistry
Sections were cut at either 20 μm (for P3-P10 animals) or 40 μm (P20 and P30 animals) on a cryostat. Sections were processed either free-floating (perfused tissue) or post-fixed with 4% fresh formaldehyde and processed on slides (flash-frozen tissue). The differences in euthanasia and fixation methods were introduced because, although immunocytochemical results were adequate, it was more difficult to achieve good-quality tissue sections from young animals using transcardial perfusion techniques. For tissue processed from both adult and young animals, there were no differences in the quality of Fos immunoreactivity seen among sections that differed in section thickness, fixation method or processing technique.
Sections were processed for Fos using standard immunocytochemical techniques (see Illig and Haberly, 2003 for detailed methods). Briefly, sections were washed several times (PBS+2% BSA+0.3% Triton X-100; 20% NGS added for final wash), incubated overnight at 22º C in primary antibody for Fos (1:30 000 in final wash buffer), rinsed, incubated in biotinylated secondary antibody for 3 h at 22º C and rinsed. Visualization of biotinylated antisera was with Vector “ABC” reagents and DAB (3–5 minutes). Sections were mounted and coverslipped. Immunolabeling was observed using light microscopy. Images were acquired using a digital camera atop an Olympus microscope with planapochromatic objectives. Photomicrographs used as figures were matched for brightness and contrast using Adobe Photoshop.
RESULTS
Olfactory Bulb
In both the developing and adult OB, odor exposure evokes specific patterns of activity in neurons associated with activated glomeruli (Sharp et al., 1975; Johnson et al., 1995; Illig and Haberly, 2003; Guthrie and Gall, 2003; Zou et al., 2005). In the present study, cells immunolabeled for Fos protein (Fos-positive) were present in the developing OB in the granule, mitral cell, external plexiform and glomerular layers following odor exposure at all ages investigated (Figure 1). Further, the spatial location of cellular activation was odor-specific; for example, following butyric acid exposure, Fos-positive cells were found in the dorsomedial quadrant of the rostral OB, and this location shifted ventrally after exposure to caproic acid. This pattern was consistent throughout development, in agreement with previous studies examining odor-evoked activity in the adult and developing animals (e.g., Sharp et al., 1975; Johnson and Leon, 2000; Illig and Haberly, 2003; Guthrie and Gall, 2003; Mirich et al., 2004; Zou et al., 2005).
Figure 1.

Patterns of odor-evoked Fos labeling in the olfactory bulb. A) Coronal section through the olfactory bulb of a P3 rat pup exposed to purified air. D=dorsal; L=Lateral. B) Matched section from a P3 pup (littermate to P3 pup shown in A) exposed to butyric acid. Fos-positive cells were present in the granule cell layer (GCL; solid arrowhead), mitral cell layer (MCL), external plexiform layer (EPL; arrow), and glomerular layer (GL; open arrowhead). Labeling following butyric acid exposure was restricted to the dorsomedial quadrant at all ages tested. These patterns were observed at P5, P7, P10 (C), P20 and P30 (D).
Anterior Piriform Cortex
In adult rats there is a distinct difference in the pattern of odor-evoked Fos labeling between anterior and posterior divisions of piriform cortex. In APC, Fos staining is organized in large rostro-caudal “bands”, while PPC exhibits spatially distributed labeling without broad organization (Illig and Haberly, 2003).
In developing animals, odor-evoked Fos labeling was observed in the APC in layers II and III at all ages tested, consistent with labeling of principal pyramidal cells in adult animals. Further, cells exhibiting Fos labeling were found to be organized in rostro-caudally oriented bands, as seen in adults; however, the concentration of positive cells within these bands varied. At P3, labeled cells were clustered in a central band, located deep to the LOT, with very few cells found in dorsal or ventral portions of APC (Figure 2). The pattern of labeling also was seen at P5 and P7. However, at P10, the location of Fos-positive cells shifted to dorsal APC, while the area deep to the LOT had become relatively free of Fos-positive cells (Figure 2), and this pattern remained at P20 and P30. Further, there was an increase in the relative number of Fos-positive cells in the ventral region adjacent to the olfactory tubercle. As shown previously for adult animals (Illig and Haberly, 2003), there were no differences seen in the location of Fos-positive cells in APC that were related to odor quality at any age.
