Abstract
This study aimed to investigate the anti-inflammatory, anti-arthritic and immuno-regulatory effects of electro-acupuncture (EA) at ST36 on Collagen-induced arthritis (CIA) in mice. Male DBA/1J mice were divided into five groups: Normal, Control, NR (needle retention), EAI and EAII. All mice except those in the normal group were immunized with Collagen II for arthritis induction. Acupuncture needles were inserted into mice ST36 and electrical currents at a frequency of 2 Hz in a continuous rectangular wave form were conducted through the needles for 15 min, 3 times a week. EA treatments were administered for 5 weeks in the EAI group and for 9 weeks in the EAII group. The mice in the NR group were acupunctured in the same manner as the EA groups and the needles were retained for 15 min without electrical stimulation. CIA incidence analysis, ELISA, histological analysis and FACS analysis were performed to evaluate the effect of EA on CIA. EA at ST36 significantly reduced CIA incidence, IL-6, TNF-a, INF-γ, collagen II antibody, IgG and IgM levels in CIA mice serum and prevented knee joint destruction. EA at ST36 also reduced CD69+/CD3e+ cells and CD11a+/CD19+ cells in CIA mice lymph nodes, and CD11b+/Gr1+ cells in CIA mice knee joints. The ratios of CD3e+ cells to CD19+ cells, and CD8+ cells to CD4+ cells were maintained closer to the normal range in the EA groups as compared with the control group or the NR group. EAII was more effective than EAI throughout all the measurements. The NR was effective as well, though less effective than EA. EA at ST36 may have an anti-inflammatory, anti-arthritic and immuno-regulatory effects on CIA in mice. The effectiveness is stronger when EA starts earlier and is applied longer. Needle retention without electrical stimulation may be effective on CIA as well, however less effective than EA. Electrical stimulation and acupoint ST36 may have synergistic effects on CIA.
Keywords: Electro-acupuncture (EA), ST36, Collagen-induced arthritis (CIA), Rheumatoid arthritis (RA)
INTRODUCTION
Acupuncture is one of the oldest therapeutic interventions in the world and is now widely accepted for pain relief not only in the East but in the West as well (1). Besides pain relief, acupuncture is also considered to be effective for nausea and vomiting (2), bronchial asthma (3), musculoskeletal disorders (3) and immuno-inflammatory conditions such as rheumatoid arthritis (RA) (5,6,7) etc.
RA is a systemic inflammatory disorder that affects the diathrodial joint (8). Although acupuncture is often used for the patients with RA in the clinical situation, scientific evidence is lacking (9–12).
In the present study, we observed the influence of acupuncture on RA. For this purpose, we decided to use electro-acupuncture (EA) to objectify the amount of acupuncture stimulation on an experimental animal model of collagen-induced arthritis (CIA).
Acupoint ST36 (stomach 36) is the most commonly used acupoint for the purpose of immune strengthening and immune regulation (strengthening and regulating Qi and Blood in oriental medical terminology) in oriental medical clinics (13). Moreover, in Oriental medical theory, since the Spleen & Stomach govern the four limbs, acupoints on the Spleen & Stomach meridians are often used for disorders of the four limbs (14). Among the acupoints on the Stomach meridian, ST36 is the ‘Sea He’ point of the stomach that is important as the entry point of the Stomach meridian energy to the Stomach organ. ST36 is also the earth natured point of the earth natured (Stomach) meridian and could be the representative point of the Stomach meridian. ST36 is therefore considered to be stronger than the other ST points (15). On the basis of these theories, ST36 seemed to be the best choice for an immune related disease with symptoms in the four limbs (distal joints), such as RA.
METHODS
1. Mice
5 weeks old male DBA/1J mice were purchased from SLC, Inc. (Shizuoka, Japan). They were allowed 1 week to adapt to the environment (22 ± 2°C, 12 hr light/dark cycle). Mice had free access to water and food (Superfeed, Korea). Our animal experiment was conducted in accordance with the Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
2. CIA mouse model
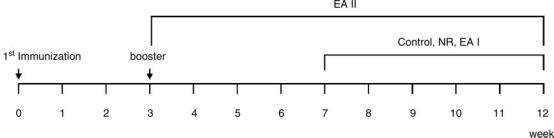
Type II collagen extracted from bovine articular cartilage (CII, Chondrex, USA) was dissolved in 0.05 M acetic acid (2 mg/ml) at 4°C overnight. Then the solution was emulsified in an equal volume of complete Freund's adjuvant (CFA, Chondrex, USA) (16,17). Mice were injected intradermally at the base of the tail with 0.2 ml of the emulsion. 21 days after the primary immunization, mice were boosted with the same volume of the emulsion (18) (Scheme 1).
