Abstract
Four compounds β-sitosterol, β-sitosterol-3-O-β-d-glucopyranoside, ursolic acid and 4′,5,6-trihydroxy-3′, 7-dimethoxyflavone were characterized from the dichloromethane extract of the aerial parts of Satureja khuzistanica (Lamiaceae), a native medicinal plant growing in Iran, on the basis of spectral analysis and comparing with the data in literature. The natural occurrence of these compounds can be conclusive for the chemotaxonomic characterization of the genus Satureja.
Keywords: daucosterin; Lamiaceae; Satureja khuzistanica; sitosterol; 4′,5,6-trihydroxy-3′, 7-dimethoxyflavone; ursolic acid
Introduction
Medicinal and aromatic plants have been used for many centuries and are still popular in today's alternative therapies. Herbal remedies and alternative medicines are used throughout the world and in the past herbs often represented the original sources of most drugs (1). Satureja khuzistanica Jamzad (Marzeh Khuzistani in Persian, family of Lamiaceae) is an endemic plant that is widely distributed in the southern parts of Iran (2). It is a subshrub, branched stem ∼30 cm high, densely leafly, broadly ovaiate-orbicular covered with white hairs. Base of the leaves is attenuate and petioliform.
This plant has been used as analgesic and antiseptic among the inhabitants of southern parts of Iran. Infusion of aerial parts of this plant is credited in folk medicine to relieve toothache. The composition of the essential oils of wild and cultivated S. khuzistanica has already been investigated (3). Antimicrobial activity, anti-inflammatory, antinociceptive effects and antioxidant, antidiabetic, antihyperlipidemic and reproduction stimulatory properties of this plant have been recently reported from Iran (4–6).
The chemical composition of the crude extract of this species has not yet been investigated. The current work examined the composition of dichloromethane extract of the aerial parts of cultivated S. khuzistanica.
Methods
General
Melting points were determined with a Buchi B-540 melting point apparatus. 1H, 13C-NMR (DEPT) and 2D-NMR were recorded on Bruker (DRX-500 Avance) NMR spectrometer. Silica gel (kieselgel 60, 70–230 mesh, Merck) was used for column chromatography. Spots were detected on TLC under UV or by heating after spraying with 5% phosphomolybdic acid in C2H5OH.
Plant Material
Aerial parts of cultivated S. khuzistanica in flowering stage from open field were prepared by Khorraman Company in industrial village of Khoramabad, Lorestan Province, Iran, November 2005 (Fig. 1). The plant was authenticated by herbal museum of the Faculty of Pharmacy, Tehran University of Medical Science, Tehran, Iran and a voucher specimen (No. 6650-THE) is deposited.
(1)  (2)
(2)  (3)
(3)  (4)
(4) 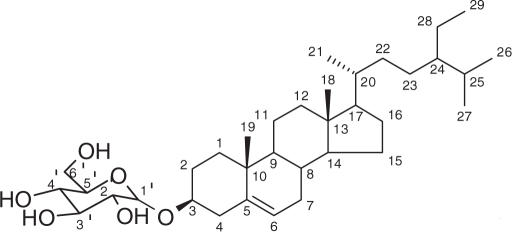
Figure 1.
Satureja khuzistanica Jamzad.
Extraction and Isolation
The air-dried aerial parts of cultivated S. khuzistanica (1 kg) was crushed and extracted with CH2Cl2 (3 × 3 liters) at room temperature for 6 days. The CH2Cl2 extracts were combined and concentrated in vacuo to yield a gummy extract. This residue treated with MeOH to remove waxy compounds.
The MeOH soluble portion (33.2 g) was subjected to a silica gel column chromatography (70–230 mesh, 800 g) with a gradient of hexane–EtOAc and then EtOAc–MeOH as eluent. Eight fractions were collected according to TLC analysis. The fraction of hexane–EtOAc (75:25) was subjected to another column chromatography using hexane–EtOAc (10:1) as the eluent. After recrystallization from MeOH, we obtained 75 mg of compound (1). The fraction of hexane–EtOAc (70:30) was rechromatographed over silica gel and eluted with hexane–EtOAc (9:1) to give 150 mg of compound (2). The fraction of hexane–EtOAc (60:40) was separated on another column chromatography and eluted with hexane–EtOAc (9:1) to give 270 mg of compound (3). From the fraction of EtOAc–MeOH (20:1), crude crystals were obtained, which gave after recrystallization from pyridine/MeOH 40 mg of pure compound (4).
