Abstract
Transcutaneous electrical nerve stimulation (TENS) has been shown to be an effective measure for pain relief. The aim of the present study was to determine the optimal intensity and interval of repeated 100 Hz TENS for the treatment of chronic inflammatory hyperalgesia in a monoarthritic pain model of the rat, and to assess the changes of the spinal substance P (SP) release in response to TENS treatment. A reliable, reproducible chronic monoarthritic pain model was produced by intra-articular injection of complete Freund's adjuvant (CFA) at single ankle joint. The efficacy of 100 Hz TENS treatments with different frequencies and intensities was compared. In the acute period (within 3 weeks) of monoarthritis, twice-a-week schedule of TENS reduced the swelling of the inflamed ankle significantly. In the stable period (4–9 weeks), however, once-a-week schedule produced a significantly better therapeutic effect on both inflammation and arthritic hyperalgesia than that of twice- or five-times-a-week schedule. Using three levels of intensity of TENS, we found that the weaker (1-1-2 mA) stimulation produced significantly better therapeutic effects. Repeated TENS produced a reduction of SP content in spinal perfusate in parallel with the progressive reduction of the arthritic pain scores. Our results suggest that (i) consecutive TENS treatments produced cumulative effect for chronic hyperalgesia, (ii) for chronic inflammatory hyperalgesia, a weaker intensity and more sparsely arranged treatment schedule may produce better therapeutic effect and (iii) a decrease in SP release may serve as one of the possible neurochemical mechanisms underlying the therapeutic effects of multiple TENS treatments on chronic inflammatory hyperalgesia.
Keywords: chronic inflammatory hyperalgesia, Freund's adjuvant, monoarthritis, substance P, transcutaneous electrical nerve stimulation (TENS)
Introduction
Pain is one of the most serious medical problems encountered today. Chronic pain, characterized by its long duration, slow recovery and difficulty to control in clinical practice, makes it even more serious than acute pain both physically and psychologically. It is thus an imperative and formidable task for researchers to demonstrate the mechanisms of chronic pain and find effective therapy for its treatment. Transcutaneous electrical nerve stimulation (TENS), which has been developed in the past 30 years, is now being used more and more extensively for pain relief (1).
Early studies of TENS-induced analgesia mainly focused on the immediate effect induced by single treatment (1). It has been demonstrated that frequency, intensity, electrode placement and pulse duration are the important impact factors on the TENS effect (2–4). However, clinical data indicate that repeated electrical stimulation could produce cumulative (long-term) effect (5,6). The optimal parameters found from acute tests may not be applicable in cases with chronic pain. It has drawn more and more attention to find the suitable parameters for the therapeutic efficacy of multiple TENS treatments (7–9). Among many factors, the less well-characterized parameter is the time interval between two consecutive sessions of TENS, which played an important role in the outcome of long-term effect in normal rats (W. Xu, PhD thesis, Department of Physiology, Beijing Medical University, Beijing, China, 1995). In the present study, we characterized the optimal interval and intensity of repeated 100 Hz TENS for the treatment of chronic hyperalgesia induced by injecting a small amount of water-in-oil type complete Freund's adjuvant (CFA) into single ankle joint in rats.
A great deal of evidence indicates that substance P (SP) as a neurotransmitter or neuromodulator is involved in the transmission of nociceptive signals in the spinal cord (10,11). It has been shown that SP release in spinal cord and peripheral tissue was increased during the development of inflammation (12,13) and that TENS or acupuncture might alter the noxious nerve stimulation-induced release (14,15) and production (16) of SP in the spinal cord, which may be frequency dependent (17). Whether spinal SP release was altered by repeated 100 Hz TENS was also characterized in the present study.
Methods
Rats
Wistar female rats obtained from the Experimental Animal Research Center, Peking University, were used throughout the study. They arrived at a weight of 150–200 g, were housed 4–6 rats per cage with sawdust bedding and given food and water ad libitum. Experiments were conducted according to the Ethical Guidelines for Investigation of Experimental Pain in Conscious Animals (18) and were approved by the local ethics committee for animal research of Peking University.
Indices obtained in the present study were observed and measured by one of the authors (H.-X. L.) throughout the experiments to avoid interobserver differences.
Induction of Monoarthritis
Preparation of Water-in-Oil Type CFA
Two 1 ml syringes were used, one filled with incomplete Freund's adjuvant (IFA) (0.85 ml paraffin oil mixed with 0.15 ml Aracel A in 1 ml, Gibco Company), and another with equal volume of suspension of killed Mycobacterium tuberculosis (Human strain, 20 mg ml−1, Chinese Inspection Institute for Biological Materials, Beijing). They were interconnected with each other through a plastic catheter of ∼6 cm long. Careful attention was paid to avoid air bubbles in the catheter and syringes. The pistons of the two syringes were pushed alternately until thick water-in-oil emulsion was formed. The preparation of the control IFA was the same as that for CFA with the exception that the suspension of killed M. tuberculosis was substituted with normal saline.
Intra-articular Injection
Intra-articular injection to the tibio–tarsal joint was performed as described by Butler et al. (19). Briefly, rats were anesthetized with 10% chlorohydrate (300 mg kg−1, i.p.). Freund's adjuvant (30 μl) was injected into the right ankle joint through a 261/2-gauge needle inserted into the articular cavity.
Transcutaneous Electrical Nerve Stimulation
The monoarthritic rats were restrained in a specially designed holder with the hind legs and tail exposed. The hair on the lower legs was removed with saturated barium sulfide (BaS) solution and rinsed thoroughly with water. After wiping and drying the legs, two pairs of round platinum electrodes (5 mm diameter) were attached tightly on both legs, one on the normal saline wetted skin of Zusanli (ST36, the lateral side of the anterior tibial tubercle of the leg at the level between upper one-third and lower two-third of the tibia) and another on the medial side at the same level to form a circuitry. The Eight channel HANS stimulator used was manufactured by the Beijing Medical University. The parameters for TENS output were as follows: 0.2 ms pulse width, 100 Hz frequency and 30 min duration per session. The intensity of stimulation was arranged at three levels as follows: weak (1-1-2 mA), medium (1-2-3 mA) or high (2-3-4 mA), with current increasing stepwise, each step lasting for 10 min. For mock stimulation, the skin electrodes were placed on the same regions but no current was delivered. To find the optimal interval of TENS treatment, once-a-week, twice-a-week or five-times-a-week schedule was used with the medium intensity stimulation.
