Introduction
Task performance elicits fMR-BOLD signal responses that reflect the vascular response to associated neural activity (Buxton et al., 2004; Fox et al., 2005a). Standard analysis strategies often assume that a linear convolution of the task period with an estimate of the hemodynamic impulse response function is an adequate model of the imaging signal. However, during extended periods of task performance (e.g. > 30s), complex features of response shapes can often be identified, for example, signal spikes or undershoots at task transitions (Hoge et al., 1999; Harms and Melcher, 2002). Sources of these features may include changing neural activity during the execution of a task, neural processing involved in switching between tasks, and dynamics of the vascular response to this neural activity. Modelling the relationship between neural activity, vascular responses, and the imaging signal may produce methods for the accurate estimation of the neural activity from imaging data (Buxton et al., 2004; Obata et al., 2004). Such modelling studies have typically focused on a small number of primary brain regions, where activity is easily controlled experimentally. However, it is important that approaches be developed that enable efficient characterisation of responses across the brain, for reliable assessment of activation in fMRI studies, and for the identification of features of the response dynamics that need to be accounted for in models of neuro-vascular coupling.
A number of recent studies have developed simple methods to systematically assess time-course features across the brain. Harms and Melcher (2002) studied activity associated with extended blocks of continuous sound, finding regional BOLD signal responses exhibiting signal spikes at the start or end of the task period, gradually ramping signal during the task period, and undershoots following the completion of the task. To parameterise this activity, they developed a linear response model with a concise set of physiologically plausible waveform components, the OSORU (Onset-Sustained-Offset-Ramp-Undershoot) model (see Figure 1) (Harms and Melcher, 2003). Employing this model in a General Linear Modelling (GLM) framework, they quantified BOLD response shapes across a number of tasks and subjects. They found the extent of spiking at the onset and offset of responses varied across regions, and was also correlated with the temporal envelope of the sound stimulus (Harms et al., 2005). Independently, Fox and coworkers have investigated region- and task-specific signal transients using an ANOVA analyses of activity not fit by the standard response model. This analysis identified regions exhibiting reduced BOLD signal spikes at task onset in schizophrenic individuals in a working memory task (Fox et al., 2005b). A separate meta-analysis mapped transient activity across the brain for a range of experimental task types (Fox et al., 2005a). A network of regions displayed transient activity for all task types, while other regions exhibited transient behaviour only at specific task-transitions. General patterns of transient dynamics remain to be fully elucidated. In this paper, we report a detailed investigation of transient dynamics in a fMRI study of motor training, assessing the spatial distribution of individual features of responses, and how responses change within and across scanning sessions.
Fig. 1.
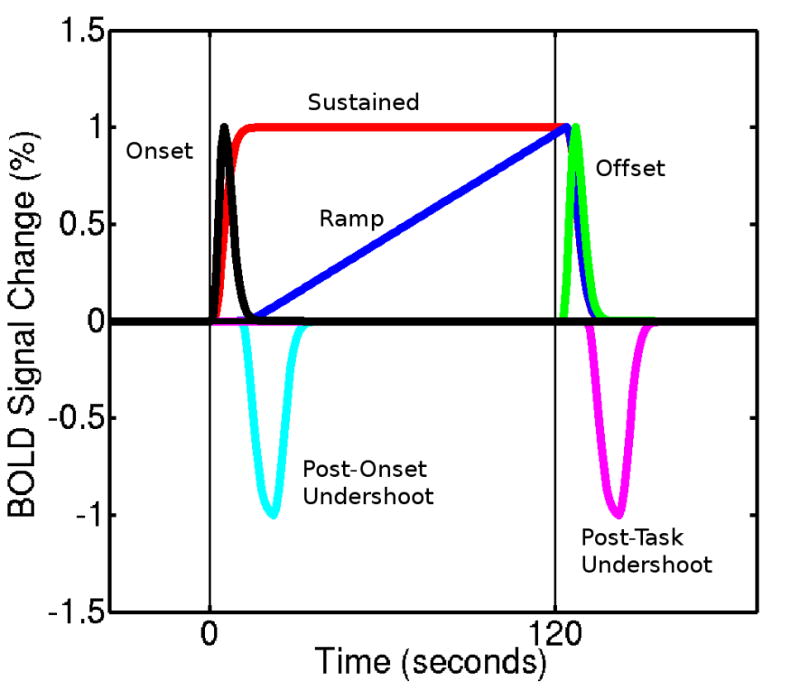
The OSORU response model (adapted from Harms and Melcher (2003)). The post-onset undershoot is an additional component, included for the present study.
A large number of fMRI studies have investigated activation dynamics associated with motor-sequence training utilising conventional models of a sustained BOLD response. A general pattern of results has emerged (Doyon et al., 2003). Performing a novel task initially demands focused attention, which is reflected in stronger activation in the dorsolateral prefrontal cortex (DLPFC) early in a first training session. This activation tends to reduce after a brief period of training. Responses in the cerebellar cortex, caudate, and sensory-motor cortex tend to reduce more gradually during an initial training session. Over the same period, activation increases have been reported in the thalamus and putamen, and dentate nucleus, which may be maintained in performance during later sessions (Floyer-Lea and Matthews, 2004, 2005; Ungerleider et al., 2002; Toni et al., 1998). The cerebellum continues to be involved if ongoing adaptation is required. With repeated training sessions, a task can be learnt to a level of automaticity, and encoded for long-term recall. Motor and sensory cortices have been shown to have relatively increased levels of activation during performance at this stage, suggesting task-specific plasticity in primary cortical regions (Karni et al., 1995; Floyer-Lea and Matthews, 2005).
However, there are inconsistencies across studies, which have been thought to be related to variations in the type of task or the learning phase studied (Doyon et al., 2003). Another explanation could be incomplete modelling of complex BOLD response shapes. While we are not aware of any systematic studies of response shapes for motor tasks, numerous studies describe transient aspects of motor responses. Electrophysiological and imaging studies have distinguished preparatory and initiation related activity from that related to the performance of a motor task (Toni et al., 1999; Cunnington et al., 2002). Adaptation and learning related changes can occur quickly with the performance of novel motor tasks, and could lead to changing activity over a single task block (Toni et al., 1998; Floyer-Lea and Matthews, 2005). Transient spikes in BOLD signal in the primary sensory cortex and supplementary motor area (SMA) at the onset of a motor sequence task were reported by Nakai et al. (2000), and investigated further by Obata et al. (2004). If only specific aspects of responses, such as transient spikes, changed with learning, inconsistencies could arise with the use of the standard response model. For short task blocks these transients would make a significant contribution to model fits, and changes in these components would be detected. However, they may not be detected in studies that employ longer duration blocks, where the sustained signal would dominate.
This paper reports a systematic characterisation of BOLD response shape dynamics for a motor sequence task, over a one month learning period. We employed the OSORU model after an initial exploration of BOLD response shapes revealed distinctive features similar to those originally characterised with this model (Harms and Melcher, 2003). Harms and Melcher (2003) discuss a range of possible approaches that could be employed to map such dynamics, including wavelet and harmonic decompositions, principle component analysis, and finite impulse response linear models. The crucial advantage of a multi-component GLM approach is the ability to explicitly model specific waveform features. In addition, GLM approaches have well developed and sophisticated statistical inference procedures, and complex multi-factorial models can be implemented using standard fMRI analysis packages.