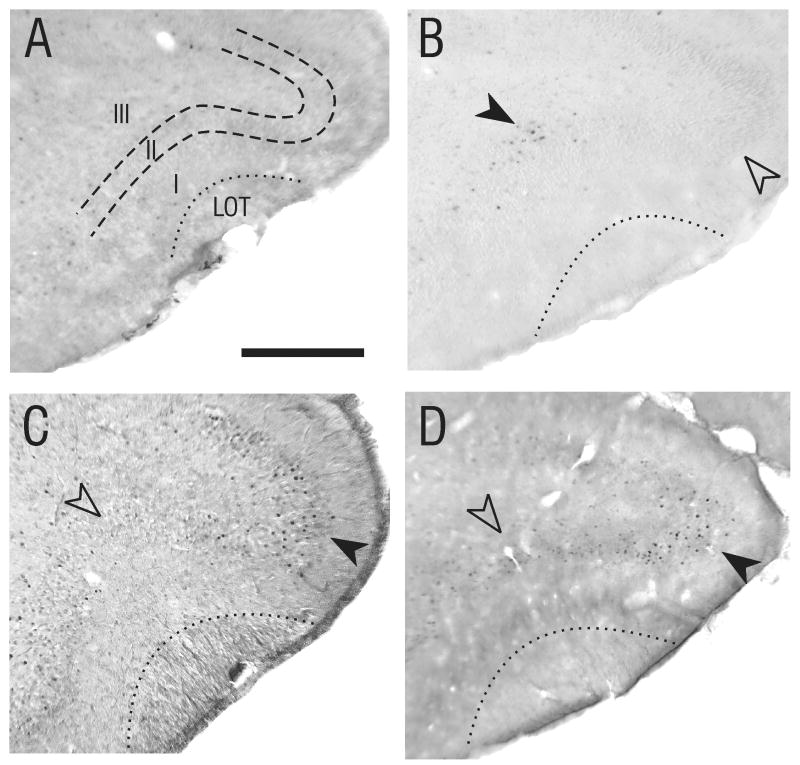
Figure 2.
Odor-evoked patterns of Fos labeling in the APC change during development. Fos labeling is found in layers II and III of piriform cortex in rats exposed to butyric acid at all ages tested (P3-P30). However, the spatial distribution of Fos-labeled cells shifted during early postnatal development. A) Coronal section (approximately 3.2mm anterior to bregma) from a P3 animal exposed to purified air only. B) Matched section from a P3 animal (littermate to pup shown in A) exposed to butyric acid. Fos-positive cells were clustered in a location deep to the LOT (solid arrowhead). This pattern also was seen in P5 and P7 animals (not shown). C) At P10, the cluster of Fos-positive cells evoked by butyric acid exposure in APC had shifted dorsally (solid arrowhead), while the area deep to the LOT had become relatively free of Fos-positive cells (open arrowhead). This pattern of labeling continued in animals to P30 (D).
Posterior Piriform Cortex
As in APC, odor-evoked Fos labeling was found in layers II and III of the developing PPC (Figure 3). However, unlike APC, cellular activation was spatially distributed, with no broad-scale clustering of Fos-positive cells. The same pattern of activity present at P3 was stable until P30, and, as shown previously for adult animals (Illig and Haberly, 2003), there were no differences seen in the location of Fos-positive cells in PPC between animals exposed to butyric or caproic acid at any age.
Figure 3.