Indices for Behavioral Observations
Behavioral observations included body weight, circumferences of the ankle joints bilaterally, leg-withdrawal threshold and scores of arthritic flexion (both plantar and dorsal flexion) pain test. The rats were weighed before putting in the holder. The other measurements were made 30 min after the rats were restrained. These measurements were taken once-a-week before TENS treatment with the observation time allocated in the morning between 8:00 a.m. and 12:00 noontime.
Measurement of Ankle Circumference
The circumferences of the ankles were measured around the lower edge of lateral and medial malleolus with a flexible ruler without elasticity.
Measurement of Leg-withdrawal Threshold
Randall–Selitto response was determined with continuously increasing pressure applied on one side of the ankle joint. The value of pressure (mm Hg) was recorded at the withdrawal of the leg. The mean value of two measurements with a 30 min interval was taken as the final result of the paw withdrawal threshold. The threshold of the contralateral side was examined 15 min later.
Scoring of Arthritic Flexion Pain Test
The ankle joint was gently flexed to the plantar direction for 5 times with an interval of 5 s. The same manipulation was used and the force onto the ankle was monitored by the color change of the thumb fingertip induced by blockade of blood circulation. The leg-withdrawal scores were measured, respectively. It was scored zero when rats showed no quick leg-withdrawal; scored 1 when the brisk withdrawal reaction appeared. A total leg-withdrawal score was obtained between 0 and 5 for each test session. The same scoring method was employed for dorsal flexion test, which was proceeded to the extent that the toes touched the front of the leg.
X-ray Examinations
Radiographic evaluation (40 kV, 2 ms) was performed under light anesthesia based on whole body radiographs and coned-down views of the hind limbs.
Radioimmunoassay of SP
Rats were anesthetized with chlorohydrate. A PE-10 catheter was inserted into the subarachnoid space through the cisterna magna with the tip reaching the level of lumbar spinal enlargement. Another piece of polyethylene tube (PE-50) was inserted 10 mm down the cisterna. Artificial cerebrospinal fluid contained bacitracin (30 mg l−1), a peptidase inhibitor, was perfused at a rate of 1.0 ml per 30 min with a push–pull pump (20). The spinal perfusate (1.0 ml) was collected in the Eppendorf vials immersed in an ice-water bath and then heated for 10 min in boiling water bath. After cooling down to room temperature, the perfusate was centrifuged at 10 000 g for 10 min. Supernatants were lyophilized and stored at −20°C.
The lyophilized perfusate was reconstituted in 250 μl H2O for the radioimmunoassay (RIA) of SP. 125I-SP RIA kits were bought from the Basic Research Institute, Chinese Academy of Medical Sciences, Beijing. The SP antiserum showed no detectable cross-reactivity with ACTH, angiotensin II and Met-enkephalin, <0.01% with eledoisin, <0.008% with eledoisin-related peptides. The non-specific binding rate was 6.18%, total binding 35.8% and IC50 112 fmol per tube. Standards (or samples) (100 μl), SP antiserum (1:6500, 100 μl) and [125I]SP (10000 dpm, 100 μl) were added to the polyethylene test tubes, mixed thoroughly and then incubated for 72 h at 4°C. For the separation of bound from free peptide, 1 ml 0.5% Dextran-T70-wrapped active carbon was added into the above assay system while mixed in an ice-water bath. The mixtures were centrifuged for 20 min at 1750 g, 4°C following 10 min of standstill on bench. The supernatants were counted using micro-γ-counter.
Data Processing and Statistical Analyses
The results were presented as mean ± SEM for continuous data (body weight, ankle circumference, withdrawal threshold of pressure to ankle, content of SP-ir) or as median ± median-derived absolute deviation (MAD) for the non-parametric data (pain scores). Continuous data were processed with two-way analysis of variance (ANOVA) followed by Duncan's test. For the discrete data (pain scores), Friedman ANOVA (repeated measures ANOVA on ranks) was used to analyze the change of pain scores with the time course within the group, Kruskal–Wallis ANOVA (one-way ANOVA on ranks) followed by Dunn's test or Mann–Whitney U-test for the comparison between the groups at the same time point. A P-value of <0.05 was considered statistically significant.
Results
Assessment of Monoarthritis as a Chronic Pain Model
CFA inoculated rats maintained a steady weight increase during the 9 weeks of observation, although the final increment was 9.7% less than the control group (data not shown). The animals appeared active generally.
Circumference of the Ankle Joint
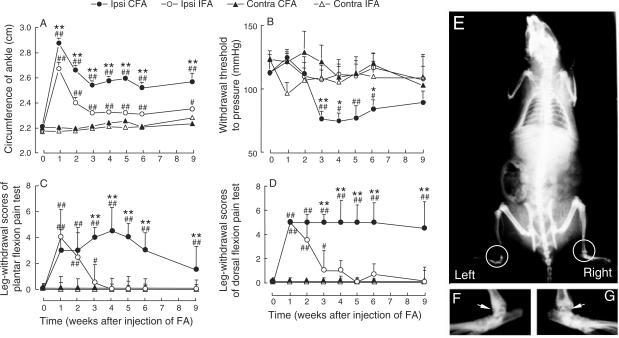
Swelling of the injected ankles occurred in a few hours after inoculation, and aggravated or even spread to the toes and lower legs in 24 h. It subsided gradually after 48 h, became limited to the ankle in a week, subsided in 3 weeks and maintained at this level for at least 6 weeks (Fig. 1A). A similar but milder response was observed in rats inoculated with IFA. No significant change of the diameter of the contralateral ankle was observed.
Figure 1.
Behavioral tests and X-ray examination in rats injected intra-articularly with the Freund's adjuvant (FA) at the right ankle joint. Ankle circumference (A), withdrawal threshold to lateral pressure (B) and pain score evaluated by the plantar (C) or dorsal (D) flexion of the hind paw were examined (n = 10 in each group). Whole body (E) radiograph and high power view of bilateral ankles of a rat were taken at the sixth week after intra-articular injection of CFA at the right ankle. (F) and (G) are the enlargements of the left and right ankle joint from E. Ipsi and contra represent the side of ankle joint ipsilateral or contralateral to that receiving intra-articular injection. IFA, incomplete Freund's adjuvant; CFA, complete Freund's adjuvant. *P < 0.05, **P < 0.01 compared with the level measured before the FA inoculation (Week 0). #P < 0.05, ##P < 0.01 compared with left ankle at the same time point.