In this paper, we extend the OSORU model with an additional undershoot component, and introduce whole-brain statistical mapping of individual OSORU components as an efficient means by which to characterise response shapes. We employ the model in a more complex GLM than has previously been performed, assessing the influence of multiple factors on individual response components. Our aims were: 1. to characterise the temporal profiles of BOLD signal responses associated with a long duration motor sequence task 2. to measure how these change with training, within and across sessions, and 3. to assess the extent to which transient signal features may impact fMRI studies of the motor and other systems.
Methods
Motor learning paradigm
We used a motor-sequence learning task previously employed by Karni and coworkers (Karni et al., 1995). Fingers were repetitively tapped upon the thumb in an ordered sequence. Two tapping orders were defined, 4-2-3-1 and the reverse, 1-3-2-4. Each subject was assigned one of these sequences for training. Subjects trained the task with their left hand, fifteen minutes per day for a period of four weeks. Subjects practised speed and accuracy of the task. Performance speed and error rates were measured from video recordings of training sessions.
Subjects participated in three functional MRI sessions, the first prior to training, and subsequently after two and four weeks of training (Fig. 2). Each of these sessions consisted of six functional imaging scans. The first and final scans were resting state, and will not be further considered here. The second through fifth scans lasted eight minutes each, during which two two-minute finger tapping periods were interposed with two minute rest periods. The task blocks consisted of either the trained task or the alternate, control tapping sequence, randomised across the session. Subjects were instructed to maintain a steady 2Hz finger tapping rate in all scanning sessions, paced by the regular gradient shifts. The scans finished during a task block, meaning only half the trials measured offset dynamics. This resulted in reduced power for tests related to offset dynamics.
Fig. 2.
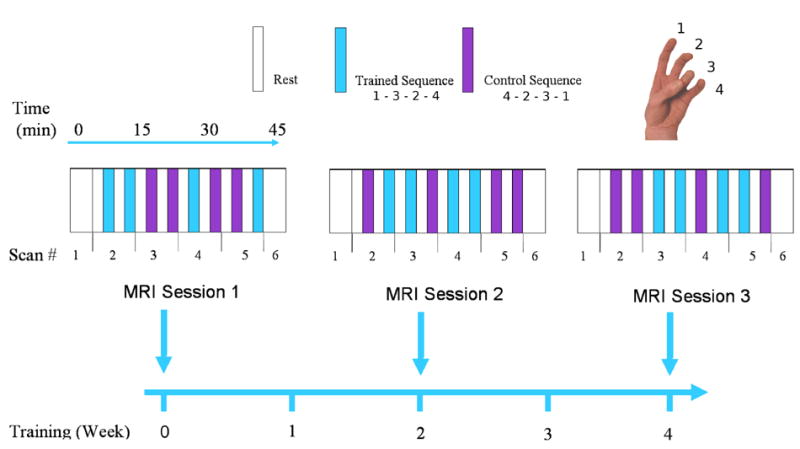
Study Scanning Schedule: Three fMRI scanning sessions were performed over a one month period. The first and final 4-minute scan of each session were resting-state scans. The second through fifth scans recorded alternating two-minute periods of task performance and rest. Task performance was randomised between two simple left handed thumb-to-finger tapping sequences, one of which was trained daily after the first session.
Acquisition
Scanning was performed on an Elscint Prestige 2 Tesla whole body scanner. During the task scans, 240 sixteen-slice whole-brain volumes were acquired using a T2*-weighted gradient-echo, echo-planar-imaging sequence (TR = 2000ms; TE = 45ms; flip angle = 70°; slice thickness = 6mm; 3.19mm X 3.21mm in-plane voxel resolution; 128*72 image matrix, FOV, 411mm X 229mm). The first four volumes were discarded to obtain stable magnetisation. At the end of fMRI data collection, spin-echo, T1-weighted anatomical images (TR = 33ms; TE = 12ms; flip angle = 60°; slice thickness = 5mm; 1mm X 1mm voxel resolution; 256 X 256 image matrix) were acquired in the same slice positions to facilitate the precise determination of the structures corresponding to the functional activation foci. For registration, a high resolution 3D image was also acquired (TE=6ms, TR=33ms, tip angle = 35°, 256 X 256 image matrix, 1mm X 1mm X 0.5mm voxel resolution).
Twelve right-handed volunteers, ranging from 18 to 45 years old, with no known neurological disorders, were recruited, trained, and imaged. Men and women were recruited in equal proportions by posted advertisement. Informed consent statement was obtained for each subject before experiment. The study was approved by the Institutional Review Board of the Research Imaging Centre, San Antonio.
OSORU model
Our GLM analysis modelled responses using a linear response model based on the OSORU set of basis functions (Harms and Melcher, 2002). Each basis function models a distinct feature of the BOLD signal response (Fig. 1). These consist of a signal spike at task Onset (O), a Sustained signal throughout the task (S), a spike at task Offset (O), linearly Ramping signal across the activation period (R) and an Undershoot following the Offset spike (Uof f ). We included an additional Undershoot component (Uons) following the Onset spike, after an initial investigation of the dataset identified signal dipping below the level of sustained activity following the onset spike. This component also adds symmetry to the model, in accounting for undershoots following activation that may occur during the rest condition.
All components of the model were defined by convolving simple functions with a single gamma hemodynamic response function, with a time to peak of 6 seconds, and dispersion parameter of 1 second. Onset and offset spikes were defined with a unit spike at task onset or offset points. Sustained activation was defined using a boxcar function equal to one during the task period and zero otherwise. The ramp function increases linearly from zero to one, over the period from the point at which the sustained component reaches its peak (eight seconds past task onset), to the end of the task period, at which time it immediately returns to zero. The undershoot functions were boxcars equal to negative one, also starting eight seconds after task onset or offset, and persisting for ten seconds. The eight second delay matches the observed time course of these signal features, and avoids possible over-fitting of onset-related signal. A negative function was used for the undershoots, as this was the expected direction of signal.Thus, a positive parameter estimate for this component indicates that the modelled response includes an undershoot component. A negative value indicates a negative undershoot component, that is, signal above baseline. In addition to the OSORU model, a linear component was included to account for baseline drift. This was required as the high-pass filter was found to induce a linear drift in activated regions, due to the fact that task blocks were not distributed evenly within each scan.
Model fitting
BOLD signal time series analysis was performed using FEAT (FMRI Expert Analysis Tool, www.fmrib.ox.ac.uk/fsl). Rigid body motion correction was followed by 5mm FWHM Gaussian spatial filtering and high-pass temporal filtering with a cutoff period of 350 seconds (Jenkinson et al., 2002). Spatial normalisation was performed by first registering each subject’s individual runs to their high resolution T1 image, and this image to the MNI template brain using an affine transformation with 12 degrees of freedom.
The statistical analysis employed a three-level estimation procedure. At the first-level, the extended OSORU model was fit individually to the BOLD signal response for each task period, using FEAT’s FILM, with local temporal autocorrelation correction (Woolrich et al., 2001). The model was not orthogonalised, as we were interested in the fit parameters of individual OSORU model components, which reflect basic features of the BOLD responses. An orthogonalised basis set would span the same range of waveforms, but the basis set would not consist of simple, interpretable, waveforms, due to the strong constraint of linearly independent components. A lack of orthogonality has the effect that model fits may not be unique, and variance cannot be uniquely assigned to individual model components. As the components of the OSORU model are not strongly correlated, we expect there to be little ambiguity in model fits, and the variance estimates calculated for individual components to be reasonably accurate.
