Abstract
Our laboratory treats guinea pigs with hyperbaric oxygen (HBO) as a model for investigating the formation of nuclear cataract. Previous analyses of lens supernatants using this model have shown an increase in disulfide (-SS-) and loss of sulfhydryl (-SH) in the lens nucleus of O2-treated animals. In this paper, we have used the non-invasive technique of Raman spectroscopy to confirm these findings in intact, freshly-excised lenses. Guinea pigs were treated 3 times per week with HBO for a total of 50 (4 months of treatment) or 85 (7 months of treatment) times to induce an increased level of lens nuclear light scattering. Intact lenses were analyzed by Raman spectroscopy using a 514.5 nm laser and collecting the scattered light in a 90° geometry. The laser beam was focused either in the lens nucleus or equatorial cortex. Changes in the levels of -SS- (503 cm−1) and -SH (2577 cm−1) vibrations were measured. Raman spectra were analyzed by fitting Lorentzian profiles to the observed data in the -SS- and -SH regions. -SS- levels in the O2-treated nucleus were found to have increased by a factor of 2.1 (p=0.0001) and 2.5 (p=0.001) after 50 and 85 HBO treatments, respectively, compared to age-matched controls. Based on previous biochemical analyses, the -SS- increase was due mainly to the formation of protein disulfide (PSSP) with contribution also from protein/thiol mixed disulfides, but not from oxidized glutathione. -SH levels in the O2-treated nucleus decreased by 13% (p=0.007) and 35% (p=0.001) after 50 and 85 HBO treatments, respectively, compared to age-matched controls. No significant increase in -SS- or loss of -SH was observed in the lens cortex of the O2-treated guinea pigs. The Raman spectroscopy results rule out the possibility that artifactual production of -SS- and loss of -SH occurred during homogenization of lenses in previous studies. The data provide additional evidence to support a link between O2, disulfide-crosslinking of lens crystallins in the nucleus, and nuclear cataract.
Keywords: Raman spectroscopy, lens, nucleus, hyperbaric oxygen, disulfide, sulfhydryl, nuclear cataract, animal model
1. Introduction
The formation of lens protein disulfide (PSSP), leading to crosslinking and precipitation of lens crystallins, is a major factor in the development of nuclear cataract (Dische and Zil, 1951; Spector, 1984; Giblin et al., 1995) . Recent studies have linked molecular oxygen (O2) with lens nuclear opacity (Holekamp et al., 2005; Simpanya et al., 2005), and it is possible that O2 is a precursor to a more active, harmful species within the lens that can generate disulfide (-SS-). We treat guinea pigs with hyperbaric oxygen (HBO) in order to investigate events leading to nuclear cataract, and have used this technique to demonstrate an association between O2-induced lens nuclear light scattering and an increase in the level of PSSP (Giblin et al., 1995; Simpanya et al., 2005). However, in these studies, PSSP analyses were conducted in supernatants of lens homogenates, a procedure that can possibly lead to artifactual production of -SS-. Thus, we considered it useful to investigate -SS- formation in isolated, intact lenses of O2-treated guinea pigs, as we have done in the present study, using the non-invasive technique of laser Raman spectroscopy.
Raman spectroscopy has been employed for many years to measure levels of sulfhydryl (-SH) and -SS- in different regions of intact lenses from a variety of species (Askren et al., 1979; Barron et al., 1988; Cai et al., 1989; DeNagel et al., 1988; East et al., 1978; Kuck et al., 1982; Ozaki et al., 1987; Ozaki et al., 1983; Ozaki and Mizuno, 1992; Yu et al., 1985b). The lens is ideal for this type of technique because of its unusually high concentration of both protein (about 35%) and -SH groups (nearly 50mM), and the fact that -SH and -SS- each have their own signature vibrational frequencies at approximately 2577 cm−1 and 503 cm−1, respectively. Raman analysis of whole lenses has contributed significantly to our understanding of the effects of age and certain types of stresses on lens -SH/-SS- levels in different species. Aging, clear lenses of the mouse and rat exhibit dramatic, unique oxidation of-SH to -SS-, in contrast to the lack of substantial -SS- increase observed in aging, normal lenses of other species such as the guinea pig, rabbit, and human [-SS- formation in the aging rodent lens has been shown to be due to intramolecular, not intermolecular, bonding of protein -SH groups (Hum and Augusteyn, 1987)]. In addition, use of Raman spectroscopy has demonstrated a substantial loss of -SH and increase in -SS- in the nucleus of lenses of guinea pigs exposed to long-term UVA light (Barron et al., 1988), while, in contrast, no increase in lens -SS- over that present in controls, was associated with aging of the Emory mouse (DeNagel et al., 1988), or with galactose-induced cataract in the rat (Cai et al., 1989). The present study represents the first time that laser Raman spectroscopy has been used to investigate effects of in vivo HBO treatment on levels of lens -SH and -SS-.