Withdrawal Threshold to Lateral Pressure
Leg-withdrawal thresholds to lateral pressure decreased significantly compared with the IFA-inoculated group or the contralateral side of the same group (P < 0.01, Fig. 1B) at the third week after CFA inoculation. The hyperalgesia maintained for ∼4 weeks, and returned to the normal level by ninth week. No significant reduction occurred in IFA-inoculated group (P > 0.05).
Leg-withdrawal Scores in Flexion Pain Test
Results obtained from the flexion pain tests revealed that (i) an increase in pain scores occurred only in the ipsilateral side, suggesting a local rather than general lesion; (ii) a similar abrupt increase in arthritic pain appeared in both the CFA- and IFA-inoculated groups, suggesting the development of acute inflammation; (iii) a decrease in pain scores of the IFA group occurred in the third week and disappeared in the fourth week, which was in contrast to the maintenance of high levels of pain scores for at least 9 weeks in the CFA group, suggesting the existence of chronic hyperalgesia (Fig. 1C and D).
X-ray Examination
A slight narrowing of the joint cavity and roughening of the articular surface of the inoculated ankle was observed 6 weeks after CFA inoculation (Fig. 1E and G). No significant change of the bone density was observed around the joint at 4 and 6 weeks following the CFA injection. No X-ray changes were seen in IFA-injected group at the same time points of observation (data not shown).
Pathological Study
Pathological study revealed that the arthritis was localized to the site of injection, accompanying swelling of the joint-capsule, narrowing of the joint cavity and roughening of the articular surfaces at 4 and 6 weeks after CFA injection (data not shown).
Optimal Parameters of Repeated 100 Hz TENS for the Treatment of Chronic Inflammatory Hyperalgesia
Consecutive TENS Treatments with Different Intervals
The schedule for the treatment was once-a-week, twice-a-week or five-times-a-week using the medium intensity stimulation (1-2-3 mA). TENS was administered (i) beginning at 24 h after the injection of CFA, and lasted for 3 weeks for the treatment of acute period of arthritis; (ii) starting at fourth week and extended to ninth week after the intra-articular injection of CFA, for the treatment of chronic period of arthritis. The indices measured include body weight, circumference of the ankle and scores of arthritic flexion pain tests, which were performed once-a-week just prior to the TENS treatment.
Acute Period of Arthritis
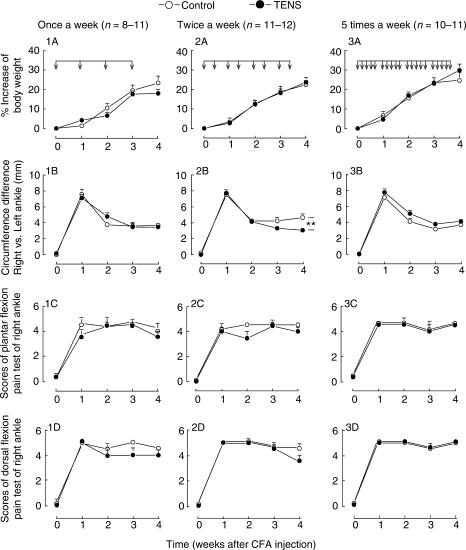
Repeated TENS treatments were delivered at different intervals as follows: once-a-week (Fig. 2: 1A–D), twice-a-week (Fig. 2: 2A–D) and five-times-a-week (Fig. 2: 3A–D) on the acute period of monoarthritis (Weeks 1–3 after intra-articular injection of CFA). The ankle circumference was decreased in the group receiving twice-a-week TENS treatment (Fig. 2: 2B). On the contrary, it increased in the five-times-a-week TENS group (Fig. 2: 3B) compared with the control group. No significant change was observed for other indices (Fig. 2).
Figure 2.
Multiple sessions of 100 Hz TENS with twice-a-week schedule reduce joint swelling in the acute period of rat monoarthritis induced by intra-articular injection of CFA at the right ankle. TENS was administered with medium intensity of stimulation (1-2-3 mA), beginning at 24 h after the injection of CFA (shown by arrows on the horizontal bar). For control group, TENS skin electrodes were placed on the same regions of skin but no current was delivered. **P < 0.01 (two-way ANOVA between two curves from the first week to the fourth week).
Chronic Period of Arthritis
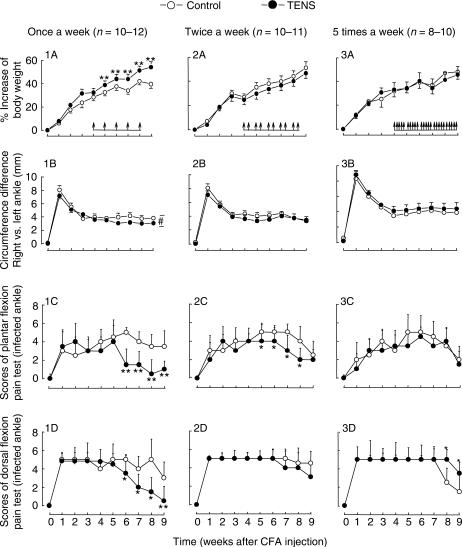
The results are shown in Fig. 3. When TENS was applied in the stable period of monoarthritis (i) the indices were improved in rats receiving TENS once-a-week as shown by an increase in the body weight at the time point of 5, 6, 7, 8 and 9 weeks after injection of CFA (Duncan's test following ANOVA, P < 0.01) (Fig. 3: 1A), a reduction of swelling of the infected ankle (Fig. 3: 1B, two-way ANOVA from fifth week through ninth week), a robust decrease in the flexion pain scores at the sixth week through ninth week (Mann–Whitney U-test, P < 0.01 for all) (Fig. 3: 1C and D). (ii) Administered twice-a-week, TENS treatment produced a moderate decrease in the plantar flexion pain score at sixth week through eighth week after injection of CFA (Mann–Whitney U-test, P < 0.05) (Fig. 3: 2C). The other three indices did not change significantly (Fig. 3: 2A, B and D). (iii) Applied five-times-a-week, there were no obvious improvements in body weight, ankle swelling and plantar pain test (Fig. 3: 3A, B and C). Rather, the dorsal flexion pain score was slightly higher than mock TENS group at eighth and ninth week after injection of CFA (Mann–Whitney U-test, P < 0.05) (Fig. 3: 3D).
Figure 3.