Scheme 1.
Experimental procedure. The total experiment took 12 weeks. The animals were immunized with CII at the beginning of the experiment and boosted 3 weeks later. Restriction within the cage in control group, needle retention in NR group and EA in EAI group started at the 7th week and were conducted for 5 weeks. EA in the EAII group started at the 3rd week and was conducted for 9 weeks.
3. Electro-acupuncture
The mice were divided into five groups: normal; control; NR (needle retention); EA (electro-acupuncture) I and EAII. Each group consisted of 10 mice. Small cages with five holes for four limbs and a tail were built. During acupuncture administration, the animals were maintained within the cage with right hind limb taken out and fastened to the wall of the cage with tape. Sterilized disposable stainless steel acupuncture needles (0.25 × 30 mm, Dongbang Acupuncture, Inc. Korea) were inserted perpendicularly as deep as 2–3 mm at right ST36. Mice ST36 was determined according to human ST36 which is located on the stomach meridian, longitudinally three body inches below the knee joint, transversely in the middle of the tibialis anterior muscle. For a better point detection in mice, we used an elastic rubber band which was stretched along a ruler and three points at 0, 3 and 16 cm on the rubber band were marked with a red pen. The rubber band was then set to mouse right hind limb with the first mark at 0 cm on the rubber band adjusted to mouse ST35 (a point located at the lower border of patellar, in the depression lateral to the patellar ligament) (19), and the last mark at 16 cm on the rubber band adjusted to mouse ST41 (a point located on the dorsum of foot, at the midpoint of the transverse crease of the ankle joint, in the depression between the tendons of m. extensor digitorum longus and hallucis longus) (19). The point that corresponded to the second (middle) mark on the rubber band was determined as ST36. After a needle was inserted at right ST36, an electrode (0.5 × 1 cm2) was attached to right ST41. An electro-stimulator (PG-306, Japan) was connected to the handle of the acupuncture needle inserted at right ST36, and to the electrode attached to right ST41 (Figure 1). The mice in the EA groups were electrically stimulated with a 2 Hz current in a rectangular wave form for 15 min, 3 times a week. The intensity (6-7mA) was adjusted until local muscle contractions were seen. The mice in EAI group were treated with EA for 5 weeks starting 4 weeks after the booster immunization, while those in EAII group were treated for 9 weeks, starting right after the booster immunization. The period of EAII was 4 weeks longer than that of EAI, and we could observe the protective effect of EA against CIA. The mice in the NR group were acupunctured in the same manner as the EA groups and the needles were retained for 15 min without electrical stimulation. The mice in the control group were maintained in the cage for 15 min without acupuncture administration (Scheme 1).
Fig. 1.
Schematic drawing of electro-acupuncture at right ST36. Acupuncture needles (0.25 × 30 mm) were inserted perpendicularly as deep as 2–3 mm at right ST36 of mice. An electro-stimulator (PG-306, Japan) was connected to the handle of the acupuncture needle inserted at right ST36 and an electrode attached to right ST41.
4. Determination of CIA incidence
Starting a week after the booster immunization, arthritic incidence of mice in each group was determined weekly based on the number of arthritic limbs and the arthritis scores with range of 0 to 4 (score: 0 = no change, 1 = light swelling and erythema of the mid foot or tarsal bone or the ankle joint, 2 = mild swelling and erythema of the mid foot or tarsal bone through the ankle joint, 3 = moderate swelling and erythema of the ankle joint through the metatarsal bone, and 4 = gross swelling and erythema of the ankle joint through the digit) (16). CIA was regarded as induced when the score was higher than 1 in more than two limbs or higher than 2 in more than one limb. CIA incidence of each group was estimated by a formula (incidence = number of CIA mice/total number of mice × 100).