2. Materials and Methods
Male retired breeder Hartley guinea pigs, initially 17 months old, were obtained from Kuiper Rabbit Ranch (Indianapolis, IN). The animals were held for one month prior to hyperbaric oxygen (HBO) treatment. During this time, the lenses of the guinea pigs were examined carefully by slit-lamp biomicroscopy, and animals with lens opacities were excluded from the study. Procedures used to treat guinea pigs with HBO have been described in detail previously (Simpanya et al., 2005). Each experimental animal was exposed to 2.5 atm absolute (ATA; 22.3 psig [pounds per square inch gauge] or 51 ft. of seawater) of 100% O2 for 2.5 hr periods, three times per week on alternate days. One group of animals received a total of 50 treatments (4 months) and the other group received 85 treatments (7 months). Age-matched control guinea pigs were included with each group of O2-treated animals. Both 50 and 85 HBO-treatments have been shown to produce an increased level of lens nuclear light scattering in guinea pigs, compared to age-matched controls (Borchman et al., 2000; Simpanya et al., 2005). A two-fold increase in lens focal length variability was observed after 70 treatments of guinea pigs with HBO (Bantseev et al., 2004).
Guinea pigs were euthanized with CO2, and the eyes isolated. The lens of one eye was removed by posterior approach and immediately placed anterior side down in 3.5 ml of Hepes-buffered Tyrode’s medium, pH 7.0, in a quartz cuvette for analysis by laser Raman spectroscopy. If the lens of the second eye was to be analyzed, the eye was stored temporarily in Tyrode’s medium at room temperature. All Raman measurements were conducted at room temperature. The Raman spectra were excited with 514.5nm radiation from an argon ion laser (Model 2016, Spectra Physics, Mountainview, CA, USA) with a final power at the sample of 120 mW. The laser light was passed up through the lens in a vertical direction and scattered light was collected at 90° to the incident radiation (Fig. 1). In order to allow lens fluorescence background to dissipate, laser light was passed through each lens for about one hour prior to analysis for -SH and -SS-.
Fig. 1.
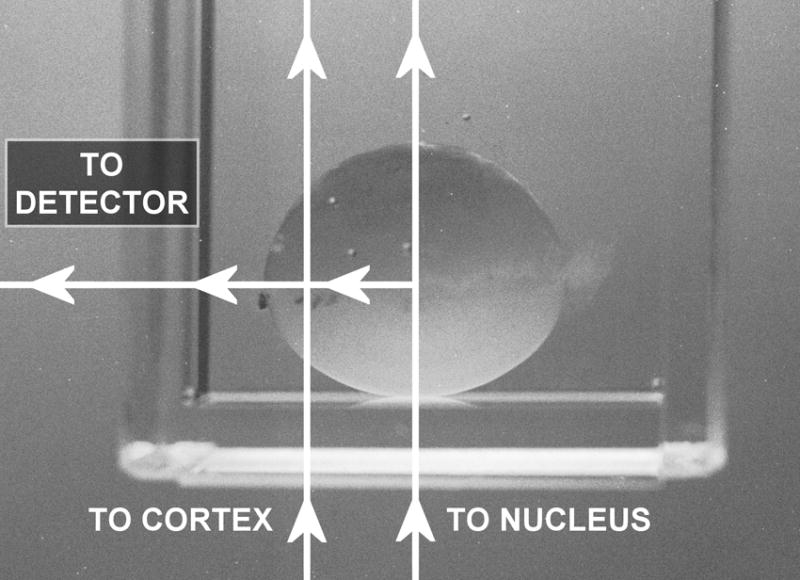
Analysis of a guinea pig lens by laser Raman spectroscopy at a 90° geometry. The lens was placed anterior side down in 3.5ml of Tyrode’s medium in a quartz cuvette. The laser beam was passed up through the center of the lens and focused either in the nucleus or equatorial cortex.
A single grating Raman spectrometer (HR460, Instruments S.A., Inc., Edison, NJ, USA) and a holographic notch filter (Kaiser Optical Systems, Inc., Ann Arbor, MI, USA) system equipped with a liquid nitrogen cooled charge-coupled device (CCD) detector and associated electronics (Instruments S.A., Inc., Edison, NJ, USA), was used to measure Raman spectra. The grating used had 1,800 grooves/mm, the entrance slit width was 100 microns, the focal length of the spectrometer was 640 mm, and the pixel size in the CCD detector was 25 microns. The spectral resolution, as determined from the full width at half the maximum intensity of the Hg green line (546.07 nm) from a spectral lamp, was 7 cm−1.