Multiple 100 Hz TENS treatments given once-a-week reduce joint swelling and pain scores in the stable period of rat monoarthritis induced by intra-articular injection of CFA at the right ankle. TENS treatments with medium intensity (1-2-3 mA) were administered from fourth week through ninth week after the injection of CFA (shown by arrows on the horizontal bar). *P < 0.05, **P < 0.01 compared with control group at the same time point [ANOVA followed by Duncan's test for the body weight and ankle circumferences in (A) and (B), Kruskal–Wallis ANOVA followed by Dunn's test for the pain scores in (C) and (D)]. #P < 0.05 compared between two groups from fifth week through ninth week (two-way ANOVA).
TENS Treatments with Different Intensities
The rats were inoculated intra-articularly at right ankle and were distributed randomly into four groups as follows: three TENS groups using three levels of intensity (low: 1-1-2 mA, medium: 1-2-3 mA and high: 2-3-4 mA) and the control group. TENS treatment was administered beginning at 24 h after the injection of CFA and lasted for 9 weeks, using once-a-week or twice-a-week schedule.
Once-a-week
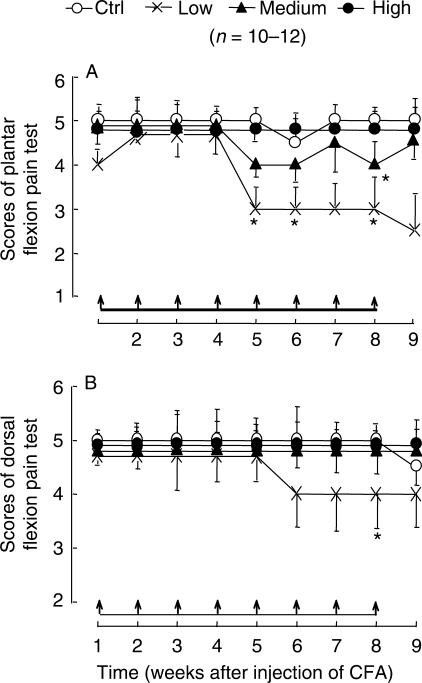
The results are shown in Fig. 4. The low intensity (1-1-2 mA) TENS treatment induced a marked and sustained decrease in the plantar flexion pain test (Friedman ANOVA on ranks, P < 0.01, P < 0.05, respectively). Medium intensity TENS induced a moderate drop of scores of flexion pain test (Friedman ANOVA on ranks, P < 0.05). It is notable that no therapeutic effect was observed with high intensity TENS. The growth rate of body weight and the circumference of the inflamed ankle showed no significant difference between the TENS group and the control group (data not shown).
Figure 4.
Weak TENS treatments reduce pain scores of plantar (A) and dorsal (B) flexion test in monoarthritic rats induced by intra-articular injection of CFA at right ankle. 100 Hz TENS was administered once-a-week started at 24 h after injection of CFA and lasted for 9 weeks (shown by arrows on the horizontal bar). The weak (1-1-2 mA) TENS treatment induced a decrease in scores of flexion pain test (repeated measures ANOVA on ranks, P < 0.01). TENS treatment with medium intensity (1-2-3 mA) induced a moderate drop of plantar flexion pain test (repeated measures ANOVA on ranks, P < 0.05). *P < 0.05 compared with control group at the same time point (one-way ANOVA on ranks). Note that the vertical bars are presented by median-derived standard error, instead of MADs.
Twice-a-week
No significant change of any indices was observed in any TENS group (data not shown).
Suppression of Spinal Release of SP by Repeated 100 Hz TENS
Monoarthritic rats produced by intra-articular injection of CFA at right ankle were randomly distributed into 12 groups, given spinal perfusion at 1, 2, 3, 5, 7 and 9 weeks, respectively, after injection of CFA. At each time point, the TENS (1-1-2 mA, once-a-week) group was accompanied by a control (mock TENS) group. At each time point 3–4 naïve rats were used, in which the above-mentioned indices were measured and spinal perfusate was collected. The results are shown in Fig. 5.
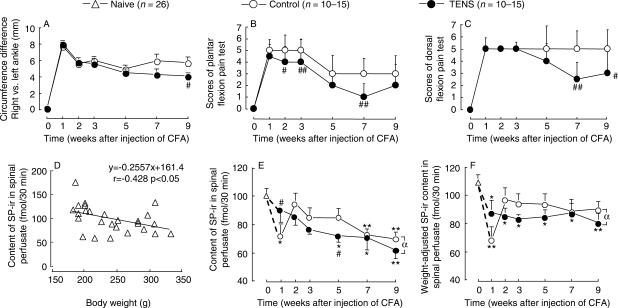
Figure 5.
Paralleled observation of the behavioral changes (top panel, A–C) and spinal SP-ir release (bottom panel, D–F). Six series of experiments were performed in 13 groups. Behavioral indices were measured and then spinal perfusion was conducted at 1, 2, 3, 5, 7 and 9 weeks after CFA injection. One group of naïve rats served as control. 100 Hz TENS was administered once-a-week started at 24 h after the injection of CFA. The intensity of stimulation was increased from 1 mA to a maximal of 2 mA for 30 min. Behavioral measurements were performed only once for each group prior to spinal perfusion. The inflamed ankles of rats received TENS treatments had a lower level of ankle swelling (A) and lower sensitivity to flexion pain tests (B and C). There was a significant decrease in SP-ir level in spinal perfusate in rats receiving TENS treatments (E and F). *P < 0.05, **P < 0.01 compared with the baseline level; #P < 0.05, ##P < 0.01 compared with the mock TENS control group at the same time point (two-way ANOVA followed by Duncan's test for ankle circumference in A, E and F; Kruskal–Wallis ANOVA followed by Dunn's test for the pain scores in B and C). αP < 0.05 compared between two groups from second week to ninth week (two-way ANOVA).
Observation of the Behavioral Indices
The TENS treatment started from the first week through ninth week. Rats were sacrificed at different stages of the experiment. The results shown in Fig. 5A–C were composed of data obtained from different groups of rats, showing the reproducibility of the TENS efficacy.
The circumferences of the inflamed ankles of rats that received TENS treatments were significantly lower than that of the control group (ANOVA, P < 0.05) (Fig. 5A); the leg-withdrawal scores of arthritic plantar and dorsal flexion pain test were also decreased significantly (Kruskal–Wallis, P < 0.05 and P < 0.01) (Fig. 5B and C). No significant difference of body weight was observed between the TENS group and the control group (data not shown).