5. Fluorescence-activated cell sorter (FACS) analysis
Inguinal lymph nodes were removed aseptically and pressed through steel mesh to make a single cell suspension with RPMI 1640. Mouse knee joints were removed and chopped in RPMI 1640. Joint cells were isolated with collagenase type II (1mg/ml; Sigma, USA) in a shaking incubator for 30 min. For double fluorescence staining, purified single cells from lymph nodes or knee joints were stained with Fluorescein (FITC)-anti-mouse CD3e plus Phycoerythrin (PE)-anti-mouse CD69, or FITC-anti-mouse CD19+ plus PE-anti-mouse CD11a, or FITC-anti-mouse CD4 plus PE-anti-mouse CD8, or FITC-anti-mouse Gr1 plus PE-anti-mouse CD11b for 1hr. After washing twice with washing buffer (1 × PBS with 2% BSA), the cells were analyzed by flow cytometory (FALCON, USA).
6. Enzyme-linked immunosorbent assay (ELISA) analysis for immunoglobulin
Serum was isolated from individual animals and frozen at −70°C. ELISA plates (Biosource, USA) were coated with 25 μg/ml bovine collagen II peptide in 100 μl of 1 × PBS overnight at 4°C. Plates were then washed with washing buffer (1 × PBS with 0.05% Tween) and blocked with 200 μl of blocking buffer (3% BSA, 0.05% Tween with 1 × PBS) for 2 h at 37°C. Plates were washed and 100 μl of diluted serum samples were added in triplicaes each for rheumatoid factor IgG and IgM and incuvated at 37°C for 90 min. Plates were washed and 100 μl of 1/5000 dilution of either 0.5 mg/ml biotin-anti-mouse IgG or IgM was added and incubated for 1hr at 37°C. Plates were washed and 100 μl of 1/1000 dilution of 1 mg/ml avidin peroxidase was added and incubated for 30 min at RT or 37°C. Plates were washed and 100 μl of TMB microwell substrate solution was added and allowed to develop for 10 min. Absorbance (450 nm) was measured with an ELISA plate reader (TECAN, Canada). The antibody levels were evaluated with an ELISA kit (Shibayagi, Japan) according to the manufacturer's protocol.
7. Enzyme-linked immunosorbent assay (ELISA) analysis for cytokines
IL-6, TNF-a, and INF-γ were detected using standard sandwich cytokine ELISA. IL-6, TNF-a, and INF-γ were detected using mouse cytokine ELISA kit from Biosource (USA) according to manufacturer's protocol. Briefly, plates were coated with anti-mouse cytokine capture antibody and supernatants were plated and allowed to incubate for 2 h. Plates were then washed and a biotinylated anti-mouse cytokine plus horseradish peroxidase conjugate was added and incubated for 1 h. Plates were then developed with TMB microwell substrate solution and stopped with TMB stop solution and allowed to develop for 10 min. The absorbance (450 nm) was measured with an ELISA plate reader (TECAN, Canada).
8. Histological analysis
The knee joint was removed from each mouse and washed in PBS, and infused with 10% formalin in PBS for fixations. Formalin-fixed explants and nonimplanted, treated knee joint samples (n = 3/group) were embedded in paraffin. Sections (6 μm) were deparaffinized in xylene followed by dehydration in a graded series of ethanol to water. Hematoxylin and eosin (H&E) staining was performed on all samples to observe general morphology of tissues. Masson's Trichrome (MT) staining (Bay Shore, NY) was used to examine collagen fibers in the tissues. Condition of cartilage surface, cynoviocytes proliferation, pannus formation, bone erosion and inflammation in the knee joint were observed at a magnification of 200×.
9. Statistical analysis
Data were expressed as mean ±SEM and analyzed using a one-way analysis variance (ANOVA) followed by a t-test. The criterion for significance was p < 0.05. Since the difference between the normal group and the control group was clearly distinguished, statistical significance between the normal group and the control group was not shown in the figures to put an emphasis on the statistical differences between the experimental groups and the control group or the NR group.
RESULTS
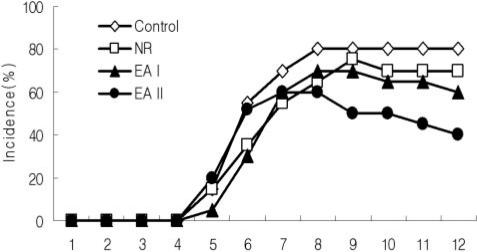
1. CIA incidence
Collagen II immunization induced arthritis in mice with the rate of 80%. The incidences of CIA in the EA groups were lower than that of the control group. CIA incidence of EAII group was the lowest and subsequently those of EAI and NR groups followed (Fig. 2).
