Abstract
Effects of static magnetic field (SMF) on the vascularization in bone were evaluated using an ischemic bone model, where rat femoral artery was ligated. Magnetized and unmagnetized samarium–cobalt rods were implanted transcortically into the middle diaphysis of the ischemic femurs. Collateral circulation was evaluated by injection of microspheres into the abdominal aorta at the third week after ligation. It was found that the bone implanted with a magnetized rod showed a larger amount of trapped microspheres than that with an unmagnetized rod at the proximal and the distal region (P < 0.05 proximal region). There were no significant differences at the middle and the distal region. This tendency was similar to that of the bone mineral density in the SMF-exposed ischemic bone.
Keywords: blood vessel, ischemic model, microsphere, static magnetic field
Introduction
The magnetic field application in orthopedics attracts the interest of scientists and clinicians. Both static magnetic fields (SMF) and pulsed electromagnetic fields (PEMF) are considered and used. Effects of the PEMF on tissue growth and repair have been reported by nervous system research groups, since Bassett et al. (1,2) demonstrated that the exposure to PEMF induced an increase in bone formation. However, Wiendl and Strigl (3) found that reduction in pseudoarthrosis caused by inflammation, non-union fracture repair of femoral head, and callus formation were promoted by the bone exposure to EMF, whereas Hanft et al. (4) confirmed that the duration of fracture healing was shortened by EMF exposure. Some researchers reported that this promotion of fractured bone union was induced by PEMF application to the electric current (5,6). They pointed out that the effects of PEMF on bone must be due to mechanisms different from those of SMF. PEMF may generate an electric current in the tissue to stimulate some biological cascades, while SMF without any movement creates no electrical potential (7).
A promoting effect of SMF on fracture repair was reported by Degen and Stetsula (8). Bruce et al. (7) also reported that the mechanical strength of fractured bone of rabbits was increased by SMF exposure. Oden et al. (9) suggested that the increase in mechanical strength of bone by SMF exposure was closely related to the increase in bone mineral density (BMD). It was assumed that SMF initiated an increase in localized calcium deposition in bone, which neutralized the net negative charge of tissues to allow for the subsequent vascularization and initiation of osteogenesis (10,11).
Our previous studies that evaluated the BMD change of bone upon implantation of a magnetized rod showed that SMF accelerates bone recovery from operative invasion or ischemia induced by artery ligation (12,13). The studies performed to minimize the relative movement between the implanted magnet and the bone by press fit fixation of a tapered rod suggested that SMF improved the blood vessel recovery from the ischemic state. As there is no direct evidence for this assumption, the present study was undertaken to evaluate the effect of SMF on the recovery from ischemia of bone using microsphere injection.
Methods
Materials
Tapered alloy rods were prepared from samarium–cobalt magnet with and without magnetization of the rod. The entire rod surface was homogeneously coated with polytetrafluoroethylene to prevent the release of metal ions from the alloys into the body. The highest strength of the magnetic field was observed to be 180 mT and localized on the polar ends of the magnet perpendicular to the rod axis, as shown in Fig. 1. Due to the geometric difference at both ends of the rod, magnetic flux density is different in the first 2 mm as shown in Fig. 1. All the rods were sterilized by immersing in 0.5% hibitane solution for 30 min before implantation.
Figure 1.
The magnetic flux density as a function of the distance from the face of the magnetized rod. S, side face of the south pole; S′, lateral side face of the south pole; N, side face of the north pole; N′, lateral side face of the north pole.
Ischemic Bone Model
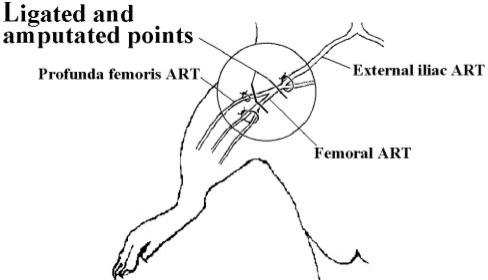
Male Wistar rats of 10 weeks old and weighing 310–360 g were used for this study. After general i.p. anesthesia with 100 mg kg−1 of ketamine hydrochloride and 10 mg kg−1 of xylazine, the skin just over the branch point of the medial femoral artery and profunda femoral artery was incised. The two arteries were ligated with a suture and amputated at the front and the post of the branch point of the medial femoral artery and profunda femoral artery, as shown in Fig. 2. Femur circulation just after the ligation was mainly supported by collateral vessels. Both of the femoral arteries in bilateral limbs were ligated and amputated in this fashion.