Results
Isolation and Identification of Compounds
In this study, for the first time we investigated the chemical composition of cultivated S. khuzistanica, a plant that was used very extensively in folk medicine of southern parts of Iran.
Dichloromethane extract of aerial parts of this plant was fractionated under silica gel column chromatography and resulted in isolation of four compounds.
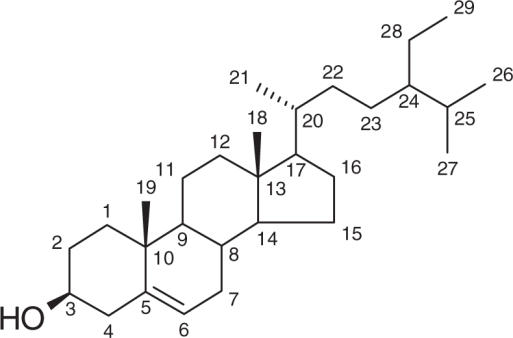
Compound (1) was obtained as colorless needles with a melting point of 133–135°C. According to the 1H, 13C, 2D NMR (H,H-COSY, HMQC, HMBC) experiments and also MS spectra and by comparing these spectroscopic data with those reported in the literatures, this compound was assigned to be β-sitosterol (7–9).
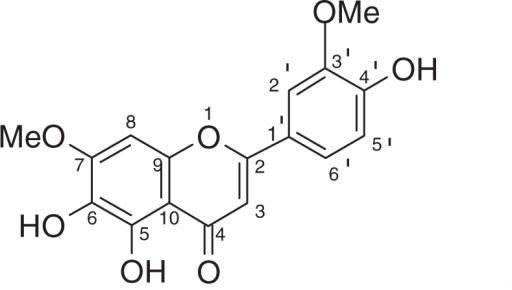
The second compound was isolated as brown crystals with a melting point of 253–255°C. By comparing the spectral data of this compound with those reported in the literatures, it was identified as 4′,5,6-trihydroxy-3′,7-dimethoxyflavone (10,11).
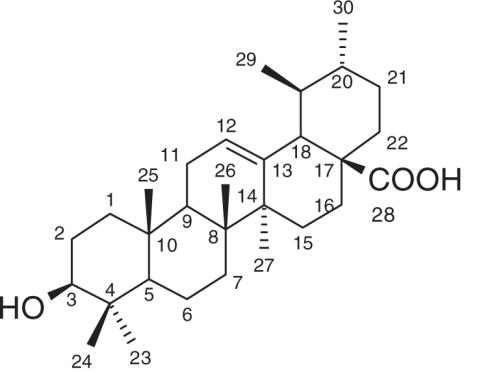
Compound (3) was obtained as a white powder with a melting point of 267–269°C. The 1H NMR and 13C NMR data of this compound were consistent with the reported data of ursolic acid (12–14).
The structure of compound (4), isolated as colorless needles (mp: 275–277°C) was established as β-sitosterol-3-O-β-d-glucopyranoside (daucosterin) by comparison of its spectral data with literature values (15,16).
β-Sitosterol (1)
Colorless needles, mp 133–135°C; 1H NMR (500 MHz, CDCl3) δ 5.39 (1H, m, H-6), 3.56 (1H, m, H-3), 1.05 (3H, s, Me-19), 0.96 (3H, d, J = 6.5 Hz, Me-21), 0.89 (3H, t, J = 7.4 Hz, Me-29), 0.87 (3H, d, J = 6.7 Hz, Me-26), 0.85 (3H, d, J = 6.7 Hz, Me-27), 0.72 (3H, s, Me-18); 13C NMR (125 MHz, CDCl3, based on DEPT, HMQC, HMBC experiments) see Table 1; EIMS m/z 414 [M]+ (39), 396 (100), 381 (21), 329 (17), 303 (19), 273 (11), 255 (24), 213 (20), 161 (18), 145 (22), 107 (22), 95 (23), 55 (21).
Table 1.