Fig. 2.
CIA incidence in mice. CIA Incidence (%) was determined by observation of mice limbs, according to the number of arthritic limbs and the arthritis scores (scores described in Materials and Methods). CIA was considered to have been induced when the score was higher than 1 in more than two limbs or higher than 2 in more than one limb. Incidence = number of CIA mice/total number of mice× 100. Control: CII injection, NR: CII injection and needle retention at ST36, EAI: CII injection and EA at ST36 for 5 weeks, EAII: CII injection and EA at ST36 for 9 weeks.
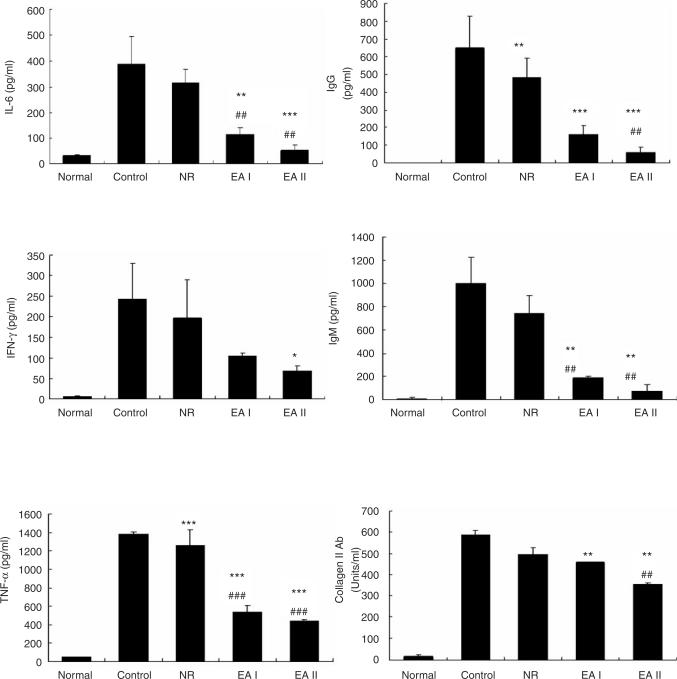
2. Levels of cytokines and antibodies in CIA mouse serum
Collagen II immunization remarkably increased IL-6, INF-γ, TNF-α, IgG, IgM and anti-collagen II antibody levels in serum as compared with those of the normal group. On the other hand, all those measures were reduced significantly by EA at ST36 in comparison with the control group or NR group. All the measurements showed greater reductions in EAII group than those in EAI group, though statistical significance was not found between EAI and EAII groups (Fig. 3).
Fig. 3.
IL-6, INF-γ, TNF-α, IgG, IgM and collagen II antibody in mouse serum. Serum IL-6, INF-γ, TNF-α, IgG, IgM and collagen II antibody levels were determined by ELISA. They were increased remarkably by CII injection and significantly reduced by EAs. Control: CII injection, NR: CII injection and needle retention at ST36, EAI: CII injection and EA at ST36 for 5 weeks, EAII: CII injection and EA at ST36 for 9 weeks. Data were expressed as mean ±SD. *: p<0.05, **: p<0.01, ***: p<0.001 compared with control group, ##: p<0.01, ###: p<0.001 compared with NR group.
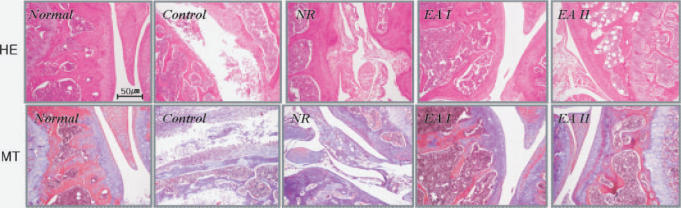
3. Histology of CIA mouse knee joint
Collagen II immunization induced severe joint destruction in mice. Severe cartilage surface erosion, cynoviocytes proliferation, pannus formation, bone erosion and inflammation were found in the control group. EA at ST36 prevented these pathological changes in CIA. (Fig. 4).
Fig. 4.