Figure 2.
Ischemic bone model indicating ligated and amputated points.
Implantation
Thirty-five rats were divided randomly into three groups; L group (bilateral sides of femoral artery were ligated), L + M or L + S group [bilateral sides of femoral artery were ligated, a magnetized rod (M) or an unmagnetized rod (S) was implanted in the middle point of the femurs], and CON group (no operations).
A 2 cm lateral skin incision was made and the femur was exposed by blunt dissection of the femoral muscle. The periosteum was incised and pushed aside to drill a hole in the distal femur from the lateral cortex to the medical cortex. The drill size was exactly identical to that of the tapered rod. A rod specimen was implanted transcortically into the hole, applying a load of 500 g for 30 s using a digital force gauge, as shown in Fig. 3.
Figure 3.
Schematic representation of implantation and regions of bone for measurement. The rod was implanted at the middle portion of the femurs.
After that, three layers of muscle, subcutaneous connective tissue and skin were sutured. Two rats were housed together in one cage (340 × 240 × 170 mm3) and free access to water and pelleted food was allowed. All the animals were bred at 23 ± 1°C and 55 ± 5% RH for 3 weeks.
Measurement of Small-Size Vessel
Microspheres (Polybead®, 10.0 micron in diameter, 2.51% solids-latex; Polysciences Inc., USA) are injected into the abdominal aorta under general i.p. anesthesia at 3 weeks after the implantation. After 5 min, the rats were euthanized with an overdose of anesthesia and then whole femurs were taken out together with the implanted rods.
The number of microspheres in the tissue was counted using the method that Vacek and Machova reported (14). Each femur was transectioned at the three parts (proximal, middle and distal) and cut in the sagittal direction at 0.1 mm interval, as shown in Fig. 4.
Figure 4.
Slice preparation in the sagittal direction at 0.1 mm interval. The rod was implanted at the middle portion of the femurs.
The number of microspheres was counted under microscopic view of 200× magnification. Statistical analysis was performed using the Student's t-test and Wilcoxon signed rank test for each of the selected regions (P < 0.05). All data were expressed as the mean ± SE.
Results
Ischemic Bone Model
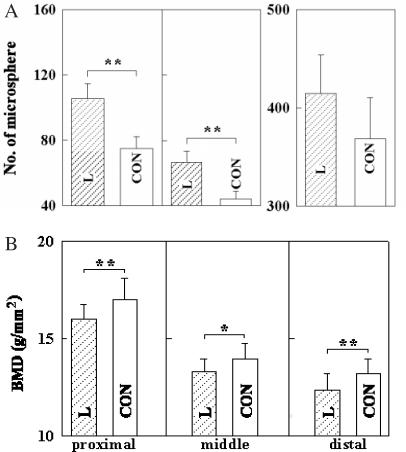
As shown in Fig. 5A, the number of the microspheres in the artery-ligated limb was higher than that in the non-ligated limb which presumably was caused by an increase in collateral circulation.
Figure 5.
Number of microspheres (Ø = 10.0 μ) (A) and the BMD (B) (13) of the rat femur at 3 weeks after operation. L, femoral artery was ligated. CON, no operation (n = 24 for each group). *P < 0.05. **P < 0.01.
Exposure to SMF
As shown in Fig. 6A, the number of the microspheres in the L + M group was larger than that for the L + S group (distal and proximal region) and is significantly different at the proximal region (P < 0.05).
Figure 6.
The number of microspheres (A) (n = 24 for each group) and the BMD (B) (L + M: n = 26; L + S: n = 22; L: n = 24) (13) of the rat femur exposed to SMF for 3 weeks. L, femoral artery was ligated. M, a magnetized rod (180 mT) was implanted. S, an unmagnetized rod was implanted. *P < 0.05. **P < 0.01.
Discussion
A number of studies have been performed to address whether or not SMF may promote bone deposition. Camilleri and McDonald (15), evaluated the effects of SMF (flux density 100 mT) on the bone remodeling and mitotic activity of osteoblasts in rat calvaria and concluded that SMF did not affect bone growth, however, thymidine uptake was significantly inhibited. Bassett (10) and Norton et al. (16) have shown that the local exposure of SMF leads to enhanced angiogenesis and ossification. However, they have not studied the field-strength dependence of the SMF effects. The effect of SMF on bone formation is still a controversial subject.