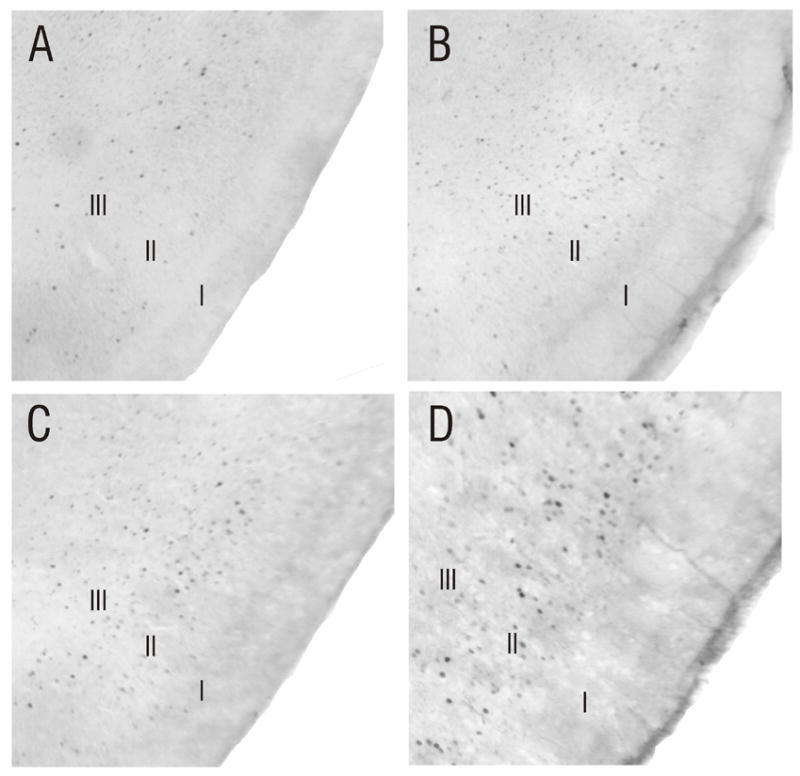
Spatially-distributed patterns of Fos labeling in the PPC do not change during development. Odor-evoked activity was found in layers II and III of PPC at all ages tested. Unlike APC, cellular activity was highly distributed spatially, with no broadscale clustering of Fos-labeled cells apparent. A) Coronal section through the PPC of a P3 animal exposed to purified air, showing minimal Fos expression. B) The number of Fos-positive cells increased following exposure to butyric acid (P3 littermate to pup shown in A). Note the spatially distributed nature of odor-evoked activity in PPC, which was seen at all ages tested, including P10 (C) and P30 (D).
DISCUSSION
Fos, the protein product of the immediate-early gene c-fos, is activated in the olfactory system upon exposure to odors (Guthrie et al., 1993; Guthrie et al., 2000; Illig and Haberly, 2003). The present experiments have demonstrated that odor-evoked patterns of neural activity are present in the OB and piriform cortex as early as P3. In the OB, these patterns are odor-specific, and the spatial location of odor-evoked activity changes little during postnatal development (Figure 1). These results are in agreement with previous studies using a variety of methods (Sharp et al., 1975; Johnson et al., 1995; Illig and Haberly, 2003; Guthrie and Gall, 2003; Zou et al., 2005). Taken together, the findings suggest that the odor-specific patterns of activity in the olfactory bulb are established in early neonatal animals, and do not undergo substantial remodeling during postnatal development.
In the piriform cortex, odor-evoked patterns of Fos labeling share some features with adult animals, but display distinct developmental characteristics. In the APC of young animals, odor-evoked activity is concentrated in a longitudinal band deep to the LOT. This pattern, present throughout the first postnatal week (Figure 2), corresponds with the “central band” observed in the adult rat (Illig and Haberly, 2003); however, this band is relatively free of Fos-labeled cells in adult rats. Adult-like patterns emerge by P10 (Figure 4), and at later ages, odor-evoked Fos expression mimics the spatial patterning of the adult, with Fos-labeled cells predominantly located in the dorsal region of APC, between the rhinal sulcus and the LOT (i.e., the “dorsal band” described by Illig and Haberly, 2003).
Figure 4.

Summary of odor-evoked patterns of Fos labeling in the developing piriform cortex. A) Schematic drawing of the ventrolateral aspect of the rat brain, showing the relative position of the APC and PPC. AON, anterior olfactory nucleus; OB, olfactory bulb; OT, olfactory tubercle; rs, rhinal sulcus. B) Diagram showing the location of highest density of Fos-positive cells following odor exposure within the first postnatal week. Compared with the patterns seen at P10 and later (C), the most notable shift in odor-evoked Fos expression during development was observed in the dorsal band (DB) and central band (CB). Odor-evoked Fos expression in the sulcal band (SB), ventral band (VB) and PPC did not appear to change during development. Scale bar in A = 2 mm.