Histology of mouse knee joint. The knee joint was obtained from each mouse. Formalin-fixed knee joint samples (n=3/group) were embedded in paraffin. Sections (6 μm) were stained with Hematoxylin and eosin (H&E) (Top) or Masson's Trichrome (MT) (Bottom). Severe cartilage destruction and other pathological changes could be found in the control group while milder changes were found in EA groups. Control: CII injection, NR: CII injection and needle retention at ST36, EAI: CII injection and EA at ST36 for 5 weeks, EAI: CII injection and EA at ST36 for 9 weeks. Magnification, ×200.
4. Immune cell population in CIA mouse lymph node and knee joint
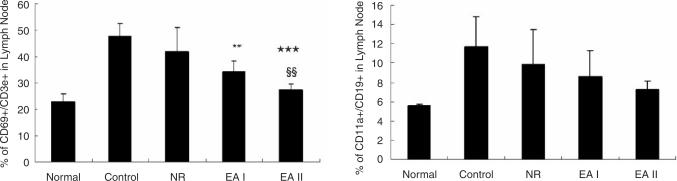
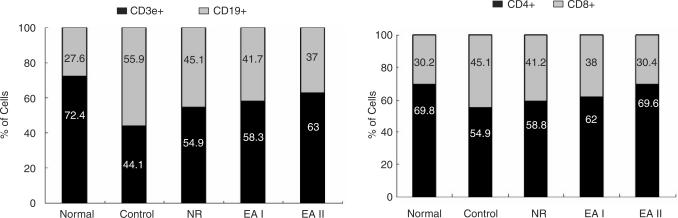
The populations of CD69+/CD3e+ cells and CD11a+/CD19+ cells in mouse lymph nodes were remarkably increased in the control group as compared with the normal group. On the other hand, CD69+/CD3e+ cells in EA groups were reduced significantly in comparison with the control group. The number of CD69+/CD3e+ cells in EAII group was significantly lower than that of EAI group. EA at ST36 reduced CD11a+/CD19+ cells as well but statistical significance was not found (Fig. 5). Also the ratios of CD19+ cell / CD3e+ cell (B cell / T cell) and CD8+/CD4+ cell (CD8 T cell/CD4 T cell) in CIA mouse lymph node were maintained closer to the normal range in EA groups as compared with the control group or NR group (Fig. 6). CD11b+/Gr1+ cell population in CIA mouse knee joint was increased by Collagen II immunization and significantly reduced by EA at ST36 (Data not shown).
Fig. 5.
CD69+/CD3e+ and CD11a+/CD19+ cell populations in mouse lymph node. The populations of CD69+/CD3e+ cells and CD11a+/CD19+ cells in CIA mice lymph nodes were analyzed by flow cytometer three times for each. Both were increased by CII administration and reduced by EA stimulation. CII injection, NR: CII injection and needle retention at ST36, EAI: CII injection and EA at ST36 for 5 weeks, EAII: CII injection and EA at ST36 for 9 weeks. Data were expressed as mean ±SD. **: p<0.01, ***: p<0.001 compared with control group, §§: p<0.01 compared with EAI group.
Fig. 6.
Ratios of CD3e+ cells to CD19+ cells and CD4+ cells to CD8+ cells in mouse lymph node. The populations of CD3e+, CD19+, CD4+ and CD8+ cells in CIA mouse lymph node were analyzed by flow cytometer three times for each, and the ratios of CD3e+ cells to CD19+ cells and CD4+ cells to CD8+ cells were estimated. The ratios were reduced by CII immunization, while they seemed to be recovered close to the normal range by EAs. Control: CII injection, NR: CII injection and needle retention at ST36, EAI: CII injection and EA at ST36 for 5 weeks, EAII: CII injection and EA at ST36 for 9 weeks.
DISCUSSION
RA is a systemic autoimmune disease characterized by chronic articular inflammation and progressive joint destruction (8). CIA is an animal model of human rheumatoid arthritis that can be induced in susceptible mice by immunization with type II collagen (CII) (20). We used CIA model to investigate the effect of EA on RA.
In our study, CIA incidence was reduced and histological destruction of joint was prevented by EA at ST36 (Fig. 2, Fig. 4). These results suggested that EA at ST36 may reduce arthritis incidence and prevent joint destruction in CIA.
RA is an immune-mediated disease for which an autoimmune pathogenesis is postulated (21). All components of the immune system are involved in mediating tissue damage and systemic inflammation in RA. T cells, B cells, and macrophages infiltrate into the synovium and form highly sophisticated lymphoid structures. Cytokines are produced by macrophages and activated tissue-resident cells. As a consequence of T cell and B cell responses, bone is eroded and cartilage is destroyed (22).