We have reported that SMF exposure did not change normal bone but accelerated recovery of the BMD of an ischemic bone. The present study examined how local SMF exposure influenced the formation of collateral vessels in an ischemic bone using the microsphere injection method introduced by Vacek and Machova (14). The influence of femoral artery ligation on the amount of microspheres trapped in the femur in Fig. 5A revealed that the bead amount increases at the third week after ligation. This increased number of trapped microspheres seems to be caused by formation of small-sized collateral vessels in the ischemic bone. The increased amount of microsphere shown in Fig. 6A is thought to reflect the collateral circulation formation.
Figure 6 shows the influence of local SMF exposure on the microsphere trap (Fig. 6A) and the BMD (Fig. 6B) (13) of the vascular-ligated bone. The value of BMD is varied with the equipment, software, radial rags and scanning speed used (12,17,18). Dual energy X-ray absorptiometry (DEXA; Aloka DCS-600; Aloka, Tokyo, Japan) was used with a software version specifically designed for small animals (small animal mode, SYS-D 162-V 6.0). Scanning was done at a speed of 10 mm s−1 with each slice in the cross-sectional direction. The BMD in the ischemic bone was increased by the SMF exposure at the proximal and the distal region, and the numbers of microspheres were also increased at the proximal region.
Those changes at the middle region are difficult to analyze because of some possible tissue reaction to the magnet implant. As blood supply to the ischemic bone is supported by the collateral circulation, it is likely that BMD of the ischemic bone is affected by the newly formed vessels. It is possible that SMF have an influence on formation of collateral circulation. Some reports show that SMF influences blood flow (19), vascular endothelial growth factors (20–27) and immunoreactivity for VEGF (28), suggesting that the increase in collateral circulation affects bone formation. However, there is no decisive explanation for these findings at present. We are now evaluating SMF exposure to angiogenesis using in vivo experimental models.
Conclusion
A samarium–cobalt magnet was implanted into the middle diaphysis of the ischemic femurs of rats. BMD and collateral circulation were valuated by microsphere injection. The BMD and number of microspheres in the ischemic femur were increased by the SMF exposure at the proximal region. This reduction of BMD in the ischemic bone may have been prevented by higher formation of collateral circulation.
References
- 1.Bassett CAL, Pawluk RJ, Becker RO. Effects of electrical current on bone in vivo. Nature. 1964;204:652–4. doi: 10.1038/204652a0. [DOI] [PubMed] [Google Scholar]
- 2.Bassett CAL, Pawluk RJ, Pilla AA. Augmentation of bone repair by inductively coupled electromagnetic fields. Science. 1974;184:575–7. doi: 10.1126/science.184.4136.575. [DOI] [PubMed] [Google Scholar]
- 3.Wiendl HJ, Strigl M. Clinical experiences in supplementary treatment of pseudarthroses using electromagnetic potentials. Fortschr Med. 1978;96:231–6. [PubMed] [Google Scholar]
- 4.Hanft JR, Goggin JP, Landsman A, Surprenant M. The role of combined magnetic field bone growth stimulation as an adjunct in the treatment of neuroarthropathy/Charcot joint: an expanded pilot study. J Foot Ankle Surg. 1998;37:510–5. doi: 10.1016/s1067-2516(98)80028-8. discussion 550–1. [DOI] [PubMed] [Google Scholar]
- 5.Bassett CAL, Mitchell SN, Gaston SR. Treatment of un-united tibial diaphyseal fractures with pulsing electromagnetic fields. J Bone Joint Surg. 1981;63A:511–23. [PubMed] [Google Scholar]
- 6.Rubin CT, Mcleod KJ, Lanyon LE. Prevention of osteoporosis by pulsed electromagnetic field. J Bone Joint Surg. 1989;71A:411–7. [PubMed] [Google Scholar]
- 7.Bruce GK, Howlen CR, Huckstep RL. Effect of a static magnetic field on fracture healing in a rabbit radius. Clin Orthop. 1987;222:300–6. [PubMed] [Google Scholar]
- 8.Degen IL, Stetsula VI. Consolidation of bone fragments in a constant magnetic field. Ortop Travmatol Protez. 1971;32:45–8. [PubMed] [Google Scholar]