Measurements were taken from two regions of each lens – first, the very center of the lens (the nucleus) was analyzed, and the cuvette containing the lens was then displaced to permit analysis at the center of one side of the lens (the equatorial cortex) (Fig. 1). The analysis volume of the focused laser beam was 0.05μ l. At each of the two lens locations, data were recorded in two spectral regions – one from 200 to 1,200 cm−1 containing the -SS- vibrational mode, and a second region from 2,100 to 2,800 cm−1 containing the -SH vibrational mode. Data were analyzed as recorded, and not smoothed. The total time required to make the four measurements in each lens was 1-2 hours. Examples of Raman spectra for the guinea pig lens nucleus of the -SS- and -SH regions, with reference peaks, for one control animal and one animal treated 85 times with HBO are shown in Fig. 2. Raman peaks present in each spectral region of interest were resolved by fitting Lorentzian profiles (Pande et al., 2002) to the observed spectra (Fig. 3) using a non-linear least square curve-fitting procedure from a spectroscopic analysis software program called DATLAB1. For reference points for the -SS- and -SH peaks, Raman peaks at 622 cm−1 (phenylalanine, Figs. 2A and 3B) and 2723 cm−1 (protein backbone, Figs. 2B and 3D), respectively, were used (Barron et al., 1988). The spectra were first sectioned into the regions of interest; viz -SS- (465-530 cm−1; Fig. 3A), -SS- reference (615-630 cm−1; Fig. 3B), -SH (2500-2610 cm−1; Fig. 3C) and -SH reference (2700-2760 cm−1; Fig. 3D). After appropriate baselines were subtracted from each of the four regions, Lorentzians were fitted in each region. Best fits to the observed Raman spectra were obtained when a single Lorentzian function was used for the -SS- reference peak (Fig. 3B), a sum of two Lorentzians was used for both the -SH reference (Fig. 3D) and -SS- (Fig. 3A) regions, and a sum of three Lorentzians was used for the -SH region (Fig. 3C). The peak positions and amplitudes of the fitted Lorentzian functions, corresponding to -SS-, -SS- reference, -SH and -SH reference, were determined for each of the lenses analyzed in the study. For all of the lenses analyzed, -SS- reference, -SH and –SH reference peak positions varied within ±1 cm−1 of 622, 2577 and 2723 cm−1, respectively. However, the -SS- peak positions ranged from 500 to 507 cm−1, and the -SS- frequency has been denoted as 503 ±3 cm−1. The ratios of the amplitudes for -SS-/-SS- reference, and -SH/-SH reference were used to calculate the data of Figures 4 and 5.
Fig. 2.
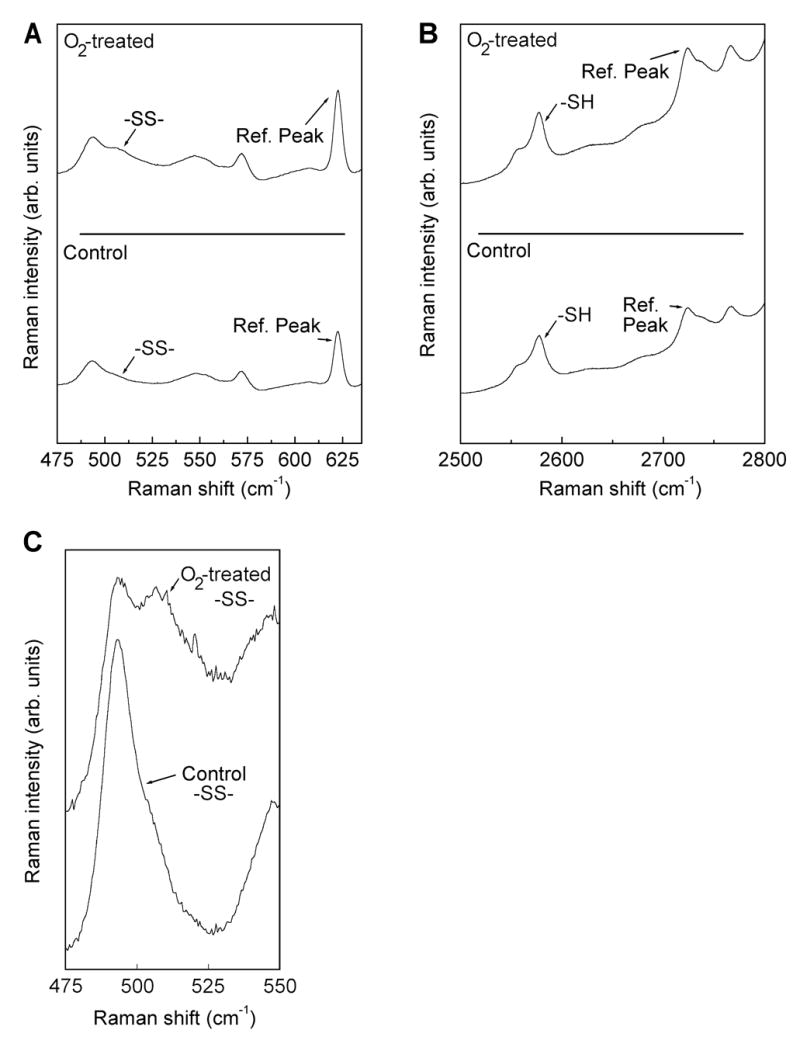
Raman spectra for the guinea pig lens nucleus of the -SS- (A) and -SH (B) regions, with reference peaks, for one control animal plus one animal treated 85 times with hyperbaric oxygen. An enlargement of the -SS- region for control and 85 treatments is also shown (C).
Fig. 3.