At subject and group levels of analysis, the OSORU waveform components were analysed separately, each in an identical manner. This is because we were primarily interested in the distribution and dynamics of the individual waveform features. For the subject level model, each of the three sessions were used as separate explanatory variables (evs). A further three evs modelled linear change in the OSORU component over the task periods of each session. Trained- and control-task periods were modelled separately, resulting in a total of twelve evs for each OSORU model component at the subject level.
For each component, a contrast was generated to detect regions where the component made a significant contribution to the model fit of the BOLD signal responses in the first scanning session. A second contrast detected regions where the component estimates exhibited linear change over the eight task periods of the first scanning session. Change unique to the first, pre-training scanning session was assessed using a contrast comparing this session with following sessions. Long-term learning effects were detected by contrasting component estimates between sessions one and two, and two and three. The generalisation of learning was assessed by contrasts comparing trained- and control-tasks in the second and third sessions.
Higher-level analyses were carried out using FLAME (FMRIB’s Local Analysis of Mixed Effects) (Beckmann et al., 2003; Woolrich et al., 2004), in which first-level fixed-effects variance is carried up to estimation of inter-subject and inter-session random-effects components of the mixed effects. At the subject level, variance was estimated separately for each scanning session. At the group level, a one-sample t-test was used to generate mean group effect maps for all contrasts. Statistical maps were corrected for multiple comparisons using a cluster detection algorithm, thresholded at z = 2.3 and with a cluster significance threshold of P = 0.05 (Worsley et al., 1992). Activation maps were overlaid on the MNI template image, and cluster locations identified according to predefined anatomical boundaries (Tzourio-Mazoyer et al., 2002). To systematically locate regions of interest (ROIs) for time course plotting, we performed an additional F-test across across all OSORU components for a given contrast, to generate a single map of regions exhibiting any significant effects.
For comparison with the OSORU model, an analysis was performed using a standard modelling approach. This incorporated the sustained component, and its temporal derivative. The temporal derivative consists of a signal overshoot at onset, and an undershoot at offset, and could therefore model a limited range of transient behaviour. An identical higher-level analysis was employed.
Regions of interest
ROIs were defined at local maxima of the thresholded group-level F-test maps. ROIs were defined on active voxels within 10mm of the local maxima, constrained to lie within the same anatomical region, based on a parcellation of MNI space (Tzourio-Mazoyer et al., 2002). ROI time-course and model plots were generated using a Region Exploration (REX) tool (Duff and Egan, 2006) developed in-house using Matlab (Mathworks, Natick, MA). ROIs were transformed to subject’s native space by inverting the spatial normalisation applied during GLM analysis. Average fit parameters for the OSORU model, at different stages of training, were derived for responses from each region of interest. Parameters were normalised to percentage signal change within individual scans, based on average signal levels during rest periods.
Results
Behavioural
Subjects were able to perform both the trained and control tasks in the scanning sessions at the required pace. Error rates were very low, not exceeding 1.5% (approximately one mistake every two minutes). All subjects exhibited speed improvements with the daily training sessions (Fig. 3). Speed improvements reduced over time, and were minimal after two weeks of training.
Fig. 3.
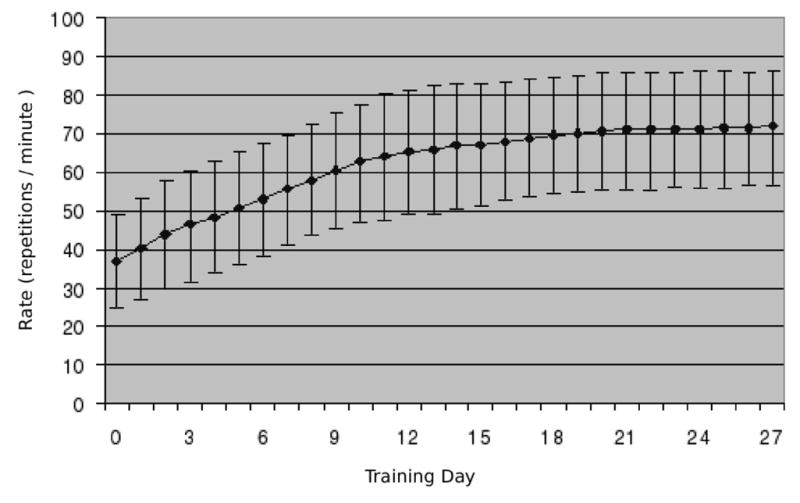
Behavioural measures: Average tapping rates were recorded during training sessions outside of the scanner. Rates increased during the first two weeks of training, after which the rate plateaued. Error rates were also recorded, but were very low. Plot shows mean and standard deviation based on all subjects.
Pre-training BOLD signal response shapes
All OSORU components contributed to the model fits of the BOLD signal from the first session. Spatial statistical maps were generated for each component, showing regions in which parameter estimates were significantly non-zero (Fig. 4). Large, transient signal features at onset and offset were widespread. Local maxima of the F-test statistical map, calculated across all model components, included major motor regions as well as regions exhibiting primarily transient activity (Table 1). Average time courses of ROIs taken around these local maxima indicate a wide variety of response shapes (Fig. 5, black plots). Average model fits to these time courses accounted very well for the features of the average time courses (Fig. 5, blue plots).
Fig. 4.
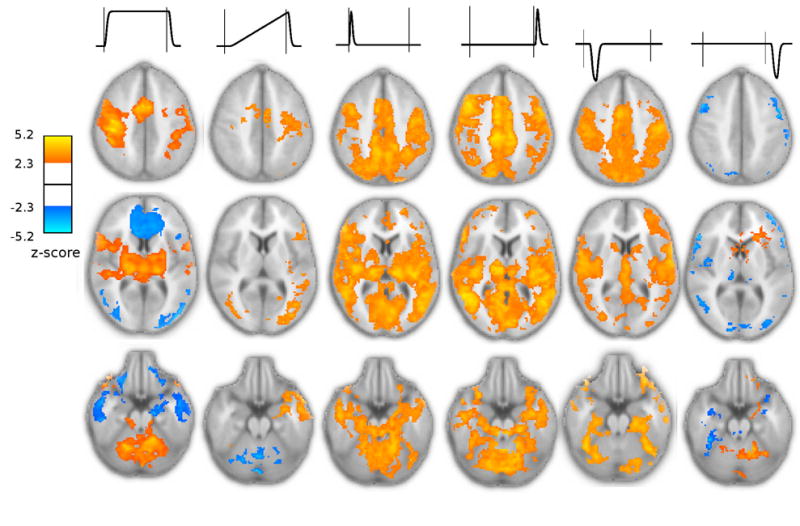
Random effects statistical maps, for the initial training session, indicating regions with significantly non-zero parameter estimates for individual OSORU components. Orange regions reflect positive parameter estimates, blue regions reflect negative parameter estimates (e.g. a decreasing ramp). Positive parameter estimates for the undershoot components reflect an undershoot of signal below baseline. Thresholding for all statistical maps in this paper is z < 2.3 with a cluster probability of P < 0.05 (corrected). Signal components are indicated by the schematics above the maps. A sustained component was significant in right M1, SMA, bilateral thalamus and cerebellum, whilst a negative sustained component was present in prefrontal Cortex. Regions with a significant positive ramp included the left sensory-motor cortex, SMA, and insula. A negative ramp occurred in the cerebellum. Onset and offset spikes were widespread, particularly through the cortical midline. The post-onset undershoot had a similar distribution to the onset spike, except in the cerebellum. A strong post-task undershoot was found in the left cerebellum. Negative parameter estimates for this component (i.e. increased signal) occurred in lateral parts of the prefrontal cortex, occipital cortex, and in the right thalamus and insula.
Table 1.