Contents of SP-ir in the Spinal Perfusate
Significant correlation of spinal SP-ir release and body weight in naïve rats. Figure 5D shows the regression curve of the contents of SP-ir in spinal perfusate and the body weight in 26 naïve rats. It indicates that within the range of 180–350 g body weight, there was a reduction of the content of SP-ir in spinal perfusate along with the increase in body weight, showing a negative correlation between the two factors (P < 0.05).
Influences of TENS on the contents of SP-ir in spinal perfusate. In the control (mock TENS) group, there was a significant decrease in SP-ir content in spinal perfusate at 1 week after the injection of CFA (ANOVA followed by Duncan's test, P < 0.05, Fig. 5E), which returned to the level of naïve rats at the second week and then gradually declined with time until the ninth week after CFA injection. In the TENS group, however, a steady gradual decrease in SP-ir content was observed. The decrease became statistically significant at fifth week to ninth week compared with the control value at Week 0. In the period from second week through ninth week, the levels of SP-ir in rats that received TENS treatments were significantly lower than that of the control group (two-way ANOVA, P < 0.05, Fig. 5D).
Weight-normalized contents of SP-ir in the spinal perfusate. In order to exclude the influence of the body weight on the contents of SP-ir, we normalized the content of SP-ir according to the regression equation (Fig. 5D) as follows: weight-normalized content of SP-ir = actual content + 0.2557 [body weight (g) − 200], where 0.2557 is the regression coefficient and 200 is about the average body weight at the beginning of the experiment. The results are shown in Fig. 5F. In the control rat, there was an abrupt decrease in SP-ir 1 week after the injection of CFA, and then returned to and remained approximately at the level of naïve rats. In the TENS group, there was a mild but significant decrease in SP-ir content, remaining there for the whole observation period of 9 weeks. Comparison between the two curves in the period from second week to ninth week after the injection of CFA showed a statistically significant difference (two-way ANOVA, P < 0.05).
Discussion
In our selection of an inflammatory pain model, we considered various options. The adjuvant-induced polyarthritis has been widely used for the study of chronic arthritic pain since its introduction in 1956 by Pearson (21). However, the general health condition in these animals could be very bad because of the widespread immunological lesions. Thus, it brings about many biochemical, physiological and behavioral disorders besides pain.
Butler et al. (19) developed a monoarthritic pain model with long-term joint hyperalgesia by injecting CFA via intra-articular route of administration. Following Butler et al.'s procedure using a water-in-oil type CFA, a monoarthritic model was produced in the present study, which fulfilled the following criteria: (i) clear manifestation of joint hyperalgesia, (ii) hyperalgesia was maintained at a plateau for at least 6–9 weeks, (iii) minimal destruction of bone tissue and (iv) no severe change in general health. The general condition of the animals was quite good as shown by the steady increase in body weight. The arthritis manifested two distinct stages. There appeared acute redness and swelling of the joint within hours after the articular injection, which peaked in 24 h and subsided in the third week, whereas the joint hyperalgesia maintained for at least six more weeks. X-ray examinations and pathological study revealed no significant change in the bone density around the site of injection. Thus, it appears to be a convenient model for the study of both acute (in the first 3 weeks) and chronic (4–9 weeks after inoculation) inflammatory hyperalgesia.
The theoretical basis of TENS was established mainly on the gate control concept developed by Melzack and Wall in 1965 (22), which suggests that activation of large diameter mechanoceptive fibers will presynaptically inhibit the activity of small caliber nociceptive afferents. According to this theory, the optimal sites for placing TENS electrodes should be chosen at the same segmental region as that of the noxious input (23–26), and the optimal parameters for TENS should be of low intensity and high frequency for preferential excitation of the large diameter fibers (23). Indeed, this has been referred to as ‘conventional TENS’ and extensively used in clinical practice (1). In contrast to the conventional TENS, electroacupuncture (EA) has often been described as a peripheral conditioning stimulation of high intensity and low frequency, which is delivered heterosegmentally (27,28). However, Wang et al. (29) found no significant difference in producing antinociception between EA and TENS at three different levels of frequencies (2, 15 and 100 Hz). It was also found that EA and TENS exhibited a profound cross-tolerance when the same frequency was applied, indicating that a similar neurochemical mechanisms may underlie both EA and TENS analgesia. The systemic study on the specificity of acupoint in normal rats using electrophysiological methods showed that the effect of EA was determined by the intensity of EA stimulation, such that weak EA produced a segmental analgesia, while strong EA produced a generalized analgesia (30,31). TENS may also act through modulating descending influences from supraspinal sites (4).
Multiple studies have shown that the frequency of TENS stimulation is an important factor for its efficacy on inflammatory hyperalgesia and neuropathic pain (4,32–37) and mechanisms underlying the effects of high (>50 Hz) and low (<10 Hz) frequency stimulation are different (1,33). We have compared the effects of low (2 Hz) and high (100 Hz) frequency of repeated TENS on the chronic inflammatory hyperalgesia and found that 100 Hz TENS produced better therapeutic effects (38).
Thus, in the present study we use high frequency (100 Hz) TENS and placed the skin electrodes near the inflammatory region to assure a full extent analgesic effect when different intensities and intervals of TENS were used.
It has been generally accepted that the activation of large diameter (Aα, Aβ) afferent fibers would produce a segmental antinociceptive effects (22), whereas high intensity (noxious) stimulation would also activate small diameter (Aδ, C) fibers to produce diffuse noxious inhibitory control (DNIC) (31). In normal rats, peripheral stimulation of 1–3 mA can produce an intensity-dependent increase in tail flick latency, i.e. the higher the intensity, the stronger the analgesic effect (30,31,39–41). However, Zhu et al. (42) found that in rats with sciatic nerve injury or arthritis, higher intensity EA did not produce a more potent analgesic effect as compared with the low intensity stimulation. In the present study, high intensity (2-3-4 mA) stimulation produced little analgesic effect in contrast to the reduction of pain scoring in the low intensity (1-1-2 mA) group (Fig. 4).