13C-NMR spectroscopic data for compounds 1–4
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1 | 37.7t | 39.2t | 38.8t | |
| 2 | 32.3t | 164.6s | 27.8t | 33.5t |
| 3 | 72.2d | 103.8d | 77.7d | 79.8d |
| 4 | 42.8t | 183.1s | 39.2s | 41.3t |
| 5 | 141.2s | 147.1s | 55.6d | 142.3s |
| 6 | 122.1d | 130.8s | 18.9t | 123.2d |
| 7 | 32.1t | 155.2s | 33.6t | 31.6t |
| 8 | 32.3d | 92.0d | 40.0s | 33.4d |
| 9 | 50.6d | 150.5s | 47.9d | 51.7d |
| 10 | 36.9s | 105.9s | 37.4s | 38.2s |
| 11 | 21.5t | 23.7t | 22.7t | |
| 12 | 40.2t | 125.4d | 40.7t | |
| 13 | 42.8s | 139.0s | 43.8s | |
| 14 | 57.2d | 42.5s | 58.1d | |
| 15 | 24.7t | 28.4t | 25.8t | |
| 16 | 28.7t | 24.7t | 29.9t | |
| 17 | 56.5d | 47.7s | 57.7d | |
| 18 | 12.4q | 53.2d | 13.5q | |
| 19 | 19.8q | 39.4d | 20.7q | |
| 20 | 36.6d | 39.3d | 37.7d | |
| 21 | 19.2q | 31.1t | 20.3q | |
| 22 | 34.4t | 37.2t | 35.5t | |
| 23 | 26.5t | 29.1q | 27.7t | |
| 24 | 46.2d | 16.1q | 47.4d | |
| 25 | 29.6d | 16.9q | 30.7d | |
| 26 | 20.2q | 17.8q | 21.3q | |
| 27 | 19.5q | 24.1q | 20.5q | |
| 28 | 23.5t | 179.1s | 24.7t | |
| 29 | 12.3q | 17.9q | 13.3q | |
| 30 | 21.9q | |||
| 1′ | 122.5s | 103.9d | ||
| 2′ | 110.8d | 76.7d | ||
| 3′ | 151.5s | 79.9d | ||
| 4′ | 148.9s | 73.0d | ||
| 5′ | 116.6d | 79.4d | ||
| 6′ | 121.2d | 64.1t | ||
| OMe | 57.1q | |||
| OMe' | 56.8q |
4′,5,6-Trihydroxy-3′,7-dimethoxyflavone (2)
Brown crystals, mp 253–255°C; 1H NMR (500 MHz, DMSO-d6) δ 12.67 (1H, brs, OH-5), 7.60 (1H, dd, J = 7.6 Hz, 2.0 Hz, H-6′), 7.58 (1H, d, J = 2.0 Hz, H-2′), 6.95 (1H, d, J = 7.6 Hz, H-5′), 6.93 (1H, s, H-8), 6.91 (1H, s, H-3), 3.92 (3H, s, OMe), 3.90 (3H, s, OMe′); 13C NMR (125 MHz, DMSO-d6, based on DEPT, HMQC, HMBC experiments) see Table 1; EIMS m/z 330 [M]+ (100), 312 (29), 284 (34), 248 (7), 148 (7), 69 (8).
Ursolic acid (3)
White powder, mp 267–269°C; 1H NMR (500 MHz, DMSO-d6) δ 5.13 (1H, m, H-12), 4.31 (1H, brs, D2O exchangeable, OH), 3.00 (1H, m, H-3), 2.10 (1H, d, J = 11.25 Hz, H-18), 1.04 (3H, s, Me-27), 0.92 (3H, d, J = 6.5 Hz, Me-30), 0.89 (3H, s, Me-24), 0.87 (3H, s, Me-25), 0.81 (3H, d, J = 6.25 Hz, Me-29), 0.75 (3H, s, Me-26), 0.68 (3H, s, Me-23); 13C NMR (125 MHz, DMSO-d6, based on DEPT, HMQC, HMBC experiments) see Table 1; EIMS m/z 456 [M]+ (5), 248 (100), 219 (6), 207 (23), 203 (33), 190 (11), 189 (13), 133 (23), 119 (10), 85 (10), 71 (14), 69 (10), 57 (19).