OSORU model fits from local maxima of the group-level statistical map of an F-test across all OSORU components, for the initial training session. F-test statistics have been converted to z-scores; coordinates refer to the position of the maxima in MNI space; the mean and standard deviations of the OSORU component parameter estimates for these voxels are tabulated in units of percentage signal change. For readability, component parameter estimates are not shown if they were not significant in GLM analysis. A positive parameter estimate for an undershoot component reflects an undershoot of signal below baseline. The “plot” column specifies corresponding average time-course plots in Figure 5, taken from ROIs centred at these voxels. The final ROI was selected to illustrate activity in the ipsilateral hand motor region.
| ROI | Z-Score | (x, y, z) | Ons | Sus. | Off | Ramp | Uons | Uof f | Plot |
|---|---|---|---|---|---|---|---|---|---|
| Right S1 | 6.89 | (42,−26,46) | 0.08(.06) | 0.86(.07) | 0.25(.09) | 0(.06) | 0.22(.05) | 0.13(.07) | - |
| Right SMA | 6.78 | (6,4,54) | 0.33(.07) | 0.72(.13) | 0.64(.10) | 0.05(.08) | 0.33(.06) | 0(.12) | a |
| Right M1 | 6.77 | (36,−18,58) | 0.11(.07) | 1.01(.16) | 0.14(.08) | 0.05(.08) | 0.23(.05) | 0.10(.06) | b |
| Left Temporal | 6.73 | (−50,−66,4) | 0.62(.09) | 0.02(.07) | 0.74(.13) | 0.02(.07) | 0.14(.05) | -0.08(.06) | c |
| Right Temporal | 6.78 | (54,−60,2) | 0.51(.07) | −0.08(.08) | 0.98(.13) | −0.19(.07) | 0.19(.07) | −0.07(.09) | - |
| Left Cerebellum | 6.78 | (−18,−50,−26) | 0.51(.10) | 1.07(.17) | 0.56(.11) | −0.22(.12) | 0.04(.06) | 0.10(.04) | d |
| Right Thalamus | 6.76 | (14,−20,2) | 0.56(.07) | 0.51(.08) | 0.45(.10) | −0.02(.09) | 0(.05) | −0.01(.02) | e |
| Right Lingual | 6.75 | (10,−34,−6) | 0.86(.09) | 0.29(.13) | 1.01(.14) | −0.21(.12) | 0.30(.09) | −0.05(.15) | - |
| Right Fr.Inf.Op. | 6.71 | (56,8,28) | 0.18(.09) | 0.54(.06) | 0.39(.12) | 0.17(.07) | 0.25(.05) | −0.11(.05) | - |
| Ant. Cingulate | 6.65 | (4,30,−2) | 0.09(.12) | −0.94(.08) | −0.33(.19) | 0.18(.13) | −0.01(.07) | 0.05(.10) | - |
| Left Occipital | 6.53 | (−42,−86,20) | 0.35(.06) | −0.44(.07) | 0.29(.10) | −0.23(.10) | 0.05(.05) | 0.12(.08) | f |
| Left M1 | 6.50 | (−56,6,34) | 0.17(.07) | 0.46(.08) | 0.19(.09) | 0.30(.09) | 0.17(.05) | −0.12(.06) | - |
| Right Insula | 6.37 | (38,−16,8) | 0.56(.07) | 0.09(.08) | 0.58(.10) | 0.21(.06) | 0.21(.05) | −0.03(.09) | g |
| Left Thalamus | 6.15 | (−10,−22,4) | 0.64(.10) | 0.45(.10) | 0.53(.11) | −0.05(.08) | 0.14(.07) | 0(.20) | - |
| Right Cerebellum | 6.05 | (14,−64,−24) | 0.26(.06) | 0.43(.08) | 0.36(.07) | −0.19(.08) | 0.03(.05) | 0(.08) | h |
| Left Pallidum | 5.96 | (−22,−6,−4) | 0.40(.06) | 0.20(.04) | 0.32(.12) | −0.01(.10) | 0.10(.04) | 0.02(.05) | i |
| Mid Cingulate | 5.91 | (12,−30,40) | 0.45(.07) | 0.13(.09) | 0.77(.11) | −0.08(.06) | 0.21(.05) | −0.16(.06) | j |
| Right Fr.Inf.Tri. | 5.89 | (56,30,10) | 0.42(.07) | 0.18(.06) | 0.60(.09) | −0.07(.10) | 0.16(.05) | −0.18(.06) | - |
|
| |||||||||
| Left M1 | 2.9 | (−36,−18,54) | 0.06(.06) | 0.15(.04) | 0.47(.08) | .10(.05) | 0.33(.04) | −0.24(.07) | k |
Fig. 5.
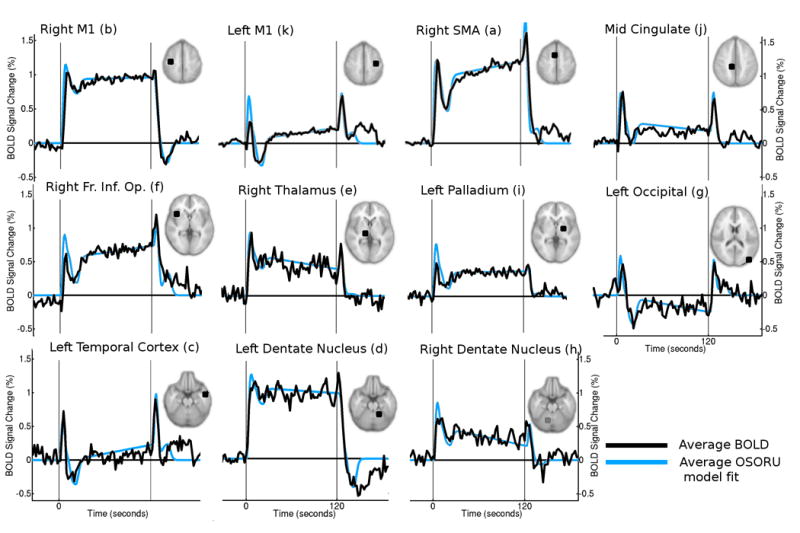
Region-of-Interest (ROI) plots of average BOLD signal responses from the first training session (black trace). Also displayed is the modelled response, generated from the average OSORU parameter estimates (blue trace). The response shapes vary considerably from region to region, and were well fit by the OSORU model. In some regions, some post-task transient activity was not accounted for by the model.
The sustained activation component was significant across a wide range of regions known to be involved with motor performance. Bilaterally activated regions included the SMA, thalamus and basal ganglia, as well as regions of premotor, parietal, insula, and inferior frontal cortices. The right (contralateral to task) primary sensory-motor cortex, and left cerebellar dentate nucleus were strongly activated. Activation was found bilaterally in pre- and sensory-motor cortices; however, the hand region of the left primary motor cortex did not reach statistical significance. There were sustained deactivations over a wide region of the superior frontal cortex as well as in the medial parietal cortex, precuneus and temporal pole. A steadily decreasing ramp was found in the cerebellum. An increasing ramp component was significant in the SMA, the left sensory-motor, insula and inferior frontal cortex and bilateral occipital cortex.