What then are the possible mechanisms underlying this seemingly paradoxical phenomenon? It has been revealed that a series of plastic changes occurred in animals with nerve injury or tissue inflammation (43–46). In the inflammatory rat model, there was an increase in the affinity of the opioid receptor and an increase in the excitability of the afferent nerve fibers not only in the spinal dorsal horn but also in many other regions of the central nervous system (47). There was also an increase in the spontaneous discharge of the spino-thalamic neurons in rats with chronic sciatic nerve injury (48). One may postulate that the signals induced by EA or TENS may be put through more easily when there is an increase in the excitability of the relevant nerve pathway, so that a weak stimulation is now recognized and processed as a moderate stimulation to produce a proper therapeutic effect, whereas a strong stimulation would be perceived as a suprastrong stimuli thereby exacerbate the hyperalgesia and allodynia. The activation of large diameter primary afferents from deep somatic tissues, not cutaneous tissues, is important in causing TENS analgesia (49).
The effects of multiple TENS have drawn significant attentions to the researchers (1). Among many factors, how frequently TENS treatments need to be given for the best outcome is less studied. In the present study we made systemic observations on the therapeutic effects of three different intervals of repeated 100 Hz TENS on the acute and stable period of CFA-induced monoarthritis, and found that for the treatment of acute period of monoarthritis (Weeks 1–3), the optimal interval is twice-a-week; while in the stable period (Weeks 4–9), once-a-week schedule appears to produce better therapeutic effect. When TENS was administered too frequently (five-times-a-week), no therapeutic effect was observed and the swelling of the inflamed ankle may even be aggravated.
Fu et al. (50) used repeated acupuncture stimulations for the treatment of experimental gastric ulcer in rats, and found that every-other-day treatment was the most effective schedule for accelerating the healing of the ulcer. Using weak EA for the treatment of neuropathic pain in the rat it was found that once a day or once every-other-day was most effective to suppress hyperalgesia and allodynia (37,42,51). Wu et al. (52) used 2/100 Hz TENS for the treatment of heroin withdrawal syndrome in drug addicts and found that in the first 2–3 days of drug abstinence, 3–4 treatments (30 min each) a day were needed to secure best results, which could then be reduced to 3 and then 2 treatments per day in the rest of the 2 week detoxification period. It has also been reported that permanent press auricular acupuncture retained in situ for 1 day after surgery reduces the post-operative analgesic requirement after ambulatory knee arthroscopy (53).
Taken together, the data indicate that diseases with acute syndrome need more frequent EA or TENS treatments as compared with chronic stage which may require more sparse treatments for best therapeutic effect. Also this may help to avoid developing TENS tolerance induced by frequent (daily) treatments (54).
Involvement of SP in the TENS Treatment on Inflammatory Hyperalgesia
To study the mechanisms of the therapeutic effect of TENS treatment on inflammatory hyperalgesia, we focused on the possible role played by SP in spinal cord in monoarthritic rats. SP immunoreactivity (SP-ir) in spinal perfusate was measured using RIA. Since the SP converting enzyme does not show an increased activity during the period of arthritis (55,56), the decreased level of SP-ir in spinal perfusate observed in the present study could be taken as an index for the decreased rate of SP release from the central nervous system, especially from the spinal cord.
Given that the negative correlation between the content of SP-ir in the spinal perfusate and the body weight in naïve rats, which has also been shown in other reports (57), may serve as the reason of the rundown of the SP-ir contents observed during the TENS treatments, we normalized the SP-ir content in spinal perfusate to exclude the impact of the animal growth (body weight increase) during the observation period of more than 2 months. An abrupt decrease in SP-ir in spinal perfusate was observed 1 week after CFA inoculation in the control rats without TENS, which is consistent with the findings obtained in spinal slices (58). Also, Sluka and Westlund (12) reported that during the acute experimental arthritis induced by injection of kaolin and carrageenan into the knee joint there was an initial depletion of SP contents in spinal dorsal horn, presumably because of a massive release of SP from the primary afferents. This may result in a depletion of SP at the spinal dorsal horn, leading to an abrupt decrease in SP-ir in spinal perfusate mentioned above.
It is interesting to note that this abrupt decrease in SP-ir was not observed in the rats receiving TENS treatment. This can be due to (i) the amelioration of inflammatory process by TENS treatment so that the inflammation-induced increase in the central SP release was not so dramatic as in the control group without TENS treatment; (ii) the suppression of SP release from the central terminal of primary afferent by the activation of large diameter afferent fiber through gate control mechanism (22). Taking the circumference of the ankle joint as an index of the ankle swelling, namely the severity of arthritis, it was obvious that no significant difference was found between the groups with and without TENS at the first week, suggesting that the inflammatory process was not significantly affected by TENS, which is obviously not in favor of the first explanation.
Starting from the second week to the ninth week, the level of SP-ir in the TENS group was significantly lower than the control group, which was in parallel with the temporal course of a significant decrease in pain scores in TENS group. This decrease was obvious in the second week in contrast to the significant improvement in joint swelling occurred only from the seventh week (Fig. 5B and C). Moreover, compared with the strong analgesic effect, the anti-inflammatory effect of TENS as revealed by the reduction of joint swelling is relatively weak. The results appear in favor of the hypothesis that a low intensity transcutaneous electric stimulation (1–2 mA) can produce a marked reduction of chronic hyperalgesia, accompanying with a suppression of the release of SP in the spinal cord. This is similar to the finding that single session TENS (50 Hz, 50 V, 5 min) shows analgesic effects by reducing the production of SP in both the dorsal root ganglion and dorsal horn of the spinal cord (59). This may add to the emerging evidences that some of the analgesic effects of TENS and acupuncture may be mediated through the alteration of neurotransmitters release, e.g. the release of aspartate, glutamate, glycine (33,37) and nitric oxide (60) and serotonergic activity (36,61,62), as well as the receptor expression, e.g. the opioid receptor (1,33), alpha2A adrenergic receptors (63) and muscarinic receptors (3,64).
Acknowledgments
This study was funded by the National Natural Science Foundation of China (NNSFC, 30330230). The authors wish to acknowledge Dr Ralph Schumacher and Dr Lan Chen (University of Pennsylvania) for their critical comments for the manuscript, and to give thanks to Dr Patty Willis for her comments and working on the manuscript.