The dorsal, central and ventral bands in APC observed in this and other studies (see Figure 4) coincide with patterns in chemo- and cytoarchitecture and connectivity with other areas (Luskin and Price, 1983; Haberly, 2001; Ekstrand et al., 2001a; Ekstrand et al., 2001b; Illig and Haberly, 2003; Illig, 2005; Brunjes et al., 2005), suggesting that the APC consists of functionally distinct subregions. While many details of these subregions are not yet known, some of the differential connection patterns suggest intriguing possibilities for their function. For instance, the ventral band is the only portion of APC that receives an input from two nuclei included in the extended amygdala: the nucleus of the lateral olfactory tract and the cortical nucleus of the amygdala. Further, the area encompassing the dorsal band receives a heavy input from the ventrolateral orbital cortex. These two areas have been shown to play a role in assessing and encoding the behavioral significance of environmental stimuli (Schoenbaum et al., 1998; Ramus and Eichenbaum, 2000; Schoenbaum et al., 2003a; Schoenbaum et al., 2003b). The activation of the dorsal and ventral bands may therefore represent the convergence of such information within piriform cortex. Finally, the central band overlaps with an area of piriform cortex that may be the only recipient zone for tufted cells (Ekstrand et al., 2001b), raising the possibility that this region of piriform cortex processes information from this unique class of bulbar cells.
The finding that differences in odor-evoked Fos labeling in the dorsal and central bands appear early in postnatal life, and that the relative activation of these bands changes during maturation of the olfactory system, suggests that these subregions of piriform cortex may play distinct roles throughout the first two weeks of life. Again, though the details of their development are unknown, the arrival of inputs from the anterior olfactory nucleus, amygdala, orbitofrontal cortex and other areas may contribute to the postnatal maturation and function of piriform cortex.
In contrast, the broad, spatially-distributed pattern of activation observed in PPC changes little with development. It should be noted that because of the spatially-distributed nature, it is possible that significant changes in the organization of odor-evoked activity could not be detected in these across-animal comparisons. Nevertheless, it appears that even at the earliest ages tested, odor-evoked activity in the PPC displays a spatially-distributed organization, in contrast to the more spatially-clustered organization found in APC. This finding suggests that PPC may be organized from early in life to function using a spatially-distributed, ensemble coding strategy for olfactory and related information (Johnson et al., 2000; Haberly, 2001).
Behavioral significance
Early neonatal rats rely on olfactory cues for feeding and survival. The olfactory system matures rapidly during this time, with ongoing myelination of the lateral olfactory tract and innervation of central olfactory structures incomplete until after P15 in both the rat and mouse (Schwob et al., 1984; Schwob and Price, 1984b; Walz et al., 2006). During this period, rat pups rapidly acquire odor preferences and display approach behaviors to classically-conditioned odors, even if the odor is paired with noxious stimuli (e.g., 0.5 mA shock; Sullivan et al., 1986; Camp and Rudy, 1988; Sullivan et al., 2000; Moriceau and Sullivan, 2004; Roth and Sullivan, 2005; Moriceau and Sullivan, 2005). This seemingly paradoxical learned odor preference may serve to prevent aversion to the mother’s odor in the event that pups experience painful stimuli while in the nest (Moriceau and Sullivan 2005). Indeed, these behaviors diminish at the close of the “sensitive period” at postnatal day 10 (P10), coinciding with the development of motor behaviors that allow the rat to leave the nest (Bolles and Woods, 1965). The experiments in this paper have demonstrated a shift in the location of Fos-positive cells in APC that coincides with the end of this sensitive period at P10. These findings raise the possibility that changes in activity within subdivisions of the piriform cortex during early postnatal development, perhaps brought about by ongoing modifications of input from the olfactory bulb, amygdala, and orbitofrontal cortex, may contribute to the shift toward adult-like olfactory learning behavior.