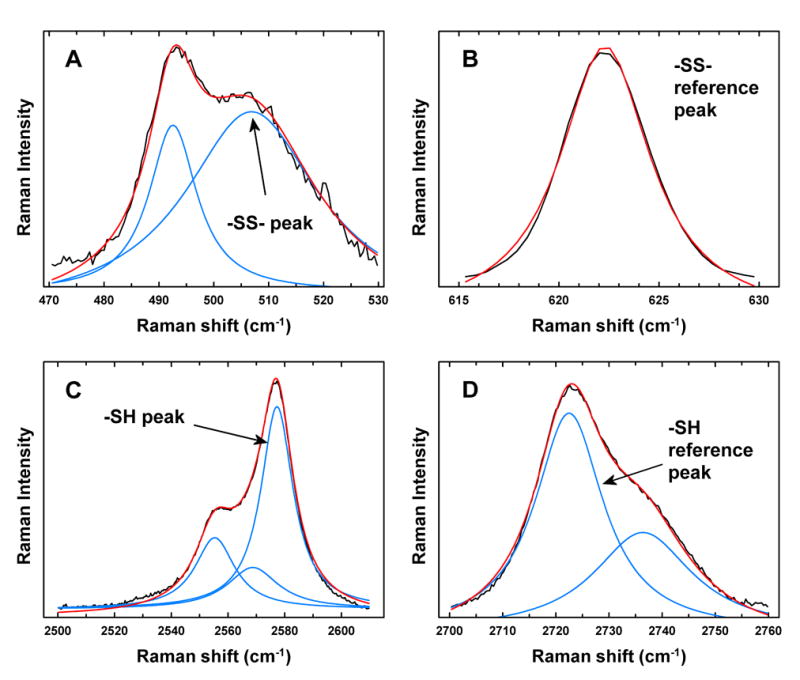
Analysis of Raman data for guinea pig lenses using Lorentzian profiles. Black lines: the observed spectra; blue lines: individual Lorentzian profiles; red lines: fitted Lorentzian profiles. (A). -SS- peak at 507 cm−1 (B) -SS- reference peak (phenylalanine) at 622 cm−1 (C) -SH peak at 2577 cm−1 (D) -SH reference peak (protein backbone) at 2723 cm−1.
Fig. 4.
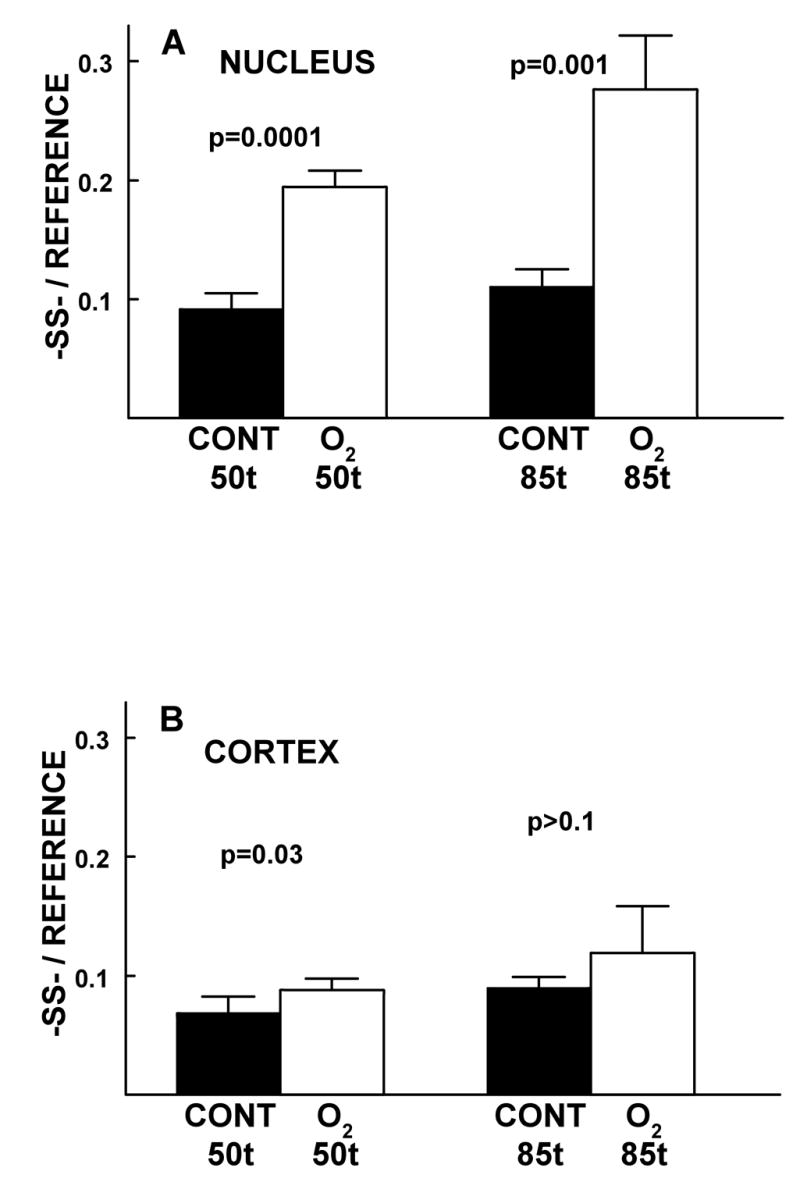
The effect of 50 and 85 HBO-treatments of guinea pigs on the relative levels of -SS- in the lens nucleus (A) and cortex (B), compared to age-matched controls. Isolated intact lenses were analyzed by laser Raman spectroscopy (see Methods). Levels of -SS- (Fig. 4) and -SH (Fig. 5) were measured in the same lenses. The ages of the animals were 22 months-old at 50 treatments and 25 months-old at 85 treatments. Results are expressed as means ± standard deviation for an n of 4, using lenses from 2-4 animals.