References
- 1.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2001;4:109–21. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 2.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–90. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 3.King EW, Sluda KA. The effect of varying frequency and intensity of transcutaneous electrical nerve stimulation on secondary mechanical hyperalgesia in an animal model of inflammation. J Pain. 2001;2:128–33. doi: 10.1054/jpai.2001.19963. [DOI] [PubMed] [Google Scholar]
- 4.Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, Leeper BJ, et al. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–7. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Chui LH, Di W. Clinical observation on acupuncture and moxibustion treatment for 66 cases of migraine. Acupunct Res. 1997;22:108–9. [Google Scholar]
- 6.Yang L. Clinical observation on treatment of senile bony gonitis by acupuncture and herbal fumigation. Acupunct Res. 1997;22:150–1. [Google Scholar]
- 7.Cheing GL, Tsui AY, Lo SK, Hui-Chan CW. Optimal stimulation duration of tens in the management of osteoarthritic knee pain. J Rehabil Med. 2003;35:62–8. doi: 10.1080/16501970306116. [DOI] [PubMed] [Google Scholar]
- 8.Cheing GL, Hui-Chan CW, Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Clin Rehabil. 2002;16:749–60. doi: 10.1191/0269215502cr549oa. [DOI] [PubMed] [Google Scholar]
- 9.Somers DL, Clemente FR. Transcutaneous electrical nerve stimulation for the management of neuropathic pain: the effects of frequency and electrode position on prevention of allodynia in a rat model of complex regional pain syndrome type II. Phys Ther. 2006;86:698–709. [PubMed] [Google Scholar]
- 10.Zubrzycka M, Janecka A. Substance P: transmitter of nociception (Minireview) Endocr Regul. 2000;34:195–201. [PubMed] [Google Scholar]
- 11.Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63:6–10. [PubMed] [Google Scholar]
- 12.Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55:367–77. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 13.Calvino B, Courand JY, Besson JM. Prevaccination with diluted Freund adjuvant prevents the development of chronic pain and transient release of cerebrospinal fluid substance P in adjuvant-induced arthritis in rats. Pain. 1994;58:211–7. doi: 10.1016/0304-3959(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhu LX, Zhao FY, Cui RL. Effect of acupuncture on release of substance P. Ann N Y Acad Sci. 1991;632:488–9. doi: 10.1111/j.1749-6632.1991.tb33166.x. [DOI] [PubMed] [Google Scholar]
- 15.Du J, He L. Alterations of spinal dorsal horn substance P following electroacupuncture analgesia—a study of the formalin test with immunohistochemistry and densitometry. Acupunct Electrother Res. 1992;17:1–6. doi: 10.3727/036012992816357882. [DOI] [PubMed] [Google Scholar]
- 16.Rokugo T, Takeuchi T, Ito H. A histochemical study of substance P in the rat spinal cord: effect of transcutaneous electrical nerve stimulation. J Nippon Med Sch. 2002;69:428–33. doi: 10.1272/jnms.69.428. [DOI] [PubMed] [Google Scholar]
- 17.Shen S, Bian JT, Tian JB, Han JS. Frequency dependence of substance P release by electroacupuncture in rat spinal cord. Sheng Li Xue Bao. 1996;48:89–93. [PubMed] [Google Scholar]
- 18.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- 20.Fei H, Xie GX, Han JS. Low and high frequency electroacupuncture release metenkephalin and dynorphin A in rat spinal cord. Sci Bull Beijing. 1987;32:1496–501. [Google Scholar]
- 21.Pearson CM. Development of arthritis, periarthritis and periostitis in rats given adjuvant. Proc Soc Exp Biol Med. 1956;91:95. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- 22.Melzack R, Wall PD. Pain mechanism: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SL, Gersh MR, Rao VR. Examination of electrode placements and stimulating parameters in treating chronic pain with conventional transcutaneous electrical nerve stimulation (TENS) Pain. 1981;11:37–47. doi: 10.1016/0304-3959(81)90137-8. [DOI] [PubMed] [Google Scholar]
- 24.Berlant SR. Method of determining optimal stimulation sites for transcutaneous electrical nerve stimulation. Phys Ther. 1984;64:924–8. doi: 10.1093/ptj/64.6.924. [DOI] [PubMed] [Google Scholar]
- 25.Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS) Pain. 1994;58:309–15. doi: 10.1016/0304-3959(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 26.Garrison DW, Foreman RD. Effects of transcutaneous electrical nerve stimulation (TENS) on spontaneous and noxiously evoked dorsal horn cell activity in cats with transected spinal cords. Neurosci Lett. 1996;216:125–8. doi: 10.1016/0304-3940(96)13023-8. [DOI] [PubMed] [Google Scholar]
- 27.Andersson SA, Holmgren E. On acupuncture analgesia and the mechanism of pain. Am J Chin Med. 1975;3:311–34. doi: 10.1142/s0192415x75000396. [DOI] [PubMed] [Google Scholar]
- 28.Willer JC, Robby A, Boulu P, Boureau F. Comparative effects of electroacupuncture and transcutaneous nerve stimulation on the human blink reflex. Pain. 1982;14:267–78. doi: 10.1016/0304-3959(82)90133-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang JQ, Mao LM, Han JS. Comparison of the antinociceptive effects induced by electroacupuncture and transcutaneous electrical nerve stimulation in the rat. Int J Neurosci. 1992;65:117–29. doi: 10.3109/00207459209003283. [DOI] [PubMed] [Google Scholar]
- 30.Li CY, Zhu LX, Ji CF. Relative specificity of points in acupuncture analgesia. J Tradit Chin Med. 1987;7:29–34. [PubMed] [Google Scholar]
- 31.Liu X. The experimental research on acupoint specificity and extensiveness of electro-acupuncture analgesia. Acupunct Res. 1997;22:66–70. [Google Scholar]
- 32.Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain. 2000;4:185–93. doi: 10.1053/eujp.2000.0172. [DOI] [PubMed] [Google Scholar]
- 33.Sluka KA, Vance CG, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem. 2005;95:1794–801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Min BI, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998:230–6. doi: 10.1016/j.brainres.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Kim SK, Park JH, Bai SJ, Kim JH, Hwang BG, Min BI, et al. Effects of electroacupuncture on cold allodynia in a rat model of neuropathic pain: mediation by spinal adrenergic and serotonergic receptors. Exp Neurol. 2005;195:430–6. doi: 10.1016/j.expneurol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Baek YH, Choi do Y, Yang HI, Park DS. Analgesic effect of electroacupuncture on inflammatory pain in the rat model of collagen-induced arthritis: mediation by cholinergic and serotonergic receptors. Brain Res. 2005;1057:181–5. doi: 10.1016/j.brainres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Somers DL, Clemente FR. The relationship between dorsal horn neurotransmitter content and allodynia in neuropathic rats treated with high-frequency transcutaneous electric nerve stimulation. Arch Phys Med Rehabil. 2003;84:1575–83. doi: 10.1053/s0003-9993(03)00290-9. [DOI] [PubMed] [Google Scholar]