Daucosterin (4)
White powder, mp 275–277°C; 1H NMR (500 MHz, C5D5N) δ 5.31 (1H, m, H-6), 5.02 (1H, d, J = 7.7 Hz, H-1′), 4.52 (1H, dd, J = 11.6 Hz, 2.1 Hz, H-6′β), 4.43 (1H, dd, J = 11.7 Hz, 5.2Hz, H-6′α), 4.23 (2H, m, H-3′, H-4′), 4.00 (1H, t, J = 7.9 Hz, H-2′), 3.92 (1H, m, H-5′), 3.87 (1H, m, H-3), 0.91 (3H, d, J = 6.4 Hz, Me-21), 0.86 (3H, s, Me-19),0.82 (3H, t, J = 7.3 Hz, Me-29), 0.80 (3H, d, J = 6.8 Hz, Me-26), 0.78 (3H, d, J = 6.9 Hz, Me-27), 0.58 (3H, s, Me-18); 13C NMR (125 MHz, DMSO-d6, based on DEPT, HMQC, HMBC experiments), see Table 1.
Discussion
Herbal remedies used in the traditional folk medicine provide an interesting and still largely unexplored source for the creation and development of potentially new drugs. But it is necessary to reveal the active principles by isolation and characterization of their constituents and to validate their possible toxicity (17,18). The chemistry of the genus Satureja has not been thoroughly analyzed. Previous studies on the chemical composition of this genus indicate the occurrence of flavonoids in Satureja thymbra and Satureja spinosa (11). Iridoid glucosides have also been reported from Satureja vulgaris (19). Mono and sesquiterpenoids and also diterpenoids belonging to labdane, isopimarene and rearranged isopimarene groups were detected in Satureja gilliesii (20,21). Oleanolic acid, ursolic acid, β-sitosterol-β-d-glucoside and flavanone glycosides have been isolated from Satureja acinos, Satureja montana and Satureja obovata (22). Two 3,4-secotriterpenoids along with a mixture of three sterols have been reported from Satureja calamintha and Satureja graeca (23,24).
From four compounds isolated from S. khuzistanica, β-sitosterol has been previously reported from S. calamintha and S. graeca (23). 4′,5,6-Trihydroxy-3′,7-dimethoxyflavone has been isolated from S. thymbra and S. spinosa (11). Also ursolic acid has been reported from S. acinos (22) and S. graeca (23). And β-sitosterol-β-d-glucoside has been isolated from S. acinos, S. montana and S. obovata (22).
The occurrence of these compounds in S. khuzistanica confirms the presence of flavones, triterpenoids and steroids in the genus Satureja (Lamiaceae). The natural occurrence of these compounds can be conclusive for the chemotaxonomic characterization of this genus.
With respect to numerous uses of this plant in folk medicine and great experiments that have been accomplished to investigate its biological properties, we decided to study the chemical composition of the crude extract. This process resulted in isolation of four known compounds. Nevertheless, we cannot attribute biological properties of S. khuzistanica to one of these compounds. Ursolic acid has been long recognized to have anti-inflammatory, antihyperlipidemic properties in laboratory animals. Recently, it has also been noted for its antitumor promotion effects (25). With regard to excessive amount of ursolic acid in S. khuzistanica, therapeutic effects of the plant may be related to the presence of ursolic acid. This compound has also been found plentifully in other species of Lamiaceae family and exact assessment of its biological effects in these species has not been reported, therefore, we believe that the biological effects of S. khuzistanica are due to all existing compounds on this plant.
Acknowledgments
The authors would like to thank Prof. Dr P. Ruedi, University of Zurich, for the MS spectra and also Dr A.N. Salehinia, Khorraman Co., for providing cultivated plants.