Onset and offset spikes were very widely distributed. Both components were significant along the midline extending from the SMA to the parietal cortex and in the posterior thalamus, bilateral precuneus, dentate nuclei and vermis of the cerebellum, the right inferior frontal, temporal and premotor cortices. Parts of the right primary sensory-motor and sensory cortices did not have a significant Onset component, in the context of strong sustained activation. A significant Offset spike was more widespread in this region. The amplitude of both components could be large. The temporal lobes exhibited large spikes in the absence of any sustained activation, while in the insula and thalamus, signal change due to spikes matched or exceeded the amplitude of a significant Sustained component. The OSORU Offset component estimates were larger that Onset components in 80% of voxels in which both features were significant, including in primary motor cortical regions such as the SMA, and the temporal lobes, despite the fact that a slightly larger OSORU Onset spike parameter than Offset parameter is required to fit equal sized spikes in the presence of sustained activation.
The two undershoot components had distinctly different spatial distributions. The post-onset undershoot was widely distributed cortically, with particularly strong clusters in bilateral primary motor, temporal and inferior frontal cortices. In the left motor cortex, and parts of the posterior cingulate, the BOLD signal dropped below baseline. The undershoot was not a significant component of the signal response in the basal ganglia, thalamus, nor in the vermis and dentate nucleus of the cerebellum. Average BOLD signal plots indicate the post-onset undershoot exhibits a consistent time-course across brain regions, distinct from any ramping of the signal. It was well fit by the extra OSORU basis function.
The post-task undershoot was significant in the left dentate nucleus, and head of the caudate. In addition, in a number of regions, including the right insula and occipital cortex, a negative parameter estimate was significant. Average time courses revealed a secondary rise in signal, following the return to baseline at task completion, lasting around thirty seconds. This unexpected feature was consistent across all subjects, with an average negative parameter estimate in 12/12 subjects in the insula.
Within-session changes in BOLD signal dynamics
Parameter estimates of the Sustained and Ramp components of regional responses were found to change over the eight trials of individual sessions (Fig. 6A). In all sessions, a symmetric network of cortical regions including SMA, sensory-motor regions, cingulate, insula, inferior frontal and temporal cortex, showed reductions in the sustained response component. There was a concurrent increase in the Ramp component across the same range of regions. Change in the Ramp component was less extensive and statistically significant, and was absent from the right primary motor cortex.
Fig. 6.
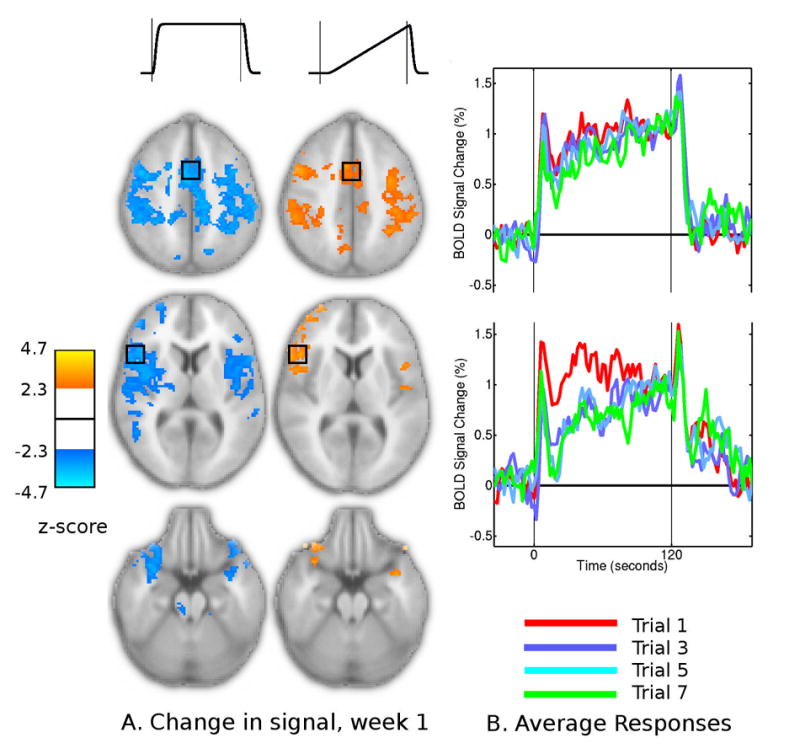
Changes in the BOLD signal responses within the first training session. (A) regions that displayed significant linear change in sustained and ramp components over the trained task performances of each session. (B) Average time courses from premotor ROI for the first session, averaged across subjects, from trials 1, 3, 5 and 7 (red, blue, aqua and green traces).
The combination of Sustained and Ramp component changes reflected a consistent change in the waveform shape (Fig. 6B). Compared to the first trial of each session, the average BOLD time-course from the final trials dropped further following the Onset spike. Over the remainder of the task period, however, the average BOLD signal level gradually ramped upwards, sometimes reaching the signal level of the initial trial. In the right primary motor cortex, reductions in sustained activation were also present but were not accompanied by strong changes in other components, with time-course plots indicating a more consistent reduction in the BOLD signal over the entire task period.
We did not detect significant differences between the changes in the first session, and changes in the second and third sessions. Nor were there significant differences between the trained and control task.
Cross-session changes in BOLD signal dynamics
After two weeks of training, changes were detected in the Sustained and Onset components of the OSORU model (Fig. 7A). Bilateral putamen and thalamus exhibited significantly increased sustained levels of activation. This was accompanied by reductions in the OSORU Onset component. Significantly decreased sustained activity was detected in bilateral inferior frontal cortex. These and further regions including the bilateral sensory-motor cortex, parietal and occipital cortex, precuneus and cerebellum also displayed significant reductions in the Onset component.
Fig. 7.
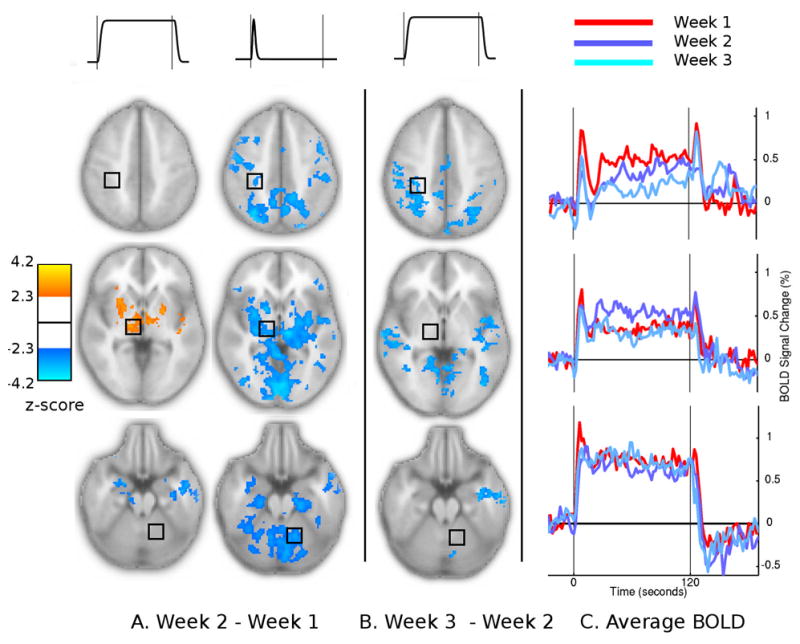
Changes in the OSORU model fits between sessions. (A) Session 2 - session 1. Changes were detected in Sustained and Onset model components (B) Session 3 - session 2. Changes were detected in the Sustained component only. (C) Average time courses from right primary sensory, thalamus and dentate nucleus in each of the three sessions
Average time courses from three ROIs illustrate these changes (Fig. 7C). In the thalamus (row 2), the average BOLD signal time courses indicate that the change in OSORU parameter estimates were primarily due to the increased level of sustained activation. In the first session, signal in the thalamus and putamen dropped off after an initial onset spike. In the second session the signal was maintained at close to the level of the peak of the onset spike, after a transient undershoot. The reduced OSORU Onset component reflected the loss of a distinct onset spike above the Sustained level of activation. In contrast, the reduction in Onset component in the cerebellum reflected an actual reduction in Onset spike in the context of a steady level of sustained activity. Figure 8 plots the time-course of the Sustained and Onset parameter estimates across all sessions and trials. Due to the limited number of subjects, trained and control trials were combined for this plot.