- 38.Jiang YX, Wang Y, Liu HX. Comparison between therapeutic effects of transcutaneous electrical nerve stimulation with the frequency of 2 Hz and 100 Hz on chronic inflammatory pain in rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2001;21:923–5. [PubMed] [Google Scholar]
- 39.Lu GW. Afferent fiber innervation on acupuncture points and its role in acupuncture analgesia. Acupunct Res. 1987;12:1–32. [PubMed] [Google Scholar]
- 40.Zhu LX, Li CY, Yang B, Ji CF, Li WW. The effect of neonatal capsaicin on acupuncture analgesia. Acupunct Res. 1990;15:285–91. [PubMed] [Google Scholar]
- 41.Wang YJ, Wang SK. Analgesic effects of electroacupuncture stimulation at different intensities and frequencies. Acupunct Res. 1993;18:44–7. [PubMed] [Google Scholar]
- 42.Zhu LX, Li WW, Ji CF, Zhang JL, Zhao FY. A comparison of therapeutic effects induced by electroacupuncture of different intensities on paralgesia in the rat. Chinese J Pain Med. 1996;2:26–32. [Google Scholar]
- 43.Menetrey D, Besson JM. Electrophysiological characteristics of dorsal horn cells in rats with cutaneous inflammation resulting from chronic arthritis. Pain. 1982;13:343–64. doi: 10.1016/0304-3959(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 44.Calvino B, Crepon-Bernard MO, Le Bars D. Parallel clinical and behavioral studies of adjuvant-induced arthritis in the rat: possible relationship with “chronic pain”. Behav Brain Res. 1987;24:11–29. doi: 10.1016/0166-4328(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 45.Danziger N, Remy P, Pidoux B, Dormont D, Samson Y, Fournier E, et al. A clinical and neurophysiological study of a patient with an extensive transection of the spinal cord sparing only a part of one anterolateral quadrant. Brain. 1996;119(Pt 6):1835–48. doi: 10.1093/brain/119.6.1835. [DOI] [PubMed] [Google Scholar]
- 46.Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106. doi: 10.1016/0304-3959(96)03105-3. [DOI] [PubMed] [Google Scholar]
- 47.Treede RD, Magerl W. Modern concepts of pain and hyperalgesia beyond the polymodal C nociceptor. Trends Parmacol Sci. 1995;16:217–28. [Google Scholar]
- 48.Laird JMA, Bennett GJ. An electrophysiological study of dorsal horn neurons in the spinal cord of rats with an experimental peripheral neuropathy. J Neurophysiol. 1993;69:2071–85. doi: 10.1152/jn.1993.69.6.2072. [DOI] [PubMed] [Google Scholar]
- 49.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-induced antihyperalgesia. J Pain. 2005;6:673–80. doi: 10.1016/j.jpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Fu ZH, Jiang Y, Yang CB, Yang ZM. A study on factors of acupuncture methods affecting effects through acupuncture treatments for experimental gastric ulcer in rats. Acupunct Res. 1995;20:40–4. [PubMed] [Google Scholar]
- 51.Liu CN, Ji CF, Zhu LX. Experimental nerve-injured hyperalgesia and its treatment by electro-acupuncture with various intervals. Acupunct Res. 1997;22:87–8. [Google Scholar]
- 52.Wu LZ, Cui CL, Han JS. Treatment on heroin addicts by four channel Han's acupoint nerve stimulator (HANS) J Beijing Med Univ. 1999;31:239–42. [Google Scholar]
- 53.Usichenko TI, Hermsen M, Witstruck T, Hofer A, Pavlovic D, Lehmann C, et al. Auricular acupuncture for pain relief after ambulatory knee arthroscopy—a pilot study. Evid Based Complement Alternat Med. 2005;2:185–9. doi: 10.1093/ecam/neh097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 55.Persson S, Post C, Weil-Fugazza J, Butler SH, Nyberg F. Decreased cerebrospinal fluid neuropeptide-converting enzyme activity in monoarthritic rats. Neurosci Lett. 1992;143:247–50. doi: 10.1016/0304-3940(92)90275-c. [DOI] [PubMed] [Google Scholar]
- 56.Probert L, Hanley MR. The immunocytochemical localisation of ‘substance-P-degrading enzyme’ within the rat spinal cord. Neurosci Lett. 1987;78:132–7. doi: 10.1016/0304-3940(87)90621-5. [DOI] [PubMed] [Google Scholar]
- 57.Chauvet N, Drian MJ, Privat A. Immunocytochemical study of phenotypic plasticity of cultured dorsal root ganglion neurons during development. Int J Dev Neurosci. 1995;13:673–83. doi: 10.1016/0736-5748(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 58.Malcangio M, Bowery NG. Spinal cord SP release and hyperalgesia in monoarthritic rats: involvement of the GABAB Receptor System. Br J Pharmacol. 1994;113:1561–6. doi: 10.1111/j.1476-5381.1994.tb17174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rokugo T, Takeuchi T, Ito H. A histochemical study of substance P in the rat spinal cord: effect of transcutaneous electrical nerve stimulation. J Nippon Med Sch. 2002;69:428–33. doi: 10.1272/jnms.69.428. [DOI] [PubMed] [Google Scholar]
- 60.Ma SH. Neurobiology of acupuncture: toward CAM. Evid Based Complement Alternat Med. 2004;1:41–7. doi: 10.1093/ecam/neh017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon YB, Kang MS, Son SS, Kim JT, Lee YH, Han HJ, et al. Different frequencies of electroacupuncture modified the cellular activity of serotonergic neurons in brainstem. Am J Chin Med. 2000;28:435–41. doi: 10.1142/S0192415X00000519. [DOI] [PubMed] [Google Scholar]
- 62.Radhakrishnan R, King EW, Kickman JK, Herold CA, Johnston NF, Spurgin ML, et al. Spinal 5-HT(2) and 5-HT(3) receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003;105:205–13. doi: 10.1016/s0304-3959(03)00207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King EW, Audette K, Athman GA, Nguyen HO, Sluka KA, Fairbanks CA. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain. 2005;115:364–73. doi: 10.1016/j.pain.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 64.Radhakrishnan R, Sluka KA. Spinal muscarinic receptors are activated during low or high frequency TENS-induced antihyperalgesia in rats. Neuropharmacology. 2003;45:1111–9. doi: 10.1016/s0028-3908(03)00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]