- 9.Oden ZM, Selvitelli DM, Hayes WC, Myers ER. The effect of trabecular structure on DXA-based predictions of bovine bone failure. Calcif Tissue Int. 1998;63:67–73. doi: 10.1007/s002239900491. [DOI] [PubMed] [Google Scholar]
- 10.Bassett CAL. Pulsing electromagnetic field: a new method to modify cell behaviors in calcified and non-calcified tissues. Calcif Tissue Int. 1982;34:1–8. doi: 10.1007/BF02411199. [DOI] [PubMed] [Google Scholar]
- 11.Norton LA, Hanley KJ, Turkewicz J. Bioelectric perturbations of bone. Angle Orthod. 1984;54:73–87. doi: 10.1043/0003-3219(1984)054<0073:BPOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Yan QC, Tomita N, Ikada Y. Effects of static magnetic field on bone formation of rat femurs. Med Eng Phys. 1998;20:397–402. doi: 10.1016/s1350-4533(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 13.Shenzhi X, Tomita N, Ohata R, Yan QC, Ikada Y. Static magnetic field effects on bone formation of rat with an ischemic bone model. Biomed Mater Eng. 2001;11:257–63. [PubMed] [Google Scholar]
- 14.Vacek L, Machova L. Distribution of microspheres in the brain of hypertensive rats. Physiol Bohemoslov. 1984;33:237–41. [PubMed] [Google Scholar]
- 15.Camilleri S, McDonald F. Static magnetic field effects on the sagittal suture in Rattus norvegicus. Am J Orthodon Dentofacial Orthop. 1993;103:240–6. doi: 10.1016/0889-5406(93)70004-8. [DOI] [PubMed] [Google Scholar]
- 16.Norton LA, Hanley KJ, Turkewicz J. Bioelectric perturbations of bone. Angle Orthod. 1984;54:73–87. doi: 10.1043/0003-3219(1984)054<0073:BPOB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi N, Kanai S, Kawamoto M, Endo H, Higashino H. Study on application of static magnetic field for adjuvant arthritis rats. Evid Based Complement Alternat Med. 2004;1:187–91. doi: 10.1093/ecam/neh024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshihara M, Masuyama R, Uehara M, Suzuki K. Reduction in dietary calcium/phosphorus ratio reduces bone mass and strength in ovariectomized rats enhancing bone turnover. Biosci Biotechnol Biochem. 2005;69:1025–8. doi: 10.1271/bbb.69.1970. [DOI] [PubMed] [Google Scholar]
- 19.Shenzhi X, Okano H, Ohkubo C. Subchronic effects of static magnetic fields on cutaneous microcirculation in rabbits. In vivo. 1998;12:383–9. [PubMed] [Google Scholar]
- 20.Darendeliler MA, Sinclair PM, Kusy RP. The effects of samarium-cobalt magnets and pulsed electromagnetic fields on tooth movement. Am J Orthodon Dentofacial Orthop. 1995;107:578–88. doi: 10.1016/s0889-5406(95)70100-1. [DOI] [PubMed] [Google Scholar]
- 21.Darendeliler MA, Darendeliler A, Sinclair PM. Effects of static magnetic and pulsed electromagnetic fields on bone healing. Int J Adult Orthodon Orthognath Surg. 1997;12:43–53. [PubMed] [Google Scholar]
- 22.Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 23.Tombran-Tink J, Barnstable CJ. Osteoblasts and osteoclasts express PEDF, VEGF-A isoforms, and VEGF receptors: possible mediators of angiogenesis and matrix remodeling in the bone. Biochem Biophys Res Commun. 2004;316:573–9. doi: 10.1016/j.bbrc.2004.02.076. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki O, Bishop AT, Sunagawa T, Katsube K, Friedrich PF. VEGF-promoted surgical angiogenesis in necrotic bone. Microsurgery. 2004;24:85–91. doi: 10.1002/micr.10190. [DOI] [PubMed] [Google Scholar]
- 25.Nakayama N, Lee J, Chiu L. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 2000;95:2275–83. [PubMed] [Google Scholar]
- 26.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension. 2001;37:386–90. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 27.Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, et al. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999;14:1290–301. doi: 10.1359/jbmr.1999.14.8.1290. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki R, Ootsuyama A, Uchida S, Norimura T. Effects of a 4.7 T static magnetic field on fetal development in ICR mice. J Radiat Res (Tokyo) 2001;42:273–83. doi: 10.1269/jrr.42.273. [DOI] [PubMed] [Google Scholar]