Fig. 8.
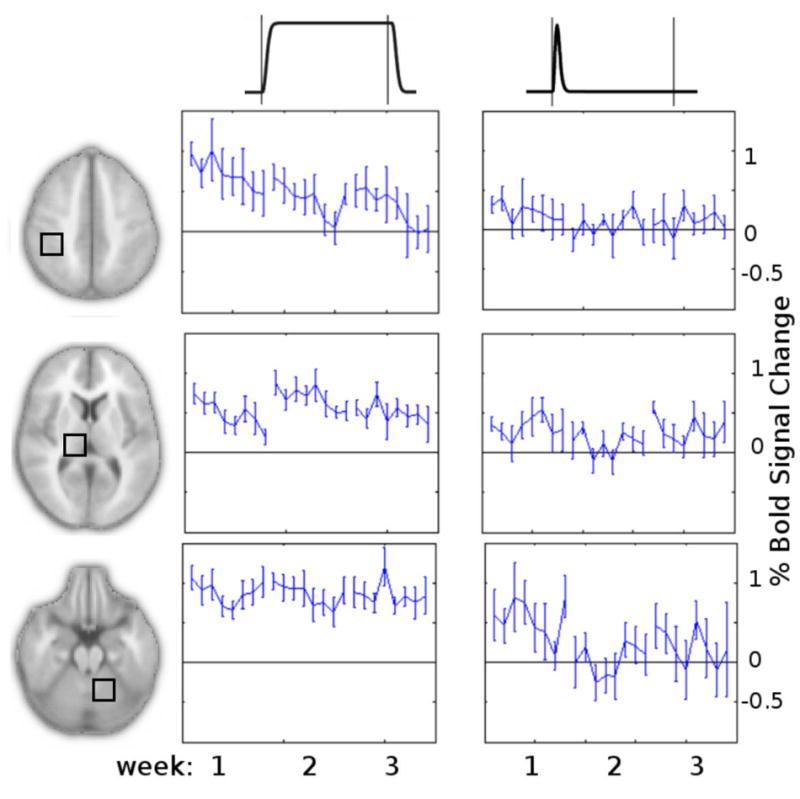
Trial-by-trial time plots of Sustained and Onset OSORU parameter estimates, average across trained and control tasks, for ROIs from Fig. 7. (A) Average sustained parameter estimate over time. (B) The onset component over time.
Further changes in Sustained activity levels were detected after four weeks of training (Fig. 7B). Reductions were detected in the parietal, occipital and sensory cortices and cerebellum. Non-significant increases in the Ramp component were present in all these regions. While not detected by the GLM contrast, ROI time course plots suggest that by the final session activity levels in the thalamus were reduced compared to their peak in the second session.
Contrasts between trained and control tasks in later sessions revealed minimal differences in brain responses. The one significant effect was a higher level of sustained activity in the dentate nucleus in the trained task in the second scanning session (not shown).
Discussion
We have quantified the spatio-temporal dynamics of extended BOLD signal responses to prolonged performance of a motor sequence task, across different stages of training, employing the OSORU model.
The speed increases over the first two weeks of training suggests that subjects learned to perform the task with proficiency. The careful pacing of the task in the scanner, and the very low error rate, suggest that motor output changed very little across weeks. Observed changes in brain responses are likely to reflect changes in the cortical activity underlying task performance, rather than any changes in the task performance itself.
The demands of the specific tasks employed in imaging studies need to be carefully considered Doyon et al. (2003). Here, the task was relatively simple, and was continuously performed for unusually prolonged periods within the scanner, which improved our ability to track transient dynamics, but reduced our ability to resolve fast changes across blocks. The extended task may have resulted in a greater rate of learning compared to other paradigms. Large onset and offset transients could be due to relatively low ongoing processing demands compared to other motor learning tasks. Long blocks can also result in a wider spatial extent of significant BOLD signal (Saad et al., 2003; Sheth et al., 2005).
OSORU analysis
The OSORU set of basis functions was used for full-brain characterisation of the BOLD responses. It was straightforward to implement in the Feat GLM package, and remarkably effective at modelling the wide range of BOLD response shapes. Overall, a wider network of responses was detected than obtained with the use of a standard response model. Distinct features of the BOLD signal responses are accounted for by separate model components, which enabled their separate statistical analysis and mapping. The additional post-onset undershoot was an important component of the model for this dataset, contributing significantly to fits across a wide range of regions. Mapping of the spatial distribution of individual features, and their dynamics within and across sessions, provided a thorough, efficient and informative summary of the response dynamics of the dataset.
The response features are not fully independent. For example, the within-session change in response shape was modelled by simultaneous changes in the Sustained and Onset components. Similarly, the onset spike of the thalamus disappeared when, in the second session, the peak signal level at onset was maintained for the whole task period. Nevertheless, the OSORU model approach provides a detailed characterisation of responses, from which complex waveform changes can be inferred. A growing awareness of the complexities of the BOLD response should be useful in directing the development of more sophisticated models based on physiologically meaningful variables, such as the balloon model, which aim to provide quantitative estimates of neural activity, a key aim of fMRI modelling Buxton et al. (2004).
The linear component modelling approach has limitations. The predefined components may not accurately model responses in all tasks or in all regions. In particular, slight variations in the timing of transient features could reduce their detectability. A common method for modelling temporal variation in response timing involves the addition of the temporal derivative of the response model. However, this approach is not feasible in the OSORU model, as such components will be correlated to the undershoot components. In general, further extensions of the OSORU will require more sophisticated modelling techniques. Another limitation of this approach is that it does not accurately characterise intermittent regional activity during continuous task performance. Additional spatial mapping of spectral band power may be an approach that could be used to detect such behaviour.
BOLD signal responses
The extended task duration revealed a wide range of BOLD response shapes across the brain, ranging from the canonical sustained response, to responses consisting of signal spikes, undershoots and ramps. A striking feature was that most regions exhibited extended periods of non-stationary dynamics, with the widespread post-onset undershoot meaning signal often did not stabilise until twenty seconds after onset. Also striking was how consistently identifiable the onset, post-onset undershoot, and offset components of responses were. The onset and offset spikes peaked around six seconds and then rapidly dropped off. The post-onset undershoot also had a consistent time course, which was maintained even when signal went below baseline (i.e. in the temporal lobe cortex, Fig. 5). A thorough investigation of responses from across the brain confirmed that this consistency was not a result of selective plotting of the most statistically significant regions.
Interpreting fMRI dynamics is not straightforward due to the dependency of the BOLD signal on the dynamics of blood flow, blood volume, and CMRO2 responses to neural activity. (Obata et al., 2004) compared fMRI BOLD and blood flow measures of responses to motor activity. In the SMA, but not motor cortex, BOLD signal responses had signal spikes at onset. However these spikes were not present in the blood flow recordings, suggesting the transient was a result delays in blood volume or CMRO2 relative to blood flow.