TNF-α is considered to be related to the process of synovititis and joint destruction in RA, and treatment of murine models of arthritis with antibodies against TNF-α ameliorates or abrogates disease (8). IL-6 also contributes to joint inflammation in RA, and has been the target of RA treatment with an anti-IL-6 receptor antibody. INF- γ is known to increase in RA (23–25). In the present study, CII immunization remarkably increased serum TNF-α, IL-6 and INF-γ levels, while, EA at ST36 significantly reduced the elevated serum TNF-α, IL-6 and INF- γ in comparison with the control group or the NR group (Fig. 3). CD11b+/Gr1+ cell population in CIA mouse knee joint was also reduced significantly by EA at ST36, indicating that EA at ST36 reduced inflammatory granulocytes in CIA mouse knee joints (Data not shown). From these results, we speculate that EA at ST36 may have an anti-inflammatory effect on CIA.
Immunization of susceptible strains of animals with native CII in adjuvant results in CIA (26,27), therefore autoimmunity to CII is implicated in pathogenesis of CIA. In the present study, CII immunization remarkably increased collagen II antibody, IgG and IgM levels in mice serum, while EA at ST36 significantly reduced these levels in comparison with the control group or the NR group. These results suggest that EA at ST36 may suppress autoimmunity in CIA (Fig. 3).
T cells and B cells are key participants in the pathogenesis of RA (8,28,29). Activated T cells promote the disease progression by inducing the secretion of proinflammatory cytokines from macrophages and synovial cells. Rheumatoid factor (RF) B cells also play a critical role in RA (30). In the present study, CD69+/CD3e+ cells and CD11a+/CD19+ cells in mouse lymph nodes were increased significantly by CII immunization and reduced remarkably by EA at ST36, indicating EA at ST36 reduced activated T cells and activated B cells in mouse lymph nodes (Fig. 5). These results suggest that EA at ST36 may have a suppressive effect on T cell and B cell activation in CIA. Besides the reduction of activated lymphocytes population, EA at ST36 seems to aid the recovery of the ratios of T cells to B cells and CD4 T cells to CD8 T cells in mouse lymph node close to the normal range (Fig. 6).
Acupuncture without electrical stimulation showed a similar tendency but was weaker than those of EA on CIA. From this result, we presume that needle retention itself is beneficial to relieve or treat CIA, but supplement of electrical stimulation intensifies its effects, indicating acupoint ST36 and electrical stimulation may have synergistic effects on CIA.
From the results of the present study, we speculate that EA at ST36 may have an anti-inflammatory and anti-arthritic effect on CIA via suppressing autoimmunity and modulating immune abnormality. The mechanism of these effects is not clear yet. Involvement of neuro-immune interaction in the mechanism of acupuncture on immune diseases such as RA is feasible. Further studies are needed on the mechanisms of these effects and the applications for human rheumatoid arthritis.
References
- 1.Eshkevari L, Heath J. Use of acupuncture for chronic pain: optimizing clinical practice. Holist Nurs Pract. 2005;19(5):217–21. doi: 10.1097/00004650-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Dundee JW, McMillan C. Positive evidence for P6 acupuncture antiemesis. Postgrad Med J. 1991;67(787):417–22. doi: 10.1136/pgmj.67.787.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jobst K, Chen JH, McPherson K, Arrowsmith J, Brown V, et al. Controlled trial of acupuncture for disabling breathlessness. Lancet. 1986;20(27):1416–9. doi: 10.1016/s0140-6736(86)92732-7. [DOI] [PubMed] [Google Scholar]
- 4.Longworth W, McCarthy PW. A review of research on acupuncture for the treatment of lumbar disk protrusions and associated neurological symptomatology. J Altern Complement Med. 1997;3(1):55–76. doi: 10.1089/acm.1997.3.55. [DOI] [PubMed] [Google Scholar]
- 5.Ruchkin IN, Burdeinyi AP. Auriculo-electropuncture in rheumatoid arthritis (a double-blind study) Ter Arkh. 1987;59(12):26–30. [PubMed] [Google Scholar]
- 6.Sun Z. A study of relation between rheumatoid arthritis (RA) and blood stasis: the effect of acupuncture promoting blood circulation to remove blood stasis. Zhen Ci Yan Jiu. 1995;20(2):71–5. [PubMed] [Google Scholar]