Acknowledgments
Many thanks to Ms. Dixie Shurling and Ms. Meghan Mayhood for technical assistance, and to Dr. Peter C. Brunjes for a critical review of this manuscript. This work was funded by NIH grant 05577 from the NIDCD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Bolles RC, Woods PJ. The ontogeny of behavior in the albino rat. Animal Behavior. 1965;12:427–441. [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex) Brain Research Reviews. 2005;50:305–335. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Ekstrand JJ, Domroese ME, Feig SL, Illig KR, Haberly LB. Immunocytochemical analysis of basket cells in rat piriform cortex. J Comp Neurol. 2001a;434:308–328. [PubMed] [Google Scholar]
- Ekstrand JJ, Domroese ME, Johnson DMG, Feig SL, Knodel SM, Behan M, Haberly LB. A new subdivision of anterior piriform cortex and associated deep nucleus with novel features of interest for olfaction and epilepsy. J Comp Neurol. 2001b;434:289–307. doi: 10.1002/cne.1178. [DOI] [PubMed] [Google Scholar]
- Guthrie K, Rayhanabad J, Kuhl D, Gall C. Odors regulate Arc expression in neuronal ensembles engaged in odor processing. Neur Rep. 2000;11:1809–1813. doi: 10.1097/00001756-200006260-00003. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Nat Acad Sci (USA) 1993;90:3329–3333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Gall C. Anatomic mapping of neuronal odor responses in the developing rat olfactory bulb. J Comp Neurol. 2003;455:56–71. doi: 10.1002/cne.10452. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: New insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Illig KR. Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Comp Neurol. 2005;488:224–231. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;437:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Odorant molecular length: one aspect of the olfactory code. J Comp Neurol. 2000;426:330–338. doi: 10.1002/1096-9861(20001016)426:2<330::aid-cne12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular resopnse in the olfactory bulb of young rats. Brain Res. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Johnson DMG, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Mirich JM, Illig KR, Brunjes PC. Experience-dependent activation of extracellular signal-related kinase (ERK) in the olfactory bulb. The Journal of Comparative Neurology. 2004;479:234–241. doi: 10.1002/cne.20325. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuit for neonatal olfactory learning. Journal of Neuroscience. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Neurobiology of infant attachment. Developmental Psychobiology. 2005;47:230–242. doi: 10.1002/dev.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polan HJ, Hofer MA. Psychobiological origins of infant attachment and separation responses. In: Cassidy J, Shaver PR, editors. Handbook of Attachment: Theory, research and clinical application. New York: Guilford Press; 1999. pp. 162–180. [Google Scholar]
- Ramus SJ, Eichenbaum H. Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. J Neurosci. 2000;20:8199–8208. doi: 10.1523/JNEUROSCI.20-21-08199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning & Memory. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behavioural Brain Research. 2003b;146:19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Haberly LB, Price JL. The development of physiological responses of the piriform cortex in rats to stimulation of the lateral olfactory tract. J Comp Neurol. 1984;223:223–237. doi: 10.1002/cne.902230206. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. J Comp Neurol. 1984a;223:177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of lamination of afferent fibers to the olfactory cortex in rats, with additional observations in the adult. J Comp Neurol. 1984b;223:203–222. doi: 10.1002/cne.902230205. [DOI] [PubMed] [Google Scholar]
- Shair NH, Masmela JR, Brunelli SA, Hofer MA. Potentiation and inhibition of ultrasonic vocalization of rat pups: Regulation by social cues. Developmental Psychobiology. 1997;30:195–200. doi: 10.1002/(sici)1098-2302(199704)30:3<195::aid-dev2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metablism in rat olfactory bulb during olfactory stimulation. Brain Res. 1975;98:596–600. doi: 10.1016/0006-8993(75)90377-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy: Ontogeny of conditioned fear and the amygdala. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Omura M, Mombaerts P. Development and topography of the lateral olfactory tract in the mouse: Imaging by genetically encoded and injected fluorescent markers. J Neurobiol. 2006;66:835–846. doi: 10.1002/neu.20266. [DOI] [PubMed] [Google Scholar]
- Zou Z, Li F, Buck LB. Odor maps in the olfactory cortex. Proc Natl Acad Sci U S A. 2005;102:7724–7729. doi: 10.1073/pnas.0503027102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]