Fig. 5.
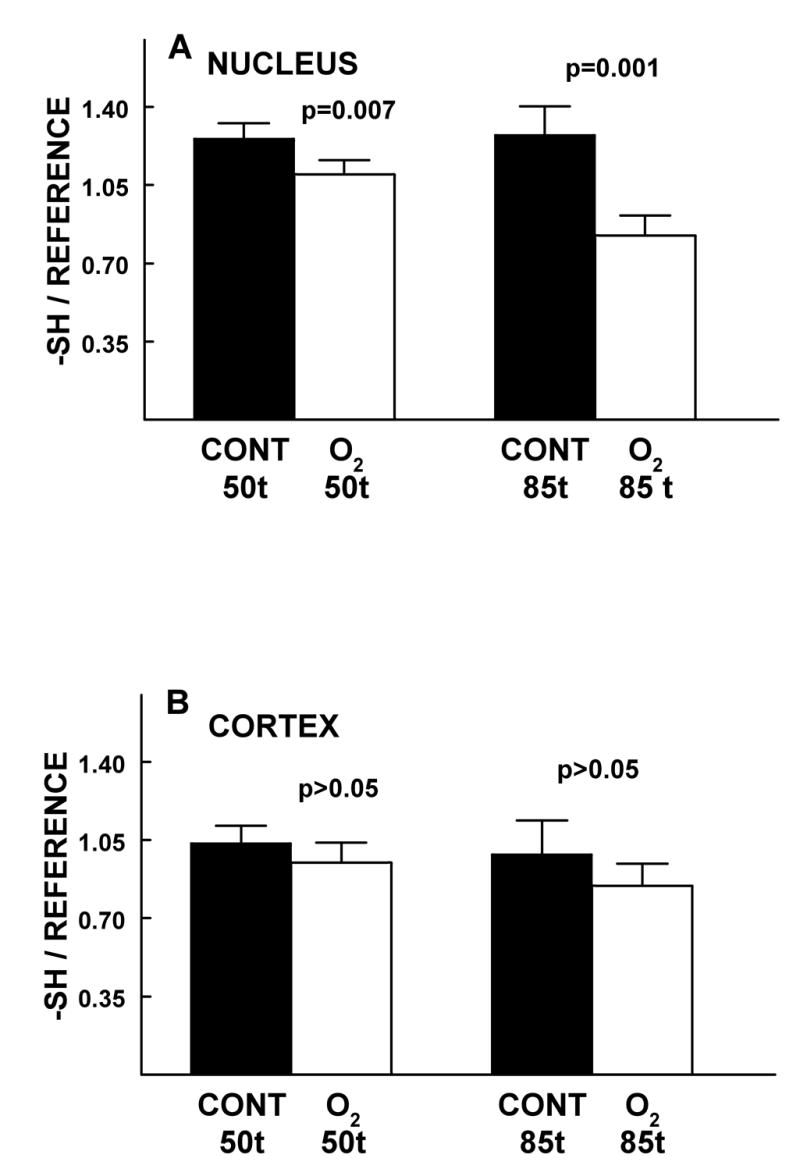
The effect of 50 and 85 HBO-treatments of guinea pigs on the relative levels of -SH- in the lens nucleus (A) and cortex (B), compared to age-matched controls. Isolated intact lenses were analyzed by laser Raman spectroscopy (see Methods). Levels of -SS- (Fig. 4) and -SH (Fig. 5) were measured in the same lenses. Ages of the animals were 22 months-old at 50 treatments and 25 months-old at 85 treatments. Results are expressed as means ± standard deviation for an n of 4 using lenses from 2-4 animals.
3. Results
As in our previous studies (Borchman et al., 2000; Simpanya et al., 2005), increased lens nuclear light scattering was also observed in the present study after 50 treatments of the guinea pigs with HBO, compared to age-matched controls, and the level of scatter intensified after 85 treatments. Since this type of slit-lamp data has been shown previously (Borchman et al., 2000; Simpanya et al., 2005), the results have not been repeated here.
Disulfide in the O2-treated nucleus increased by a factor of 2.1 (p=0.0001) and 2.5 (p=0.001) after 50 and 85 HBO-treatments, respectively, compared to age-matched controls (Fig. 4A). The 1.4-fold increase in -SS- occurring in the experimental nucleus after 85 HBO-treatments, compared to 50 treatments (Fig. 4A), was significant at a level of p=0.007. In the O2-treated cortex, there was a minimal increase in -SS-, compared to the controls (1.3 times the control for both 50 and 85 HBO-treatments; p=0.03 and p>0.1, respectively) (Fig. 4B). There was also a minimal increase in control nuclear and control cortical -SS- (1.2-1.3 fold; p=0.05 and p=0.02 respectively, as the guinea pigs aged from 22 to 25 months-old during the course of the study (Figs. 4A and 4B).