However, our results suggest many of the transient features have neural correlates. Onset and offset spikes appeared in the absence of sustained activity in some regions, and were very large relative to sustained activity in many others. A vascular explanation for such signal is unlikely. Transient neural activity could relate to the initiation and termination of the motor task, or more general set-shifting or task-switching related activity (Fox et al., 2005a). The significant reduction of the size of the onset component by the second scanning session, in a number of regions, suggests that neural processing underlying these regions altered with learning.
The relative sizes of the onset and offset spikes varied, although they where often similar. The offset-spike OSORU estimate was larger than that at the onset in a majority of voxels (80%), which included both regions exhibiting significant sustained activity, such as the SMA, and regions exhibiting primarily transient signal at task transitions, such as the temporal lobes. This is surprising, as the processing involved in the initiation of the motor task might be expected to be greater than that terminating it. For regions with significant sustained signal, the effect may be related to the fact that the the onset spike occurs in the context of the task-induced increase of signal from baseline, while the offset spike is initiated when the signal is already high. The offset spike did not exhibit any significant changes within or across sessions, which could be related to the smaller number of trials in which the offset spike was recorded (scanning finished at the end of the second task block). It may also indicate that the underlying neural activity is not affected by learning.
The post-onset undershoot was a prominent signal feature across many regions. This feature has not previously been identified, perhaps as it likely to be difficult to distinguish from ramping signal when the task duration is less extended. It usually followed a significant onset spike, however it was present in parts of the right primary motor cortex, and bilateral cerebellar hemispheres where no distinct onset spike was apparent. The undershoot may reflect a stage in the adjustment of hemodynamics to a new steady-state of neural activity.
In the left motor cortex the undershoot took the signal below baseline, after which the signal returned to a low, positive level of sustained signal. Previous studies have reported deactivation in this region during low-force motor tasks, but activation with high-force tasks (Hamzei et al., 2002; Dettmers et al., 1995). Evidence from TMS/PET, ASL, and simultaneous BOLD and field potential recordings suggest that this negative BOLD signal reflects a reduction in neural activity, which may be due to transcallosal inhibition (Liepert et al., 2001; Stefanovic et al., 2004; Shmuel et al., 2006). The present data suggest the paced motor sequence task may produce a mixture of inhibitory and excitatory drives into the left motor cortex, which take some time to stabilise. Undershoots below baseline were also observed in the posterior cingulate and inferior temporal regions, both of which have been reported to exhibit deactivation during the performance of attention demanding tasks (Fox et al., 2005c). It is also possible that inhibitory dynamics contribute to the undershoot in regions where the signal does not reach baseline.
Undershoots following task-completion were less widespread, which again may be related to the reduced statistical power for offset components in this analysis. These undershoots were most prominent in the cerebellum and the left insula. The presence of large draining veins in the cerebellum and insula could contribute to these effects, given the known sensitivity of gradient-echo protocols to large vessels (Yacoub et al., 2003). A recent study found evidence that CBF and CMRO2 changes in surface veins contribute to undershoots in signal (Yacoub et al., 2006). Significant undershoot components in the prefrontal cortex related to sustained deactivations which persisted beyond the end of the task. Parts of bilateral sensory-motor, pre-motor the insula cortex and posterior parts of the thalamus, exhibited a secondary increase in BOLD signal, after the completion of the task and offset spike, lasting up to forty seconds. Like the post-onset undershoot, this effect may also relate to a stabilisation of hemodynamics after the large reduction in flow at the end of the task period. Future studies will determine whether this is a consistent feature of BOLD dynamics.
Both positively and negatively ramping signal was detected. In many regions, positive signal ramps increased in later trials of sessions, as the signal dipped after the onset spike. Ramping activity has not been consistently observed in studies of BOLD signal. This could be due to the focus on primary cortical regions (Bandettini et al., 1997). Ramps were visible in both the BOLD and flow plots from the SMA and M1 in the report of Obata et al. (2004). An early study posited a gradual change from anaerobic to aerobic energy consumption as a potential source of gradually increasing blood flow (Frahm et al., 1997). Neural adaptation, or changes in the likelihood of intermittent activity, could also induce ramps in average signal.
BOLD response dynamics during motor learning
The responses of many regions changed in shape over the eight trials of each session. These regions included bilateral pre- and sensory-motor and insula cortices, and SMA and cingulate. Across these regions the change in shape was consistent. As trials progressed, a dip in signal level following the onset spike became more pronounced. After this dip, the signal gradually recovered upwards towards the sustained level of earlier trials. Onset and offset spikes did not change appreciably. The primary motor cortex did not exhibit this change in shape, showing a straightforward reduction in sustained activity. The regions displaying significant changes correspond to cortical regions previously reported to exhibit reduced activation over short term learning (Doyon et al., 2002, 2003). Past studies have reported, in addition, decreases in the contralateral cerebellum and increases in the contralateral thalamus, putamen and ipsilateral cerebellar dentate during an initial training session (Doyon et al., 2003; Floyer-Lea and Matthews, 2005; Jueptner et al., 1997). We detected no overall change in the activity of these regions in the present paradigm.
There were no significant differences in within-session changes between each of three scanning sessions, indicating the effects reflect gradual, yet sizable, neural or hemodynamic adaptation with over the scanning period, unrelated to motor learning. The lack of significant differences between sessions is surprising, as many studies have found response changes specific to early stages of learning a motor task(Toni et al., 1998; Doyon et al., 2003). It is likely that simple task and its extended duration may have resulted in session-specific effects occurring faster than the extended block design of the present study could resolve. Interestingly, only a few studies have tracked changes across the full learning period, permitting direct comparisons of within-session response changes (Karni et al., 1995; Floyer-Lea and Matthews, 2005).
At the second scanning session, we found a strong increase in sustained activity in bilateral thalamus and putamen. Again, the overall changes in the BOLD signal shape were complex, with an increase in the sustained signal reducing the distinct onset spike. A possible explanation is that these regions became more highly involved in the ongoing performance of the task, while the steady onset peak reflects a stable level of synaptic input and initiation-related activity. Increasing involvement of the thalamus and putamen as a motor task is learnt has been reported in many motor learning studies, and suggests a greater involvement of the cortico-striatal loop in the execution of movement (Doyon et al., 2002; Jueptner et al., 1997; Floyer-Lea and Matthews, 2005). Plots of the trial-by-trial time course of OSORU sustained component parameter estimates from the thalamus indicate reduced signal levels in the final session, relative to the second, although this change did not reach statistical significance (Fig. 8).
There was a significant reduction in the OSORU onset component across a wide range of regions in the second scanning session. While this effect accompanied increases in the sustained component in some regions, it was prominent in other regions, including bilateral cerebellum and sensory-motor cortex, where such changes were not present. Studies have reported a reduction in activity in cerebellum after the fast-learning period, if the task does not require high levels of ongoing motor adaptation, with the region involved in the adjustment of motor kinematics based on sensory feedback (Doyon et al., 2003, 2002; Jueptner et al., 1997). Our results concur with this interpretation, suggesting a fast reduction in such activity once the well trained task has been initiated. The simple task may explain the limited changes observed in the cerebellum and its nuclei over the course of experiment Previous studies have detected both increases and decreases in activity in cortical regions after extended practice Doyon et al. (2002); Karni et al. (1995); Floyer-Lea and Matthews (2005). Reductions in sustained responses were detected in the final scanning session, including in the parietal, sensory, and cingulate cortices. These changes may reflect lower attentional demands as task performance becomes automatic. Previously reported increases in primary motor cortex were not detected.
In contrast to previous studies of the same task, training induced changes in task response were also found in the control task (Karni et al., 1995). This generalisation of learning may be due to the extended period of continuous practice involved in the scanning sessions.