References
- 1.Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evid Based Complement Alternat Med. 2005;2:475–9. doi: 10.1093/ecam/neh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamzad Z. A new species of the genus Satureja (Labiatae) from Iran. Iran J Bot. 1994;6:215–8. [Google Scholar]
- 3.Farsam H, Amanlou M, Radpour MR, Salehinia AN, Shafiee A. Composition of the essential oils of wild and cultivated Satureuja khuzistanica Jamzad from Iran. Flavour Fragr J. 2004;19:308–10. [Google Scholar]
- 4.Amanlou M, Fazeli MR, Arvin A, Amin HG, Farsam H. Antimicrobial activity of crude methanolic extract of Satureja khuzistanica. Fitoterapia. 2004;75:768–70. doi: 10.1016/j.fitote.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Amanlou M, Dadkhah F, Salehinia AN, Farsam H, Dehpour AR. An anti-inflammatory and anti-nociceptive effect of hydroalcoholic extract of Satureja khuzistanica Jamzad extract. J Pharm Pharmceut Sci. 2005;8:102–6. [PubMed] [Google Scholar]
- 6.Abdollahi M, Salehnia A, Mortazavi SH, Ebrahimi M, Shafiee A. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja khuzistanica in rat in vivo: an oxicopharmacological study. Med Sci Monit. 2003;9:BR331–5. [PubMed] [Google Scholar]
- 7.Manoharan KP, Huat Benny TK, Yang D. Cycloartane type triterpenoids from the rhizomes of Polygonum bistorta. Phytochemistry. 2005;66:1168–73. doi: 10.1016/j.phytochem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Nes WD, Nortom RA, Benson M. Carbon-13 NMR studies on sitosterol biosynthesized from [13C] mevalonates. Phytochemistry. 1992;66:805–11. [Google Scholar]
- 9.Greca MD, Monaco M, Previtra L. Stigmasterols from Typha latifolia. J Nat Prod. 1990;53:1430–5. [Google Scholar]
- 10.Miski M, Ulubelen A, Mabry TJ. 6-Hydroxyflavones from Thymbra spicata. Phytochemistry. 1983;22:2093–4. [Google Scholar]
- 11.Skoula M, Grayer RJ, Kite GC. Surface flavonoids in Satureja thymbra and Satureja spinosa. Biochem Syst Ecol. 2005;33:541–4. [Google Scholar]
- 12.Chiang YM, Chang JY, Kuo CC, Chang CY, Kuo YH. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry. 2005;66:495–501. doi: 10.1016/j.phytochem.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Kriwacki RW, Pitner TP. Current aspects of practical two dimentional (2D) nuclear magnetic resonance (NMR) spectroscopy, application to structure elucidation. Pharm Res. 1989;6:531–700. doi: 10.1023/a:1015941128608. [DOI] [PubMed] [Google Scholar]
- 14.Romeo G, Giannetto P, Aversa MC. Constituents of Satureia calamintha, application of Eu(fod)2 to the assignments of methyl resonances of triterpenes related to 12-ursene. Org Magn Reson. 1977;9:29–34. [Google Scholar]
- 15.Schwarzmaier U. Prunasin, daucosterin and sitosterol from the bitter seeds of prunus amygdalus. Phytochemistry. 1972;11:2358. [Google Scholar]
- 16.Swift LJ. Isolation of β-sitostryl-d-glucoside from the juice of flurida Valencia oranges (Citrus sinensis L.) J Am Chem Soc. 1952;74:1099–100. [Google Scholar]
- 17.Lindequist U, Niedermeyer THJ, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Fatimi MAA, Jülich WD, Jansen R, Lindequist U. Bioactive components of the traditionally used mushroom Podaxis pistillaris. Evid Based Complement Alternat Med. 2006;3:87–92. doi: 10.1093/ecam/nek008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianco A, Lamesi S, Passacantilli P. Iridoid glucosides from Satureja vulgaris. Phytochemistry. 1984;23:121–3. [Google Scholar]
- 20.Labbe C, Castillo M, Connolly JD. Mono and sesquiterpenoids from Satureja gilliesii. Phytochemistry. 1993;2:441–4. [Google Scholar]
- 21.Labbe C, Castillo M, Fainia F, Coll J, Connolly JD. Rearranged isopimarenes and other diterpenoids from Satureja gilliesii. Phytochemistry. 1994;36:735–8. [Google Scholar]
- 22.Escudero J, Lopez C, Rabanal RM, Valverde S. Secondary metabolites from Satureja species. New triterpenoid from Satureja acinos. J Nat Prod. 1985;48:128–31. [Google Scholar]
- 23.Giannetto P, Romeo G, Aversa MC. Calaminthadiol, a new 3,4-secotriterpenoid from Satureia calamintha and Satureia graeca. Phytochemistry. 1979;18:1203–5. [Google Scholar]
- 24.Romeo G, Giannetto P, Aversa MC. A new 3,4-secopentacyclic triterpenoid from the genus satureia. Phytochemistry. 1980;19:437–9. [Google Scholar]
- 25.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]