Sulfhydryl levels in the O2-treated nucleus decreased by 13% (p=0.007) and 35% (p=0.001) after 50 and 85 HBO-treatments, respectively, compared to age-matched controls (Fig. 5A). The 25% decrease in -SH occurring in the experimental nucleus after 85 HBO-treatments, compared to 50 treatments (Fig. 5A), was significant at a level of p=0.001. In the O2-treated cortex, the observed slight decreases in -SH levels compared to controls, after 50 and 85 HBO-treatments, were each not statistically significant (p>0.05) (Fig. 5B). In addition, control nuclear and control cortical -SH levels did not change significantly (p>0.1) as the guinea pigs aged from 22 to 25 months-old during the course of the study (Figs. 5A and B).
4. Discussion
We have confirmed by non-invasive Raman spectroscopy that an increase in lens nuclear -SS- occurs in guinea pigs treated with HBO (Fig. 4A). These results are in agreement with those obtained previously through biochemical analysis of lens supernatants, which showed increases in lens nuclear PSSP and protein/thiol mixed disulfides following HBO-treatment of guinea pigs (Giblin et al., 1995; Simpanya et al., 2005). Thus, possible artifactual production of -SS- occurring during lens homogenization in previous studies has been ruled out. When coupled with the reported in vivo detection of very large protein-aggregates in the lens nuclei of HBO-treated guinea pigs (Simpanya et al., 2005), the present data link molecular O2 with disulfide-crosslinking of lens nuclear crystallins, leading to a loss of lens nuclear transparency. Treatment of guinea pigs with HBO has been shown to produce a two-fold increase in lens focal length viability, compared to age-matched controls (Bantseev et al., 2004). The results help to explain the observed induction of nuclear cataracts in human patients treated with HBO (Palmquist et al., 1984), and may also be relevant to the development of maturity-onset and vitrectomy-induced human nuclear cataracts since these types of cataracts are also thought to be caused by O2 (Dische and Zil, 1951; Holekamp et al., 2005; Ogura et al., 1991). The level of increase of -SS- in the lens nucleus of O2-treated guinea pigs, compared to age-matched controls, was found to be more than two-fold (Fig. 4A). Other investigators using Raman analysis have reported the same degree of -SS- increase in the lens nucleus of guinea pigs exposed to a low level of UVA light for 9 months (Barron et al., 1988). In a 2003 study, we showed with the use of biochemical analysis that chronic exposure of guinea pigs to UVA light produced increases in lens nuclear PSSG and PSSP, as well as an increase in lens nuclear light scattering. Thus, O2 as well as UVA light appear able to induce -SS- formation and loss of transparency in the guinea pig lens nucleus.
Raman analysis is not able to distinguish between various types of -SS- such as PSSP, protein/thiol mixed disulfides and oxidized glutathione (GSSG). However, since 90% of the -SH content of the guinea pig lens nucleus is PSH (Giblin et al., 1995), it is probable that the majority of the observed O2-induced increase in nuclear -SS- (Fig. 4A) was the result of PSSP formation. HBO-treatment has been reported not to produce increased levels of GSSG in the lens nucleus (Giblin et al., 1995). However, substantial amounts of protein-bound glutathione (PSSG) and protein-bound cysteine (PSSC) do accumulate in the nucleus following HBO-treatment (Giblin et al., 1995), and thus these compounds would have contributed to some extent to the observed O2-induced increase in nuclear -SS- (Fig. 4A). PSSG and PSSC levels have been shown previously not to increase further in the nucleus after reaching a maximum at 30 HBO-treatments (Giblin et al., 1995). Thus, the significant 1.4-fold increase in -SS- occurring in the experimental nucleus after 85 HBO-treatments, compared to 50 treatments (Fig. 4A), would have been due entirely to PSSP formation. This finding links increased PSSP with the increase in lens nuclear light scattering that is observed as the number of HBO treatments increases (Borchman et al., 2000; Palmquist et al., 1984). The 2.5-fold increase in lens nuclear -SS- at 85 HBO-treatments (Fig. 4A) is actually lower than expected, based on the observed change in nuclear -SH levels from 50 to 85 HBO-treatments (Fig. 5A). It is possible that at higher levels of lens nuclear -SS-, Raman analysis yields lower-than-actual -SS- levels. Other researchers have had difficulty using Raman to analyze healthy human lenses older than 58 years (Yu et al., 1985a).