Implications for fMRI experimental design and analysis
The observed dynamics have implications for studies of motor learning, which usually employ shorter trial durations to increase statistical power and to resolve fast changes across individual responses (i.e. over tens of seconds) (Karni et al., 1995; Doyon et al., 2002; Floyer-Lea and Matthews, 2005). Based on our observations here, BOLD activity in many regions may fail to stabilise over these periods. In studies in which blocks consist of the discontinuous repetition of a task, repeated signal spikes may occur. Investigations of these responses will likely reflect only certain aspects of responses.
In the presence of transients, use of the standard response model is inadequate. The fit of a standard sustained will give a measure of the average signal level across the task. The task duration will determine the contribution of different signal components to this fit, and whether specific signal changes will be detected. For example, the within session change detected in the insula in the present study would be likely to be characterised as a deactivation if the task duration was greater than fifteen seconds, but would be missed if the onset spike dominated the model fit. Similarly, the change in cerebellum with training would only be detected if the task duration was reasonably brief.
Taken with the recent studies of Harms et al. (2005) and Fox et al. (2005a), the present results suggest that BOLD transient dynamics may be a widespread phenomena. It is important that all systems studied with fMRI are investigated for these features. If present, closer attention to experimental design and the choice of tasks. While extended duration trial periods are not optimal for spatial localisation or statistical power, they can reveal much about transient dynamics. Routinely incorporating such trials into studies could aid in the design of realistic hemodynamic response models and help determine ideal task durations. Multi-component models such as OSORU are an efficient, straight-forward and easy to implement method for the characterisation of complex responses. It may be possible to fit the OSORU model across trials of different durations. Finally, to make realistic estimates of neural activity from BOLD signal, the sources of these observed signal transients need to be carefully assessed and incorporated into physiologically realistic models. This will require further multi-modal studies, as well as a comprehensive studies of how various experimental factors affect different aspects of the BOLD response.
Acknowledgments
JX is financially supported by NSF (BCS 05-09626) and NIH (5 RO1 NS046082) grants
ED’s research has been supported by The Cooperative Research Centre for Sensor Signal and Information Processing, funded by The Commonwealth Government of Australia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandettini P, Kwong K, Davis T, Tootell R, Wong E, Fox P, Belliveau J, Weisskoff R, Rosen B. Characterization of Cerebral Blood Oxygenation and Flow Changes During Prolonged Brain Activation. Human Brain Mapping. 1997;5:93–109. [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fmri. Neuroimage. 2003;20 (2):1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Buxton R, Uludag K, Dubowitz D, Liu T. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–33. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fmri. Neuroimage. 2002;15 (2):373–85. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink G, Lemon R, Stephan K, Passingham R, Silbersweig D, Holmes A, Ridding M, Brooks D, Frackowiak R. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74 (2):802–15. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41 (3):252–62. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci U S A. 2002;99 (2):1017–22. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff E, Egan G. REX: Systematic Response-Waveform Exploration and Analysis for Functional Imaging Experiments; Poster, Human Brain Mapping Conference; Florence, Italy. 2006. [Google Scholar]
- Floyer-Lea A, Matthews P. Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol. 2004;92 (4):2405–12. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews P. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94 (1):512–8. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder A, Barch D, Gusnard D, Raichle M. Transient BOLD responses at block transitions. Neuroimage. 2005a;28 (4):956–66. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder A, McAvoy M, Barch D, Raichle M. The BOLD onset transient: identification of novel functional differences in schizophrenia. Neuroimage. 2005b;25 (3):771–82. doi: 10.1016/j.neuroimage.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005c;102 (27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Kruger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal activation in man. Magn Reson Med. 1997;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C. Reduction of excitability (”inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage. 2002;17 (1):490–6. doi: 10.1006/nimg.2002.1077. [DOI] [PubMed] [Google Scholar]
- Harms M, Guinan J, Jr, Sigalovsky I, Melcher J. Short-term sound temporal envelope characteristics determine multisecond time patterns of activity in human auditory cortex as shown by fMRI. J Neurophysiol. 2005;93 (1):210–22. doi: 10.1152/jn.00712.2004. [DOI] [PubMed] [Google Scholar]
- Harms M, Melcher J. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J Neurophysiol. 2002;88 (3):1433–50. doi: 10.1152/jn.2002.88.3.1433. [DOI] [PubMed] [Google Scholar]
- Harms M, Melcher J. Detection and quantification of a wide range of fMRI temporal responses using a physiologically-motivated basis set. Hum Brain Mapp. 2003;20 (3):168–83. doi: 10.1002/hbm.10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of bold signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999 Nov;42 (5):849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17 (2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith C, Brooks D, Frackowiak R, Passingham R. Anatomy of Motor Learning. II. Subcortical structures and learning by trial and error. Journal of Neurophysiology. 1997;77:1313–24. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional mri evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377 (6545):155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Liepert J, Dettmers C, Terborg C, Weiller C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112 (1):114–21. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- Nakai T, Matsuo K, Kato C, Takehara Y, Isoda H, Moriya T, Okada T, Sakahara H. Post-stimulus response in hemodynamics observed by functional magnetic resonance imaging–difference between the primary sensorimotor area and the supplementary motor area. Magn Reson Imaging. 2000;18 (10):1215–9. doi: 10.1016/s0730-725x(00)00217-4. [DOI] [PubMed] [Google Scholar]
- Obata T, Liu TT, Miller KL, Luh WM, Wong EC, Frank LR, Buxton RB. Discrepancies between bold and flow dynamics in primary and supplementary motor areas: application of the balloon model to the interpretation of bold transients. Neuroimage. 2004;21 (1):144–53. doi: 10.1016/j.neuroimage.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Ropella KM, DeYoe EA, Bandettini PA. The spatial extent of the bold response. Neuroimage. 2003;19 (1):132–144. doi: 10.1016/s1053-8119(03)00016-8. [DOI] [PubMed] [Google Scholar]
- Sheth S, Nemoto M, Guiou M, Walker M, Toga A. Spatiotemporal evolution of functional hemodynamic changes and their relationship to neuronal activity. J Cereb Blood Flow Metab. 2005;25 (7):830–41. doi: 10.1038/sj.jcbfm.9600091. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis N. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9 (4):569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking J, Pike G. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage. 2004;22 (2):771–8. doi: 10.1016/j.neuroimage.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fmri study. Neuroimage. 1998;8 (1):50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- Toni I, Schluter ND, Josephs O, Friston K, Passingham RE. Signal, set- and movement-related activity in the human brain: an event-related fmri study. Cereb Cortex. 1999;9 (1):35–49. doi: 10.1093/cercor/9.1.35. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15 (1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78 (3):553–64. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for fmri group analysis using bayesian inference. Neuroimage. 2004;21 (4):1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fmri data. Neuroimage. 2001;14 (6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley K, Evans A, Marrett S, Neelin N. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Duong TQ, Moortele PFVD, Lindquist M, Adriany G, Kim SG, Ugurbil K, Hu X. Spin-echo fmri in humans using high spatial resolutions and high magnetic fields. Magn Reson Med. 2003 Apr;49 (4):655–664. doi: 10.1002/mrm.10433. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Ugurbil K, Harel N. The spatial dependence of the post-stimulus undershoot as revealed by high-resolution bold- and cbv-weighted fmri. J Cereb Blood Flow Metab. 2006 May;26 (5):634–644. doi: 10.1038/sj.jcbfm.9600239. [DOI] [PubMed] [Google Scholar]