- 7.Zherebkin VV. The use of acupuncture reflexotherapy in treating patients with rheumatoid arthritis. Lik Sprava. 1997;6:175–7. [PubMed] [Google Scholar]
- 8.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 9.Casimiro L, Brosseau L, Milne S, Robinson V, Wells G, et al. Acupuncture and electroacupuncture for the treatment of RA. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD003788. 3:CD003788. [DOI] [PubMed] [Google Scholar]
- 10.David J, Townsend S, et al. The effect of acupuncture on patients with rheumatoid arthritis: a randomized, placebo-controlled cross-over study. Rheumatology (Oxford) 1999;38(9):864–9. doi: 10.1093/rheumatology/38.9.864. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Jun H, Chae Y, Park HJ, Kim BH, et al. The practice of Korean medicine: an overview of clinical trials in acupuncture. Evid Based Complement Alternat Med. 2005;2(3):325–52. doi: 10.1093/ecam/neh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JD, Park HJ, Chae Y, Lim S. An overview of bee venom acupuncture in the treatment of arthritis. Evid Based Complement Alternat Med. 2005;2(1):79–84. doi: 10.1093/ecam/neh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lade A. Washington: 1998. Acupuncture points images & functions. Eastland press. 1998, 230. [Google Scholar]
- 14.Giovanni Maciocia. London: Churchill Livingstone; 1989. The Foundations of Chinese Medicine; A Comprehensive Text for Acupuncturists and Herbalists; pp. 89–90. [Google Scholar]
- 15.Choe YT, Yu CH, Kim CH, Kim DH. Oriental medicine series, Vol 2; Acupuncture & moxibustion. Seoul: Res Inst of Oriental Med Inc. 1987 160. [Google Scholar]
- 16.Lee JD, Kim SY, Kim TW, Lee SH, Yang HI, et al. Anti-inflammatory effect of bee venom on type II collagen-induced arthritis. Am J Chin Med. 2004;32(3):361–7. doi: 10.1142/S0192415X04002016. [DOI] [PubMed] [Google Scholar]
- 17.Tan Sardjono C, Mottram PL, van de Velde NC, Powell MS, Power D, et al. Development of spontaneous multisystem autoimmune disease and hypersensitivity to antibody-induced inflammation in Fcgamma receptor IIa-transgenic mice. Arthritis Rheum. 2005;52(10):3220–9. doi: 10.1002/art.21344. [DOI] [PubMed] [Google Scholar]
- 18.Tomita T, Kakiuchi Y, Tsao PS. THR0921, a novel peroxisome proliferator-activated receptor gamma agonist, reduces the severity of collagen-induced arthritis. Arthritis Res Ther. 2005;8(1):R7. doi: 10.1186/ar1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L, Gan Y, He S, Ji X, Li Y, et al. Chinese acupuncture and moxibustion, Beijing: Foreign languages press. 1997;54:71–74. 208, 325-327. [Google Scholar]
- 20.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, et al. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 21.Panayi GS, Corrigall VM, Pitzalis C. Pathogenesis of rheumatoid arthritis, The role of T cells and other beasts. Rheum Dis Clin North Am. 2001;27(2):317–34. doi: 10.1016/s0889-857x(05)70204-0. [DOI] [PubMed] [Google Scholar]
- 22.Weyand CM, Fulbright JW, Goronzy JJ. Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp Gerontol. 2003;38(8):833–841. doi: 10.1016/s0531-5565(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 23.Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46(12):3143–50. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 24.Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36(12):1681–90. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 25.Miossec P. An update on the cytokine network in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16(3):218–22. doi: 10.1097/00002281-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Cook AD, Gray R, Ramshaw J, Mackay IR, Rowley MJ. Antibodies against the CB10 fragment of type II collagen in rheumatoid arthritis. Arthritis Res Ther. 2004;6(5):R477–83. doi: 10.1186/ar1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol. 2003;15(3):246–52. doi: 10.1097/00002281-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 30.Li JM, Isler P, Dayer JM, Burger D. Contact-dependent stimulation of monocytic cells and neutrophils by stimulated human T-cell clones. Immunology. 1995;84(4):571–6. [PMC free article] [PubMed] [Google Scholar]