Only a minimal increase in lens cortical -SS- was observed following HBO-treatment (Fig. 4B). This result is in accord with our previous biochemical data showing little or no effect of in vivo HBO-treatment on the levels of PSSP, PSSG, PSSC and GSSG in the guinea pig lens cortex (Giblin et al., 1995; Simpanya et al., 2005). The result also agrees with our previous in vitro studies showing few effects of HBO on the cortex or epithelium of cultured lenses (Padgaonkar et al., 1993; Padgaonkar et al., 1989). The guinea pig lens cortex is very well-protected against PSSP formation since it contains a high ratio of GSH to PSH, 1:1, compared to a ratio of only 1:10 existing in the lens nucleus (Giblin et al., 1995). Thus, any PSSP forming in the O2-treated cortex would be rapidly reduced back to PSH by action of GSH and the glutathione redox cycle, following removal of the oxidative challenge (Giblin, 2000). We observed a slight but only minimally significant increase in -SS- level in both the nucleus and cortex of control guinea pigs as they aged from 22 to 25 months-old (Fig. 4A and 4B). Other researchers have used Raman spectroscopy to show only slight increases in the levels of lens nuclear -SS- in 3-5 year-old guinea pigs and rabbits due to normal aging (Yu et al., 1985b; Ozaki and Mizuno, 1992) The same type of result was reported for the normal lens of a 65 year-old human, compared to a 9 year-old (Yu et al., 1985b). This is in contrast to the aging mouse lens which shows a dramatic increase in -SS- level as a result of intramolecular PSSP formation (Yu et al., 1985b; Hum and Augusteyn, 1987). Thus, the aging guinea pig lens appears to be identical to the aging human lens with regard to an absence of substantial increase in -SS- level, as long as the lens remains transparent. It has been believed for many years that lens crystallins will not disulfide-crosslink until the level of GSH in a particular region of the lens has reached a critically low level (Kinoshita and Merola, 1959; Giblin et al., 1979; Giblin, 2000). It is thought that when the level of GSH drops below 1mM in the lens nucleus, PSSP formation and eventual nuclear cataract is initiated. More than 90% of -SH groups in the human lens are oxidized to -SS- in mature nuclear cataracts (Truscott and Augusteyn, 1977; Garner and Spector, 1980).
A substantial loss of -SH was observed in the O2-treated lens nucleus compared to age-matched controls at both 50 and 85 treatments with HBO (Fig. 5A). Most of this loss was presumably associated with loss of PSH due to formation of PSSP, and to some extent also to formation of PSSG and PSSC. Previous biochemical analyses have shown substantial loss of PSH in the lens nuclei of HBO-treated guinea pigs (Giblin et al., 1995). The lens nuclear levels of GSH in both control and HBO-treated guinea pigs have been reported to be low and not substantially different from each other (Giblin et al., 1995), and thus GSH loss would not have contributed significantly to the observed O2-induced decrease in -SH level. The extent of the lens nuclear -SH loss observed after 85 O2-treatments, amounting to 35% (Fig. 5A), is nearly equal to that obtained by Raman analysis of lenses of guinea pigs exposed to UVA light for 9 months (Barron et al., 1988). We observed a slight but insignificant loss of -SH in the O2-treated lens cortex compared to the age-matched control (Fig. 5B). Similar results were obtained previously for biochemical analysis of PSH and GSH in the lens cortex of HBO-treated guinea pigs (Giblin et al., 1995). Our Raman data indicate that -SH is about 1.2-fold higher in the normal guinea pig lens nucleus compared to the cortex (Fig. 5A and B: 1.24 -SH in the control nucleus, compared to 1.05 -SH in the control cortex). This result matches the Raman data of Yu et al., 1985b, for the guinea pig lens. However, when results of biochemical analyses for PSH and GSH in the guinea pig lens are considered (Giblin et al., 1995), -SH should actually be about 1.1x higher in the cortex than the nucleus, because of the nearly 25 mM GSH present in the cortex. The reason for the discrepancy between Raman and biochemical results for lens -SH is not known.
We observed no significant change in -SH level in either the control nucleus or cortex as the animals aged from 22 to 25 months old (Figs. 5A and 5B). Other researchers have used Raman spectroscopy to show a 50% loss of -SH in the guinea pig lens nucleus from age 3 weeks to age 5 years, presumably due to a decrease in the level of GSH with normal aging (Yu et al., 1985b). The relatively short time investigated in the current study (3 months) would not have been sufficient to detect a significant -SH loss.
In summary, we have used the non-invasive technique of Raman spectroscopy to confirm a substantial increase in -SS- and loss of -SH in the lens nucleus of guinea pigs treated with HBO. Possible artifactual production of -SS- occurring during lens homogenization in previous HBO/guinea pig studies has thus been ruled out. The results provide additional evidence to support a link between O2, disulfide-crosslinking of lens crystallins in the nucleus, and nuclear cataract.
Acknowledgments
This work was supported by National Eye Institute grants R01-EY02027 and R24-EY014803, and by the Office Québec-Amériques pour la jeunesse.
Footnotes
DATLAB was developed by Prof. K. Syassen, Max-Planck-Institut fluer Festkoerperforschung, Stuttgart, Germany.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askren CC, Yu NT, Kuck JF., Jr Variation of the concentration of sulfhydryl along the visual axis of aging lenses by laser Raman optical dissection technique. ExpEye Res. 1979;29:647–654. doi: 10.1016/0014-4835(79)90020-4. [DOI] [PubMed] [Google Scholar]
- Bantseev V, Oriowo OM, Giblin FJ, Leverenz VR, Trevithick JR, Sivak JG. Effect of hyperbaric oxygen on guinea pig lens optical quality and on the refractive state of the eye. ExpEye Res. 2004;78:925–931. doi: 10.1016/j.exer.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Barron BC, Yu NT, Kuck JF., Jr Raman spectroscopic evaluation of aging and long-wave UV exposure in the guinea pig lens: a possible model for human aging. ExpEye Res. 1988;46:249–258. doi: 10.1016/s0014-4835(88)80082-4. [DOI] [PubMed] [Google Scholar]
- Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D, Li L. Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest OphthalmolVisSci. 2000;41:3061–3073. [PubMed] [Google Scholar]
- Cai MZ, Kuck JF, Jr, Yu NT. Galactose-induced cataract in rat: Raman detection of sulfhydryl decrease and water increase along an equatorial diameter. ExpEye Res. 1989;49:531–541. doi: 10.1016/s0014-4835(89)80052-1. [DOI] [PubMed] [Google Scholar]
- DeNagel DC, Bando M, Yu NT, Kuck JF., Jr A Raman study of disulfide and sulfhydryl in the Emory mouse cataract. Invest OphthalmolVisSci. 1988;29:823–826. [PubMed] [Google Scholar]
- Dische Z, Zil H. Studies on the oxidation of cysteine to cystine in lens proteins during cataract formation. AmJOphthalmol. 1951;34:104–113. doi: 10.1016/0002-9394(51)90013-x. [DOI] [PubMed] [Google Scholar]
- East EJ, Chang RC, Yu NT, Kuck JF., Jr Raman spectroscopic measurement of total sulfhydryl in intact lens as affected by aging and ultraviolet irradiation. Deuterium exchange as a probe for accessible sulfhydryl in living tissue. J Biol Chem. 1978;253:1436–1441. [PubMed] [Google Scholar]
- Garner MH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. ProcNatlAcadSci USA. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ. Glutathione: a vital lens antioxidant. JOculPharmacolTher. 2000;16:121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Chakrapani B, Reddy VN. The effects of X-irradiation on lens reducing systems. Invest OphthalmolVisSci. 1979;18:468–475. [PubMed] [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE, Jr, Reddy VN. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. ExpEye Res. 1995;60:219–235. doi: 10.1016/s0014-4835(05)80105-8. [DOI] [PubMed] [Google Scholar]
- Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. AmJOphthalmol. 2005;139:302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Hum TP, Augusteyn RC. The nature of disulphide bonds in rat lens proteins. CurrEye Res. 1987;6:1103–1108. doi: 10.3109/02713688709034882. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH, Merola LO. The reactivity of sulfhydryl groups in bovine lenses. ArchBiochemBiophys. 1959;81:395–403. doi: 10.1016/0003-9861(59)90218-8. [DOI] [PubMed] [Google Scholar]
- Kuck JF, Yu NT, Askren CC. Total sulfhydryl by raman spectroscopy in the intact lens of several species: variations in the nucleus and along the optical axis during aging. ExpEye Res. 1982;34:23–37. doi: 10.1016/0014-4835(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Takanashi T, Ishigooka H, Ogino N. Quantitative analysis of lens changes after vitrectomy by fluorophotometry. AmJOphthalmol. 1991;111:179–183. doi: 10.1016/s0002-9394(14)72256-1. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Mizuno A. Molecular aging of lens crystallins and the life expectancy of the animal. Age-related protein structural changes studied in situ by Raman spectroscopy. BiochimBiophysActa. 1992;1121:245–251. doi: 10.1016/0167-4838(92)90153-5. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Mizuno A, Itoh K, Iriyama K. Inter- and intramolecular disulfide bond formation and related structural changes in the lens proteins. A Raman spectroscopic study in vivo of lens aging. JBiolChem. 1987;262:15545–15551. [PubMed] [Google Scholar]
- Ozaki Y, Mizuno A, Itoh K, Yoshiura M, Iwamoto T, Iriyama K. Raman spectroscopic study of age-related structural changes in the lens proteins of an intact mouse lens. Biochemistry. 1983;22:6254–6259. doi: 10.1021/bi00295a033. [DOI] [PubMed] [Google Scholar]
- Padgaonkar V, Giblin FJ, Reddan JR, Dziedzic DC. Hyperbaric oxygen inhibits the growth of cultured rabbit lens epithelial cells without affecting glutathione level. ExpEye Res. 1993;56:443–452. doi: 10.1006/exer.1993.1057. [DOI] [PubMed] [Google Scholar]
- Padgaonkar V, Giblin FJ, Reddy VN. Disulfide cross-linking of urea-insoluble proteins in rabbit lenses treated with hyperbaric oxygen. ExpEye Res. 1989;49:887–899. doi: 10.1016/s0014-4835(89)80047-8. [DOI] [PubMed] [Google Scholar]
- Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. BrJOphthalmol. 1984;68:113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande J, Hanlon E, Pande A. A comparison of the environment of thiol groups in bovine and human gamma crystallins using Raman spectroscopy. ExpEye Res. 2002;75:359–363. [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen Guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest OphthalmolVisSci. 2005;46:4641–4651. doi: 10.1167/iovs.05-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. The search for a solution to senile cataracts. Proctor lecture Invest OphthalmolVisSci. 1984;25:130–146. [PubMed] [Google Scholar]
- Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. BiochimBiophysActa. 1977;492:43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Yu NT, Bando M, Kuck JF., Jr Fluorescence/Raman intensity ratio for monitoring the pathologic state of human lens. Invest OphthalmolVisSci. 1985a;26:97–101. [PubMed] [Google Scholar]
- Yu NT, DeNagel DC, Pruett PL, Kuck JF., Jr Disulfide bond formation in the eye lens. ProcNatlAcadSci USA. 1985b;82:7965–7968. doi: 10.1073/pnas.82.23.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]


