Abstract
A novel application of fluorescent magnetic nanoparticles was made to visualize a new tissue which had not been detectable by using simple stereomicroscopes. This unfamiliar threadlike structure inside the lymphatic vessels of rats was demonstrated in vivo by injecting nanoparticles into lymph nodes and applying magnetic fields on the collecting lymph vessels so that the nanoparticles were taken up by the threadlike structures. Confocal laser scanning microscope images of cryosectioned specimens exhibited that the nanoparticles were absorbed more strongly by the threadlike structure than by the lymphatic vessels. Further examination using a transmission electron microscope revealed that the nanoparticles had been captured between the reticular fibers in the extracellular matrix of the threadlike structures. The emerging technology of nanoparticles not only allows the extremely elusive threadlike structures to be visualized but also is expected to provide a magnetically controllable means to investigate their physiological functions.
Keywords: acupuncture meridian, Bonghan duct, lymph, nanoparticle, transmission electron microscope
Introduction
Acupuncture is increasingly accepted worldwide as an effective alternative medicine (1,2), yet the scientific basis of its mechanism has not been unveiled. Even though evidence of its therapeutic functions, especially in pain, has been reported (3), the structural support for its functions is still lacking. Once the anatomical structure corresponding to the acupuncture meridian is established the science of acupuncture should have a solid foundation. Many observations have supported the physical reality of human acupuncture points and meridians such as low electrical impedance at the acupoints (4) thermal and sense propagation (5), and radioisotope tracing (6). Until now, however, no anatomical or histological structures corresponding to the acupoint have been found (7–9). The only claim ever made in this regard was by Bong Han Kim (10) in the early 1960s who discovered anatomically distinctive tissue at the acupoints, and threadlike ducts at the meridians. He traced the ducts and found that they formed a network throughout the body. He reported that the ducts float inside blood and lymph vessels, and on the surfaces of internal organs (10). One of the serious objections raised by medical or veterinarian experts to the existence of the Bonghan ducts was the possibility of confusion with lymphatic vessels (11,12). In the case of the threadlike ducts on the surfaces of internal organs, Feulgen reaction study provided the method to allow the visualization of features that distinguishes the Bonghan duct from a lymphatic vessel (13).
A striking confirmation of Bong Han Kim's claim was the demonstration of a floating threadlike structure inside lymphatic vessels in vivo and in situ (14), which was made possible by the discovery of the specific dye, Janus Green B, that preferentially stained the threadlike structure rather than the wall of the lymphatic vessel. In this article, we present another method that uses the technique of multifunctional nanoparticles to reveal the barely detectable transparent threadlike structures inside lymphatic vessels. This corroborating evidence provides strong support for the existence of a new anatomical structure that is not known in Western medicine, and is deeply connected to the physical basis of acupuncture meridians.
Magnetic nanoparticles have been increasingly used in various fields, especially in biomedical research, as magnetic carriers for bioseparation and for enzyme and protein immobilization and as a contrast-enhancing medium (15). When coupled to affinity ligands, nanoparticles can function as sensitive biological nanosensors. For example, gold nanoparticles (16,17) have been conjugated with synthetic oligonucleotides, proteins and other ligands to create colorimetric and fluorescent nanosensors. Recently, magnetic nanoparticles have been used in magnetic resonance (NMR/MRI) techniques to detect molecular interactions (18). Imaging applications of nanobiotechnology are mostly focused on the cellular or molecular structures. In this article, we report a rare and novel use of magnetic nanoparticles to reveal and visualize threadlike tissues that are of much larger scale than the cellular structures. We were able to detect the threadlike structures of 20 µm in thickness, which were not detectable with a light microscope, inside the lymphatic vessels of rats.
Incorporation of a fluorescent dye into the magnetic ferrite nanoparticles enabled the detection and visualization, in situ, of the threadlike structures under an external magnetic field. We injected the nanoparticles into two lumbar lymph nodes and placed a magnet for 20 min on the lymphatic vessels connected to the nodes. Owing to the external magnetic field, the injected nanoparticles were attracted to and stayed inside the lymphatic vessel. Without such a field, the particles would have flowed away with the lymphocytes rather than having been taken up by the threadlike structures.
The significance of the present work is the novel application of multifunctional nanoparticles to detect and visualize in vivo and in situ new threadlike structures inside collecting lymphatics. These threadlike structures have hitherto been unknown in lymphology and, thus, are expected to play a profound role in the physiology of lymphatic systems (19).
Materials and Methods
Preparation of Rats and Surgical Procedure
Eleven Wistar rats (ten males and one female) and two Sprague-Dawley males of ∼200 g were obtained from Jung Ang Laboratory Animal Company for use in this study. The rats were housed in a constant-temperature controlled environment (23°C) with 60% relative humidity under a 12 h light/dark cycle. All of the rats had ad libitum access to food and water. The procedures involving rats and their care were in full compliance with current international laws and policies (Guide for the Care and Use of Laboratory Animals, National Academy Press, 1996) (20). The rats were anesthetized with urethane (1.5 g kg−1) administered intraperitoneally, and all surgical procedures were performed under general anesthesia. Under deep anesthesia the abdominal sides of the rats were incised, and the lumbar nodes near the caudal vena cava were located and exposed by removing the surrounding peritonea and fat. A fluorescent magnetic nanoparticle solution (0.03 ml for each node) was slowly injected into the two nodes (over 2–4 min) by using a 30-gauge needle.
Nanoparticles
Employing a modified polyvinylpyrrolidone method, we synthesized cobalt–ferrite magnetic nanoparticles coated with a shell of amorphous silica. A luminescent organic dye, rhodamine B isothiocyanate (RITC, orange, λmax(abs) = 555 nm), was inside the silica shell and biocompatible poly(ethylene glycol) (PEG) was on the outside (21). The average size of the water-soluble bare cobalt–ferrite magnetic nanoparticles was ∼9 nm. The total size of the core-shell structure was ∼50 nm. The concentration of nanoparticles was 2.0 mg cm−3, and they were suspended in a sterile saline solution at pH 7.4. The volume of injected dye was 0.03 ml for each node.
Magnetic Fields and Illumination
A disk-shaped Nd-Fe-B magnet (diameter 14 mm; thickness 5 mm; field strength 4000 Gauss at the surface) was placed just above the lymphatic vessels for 20 min. The target area was illuminated with optical fibers of 3 mm in diameter, and the light sources were halogen and mercury lamps.
Microscope
The observations of the threadlike structures were done with a stereomicroscope (SZX12, Olympus). Confocal laser scanning microscope images were obtained with a Zeiss LSM 510 CLSM model.
Transmission Electron Microscopy
Small segments (∼1 mm × 3 mm in size) of the specimens were fixed in a solution containing 2% paraformaldehyde and 2% glutaraldehyde in a 0.05 M sodium-cacodylate buffer (pH 7.2) for 4 h at 4°C, followed by post-fixation in 1% osmium tetroxide in a 0.05 M sodium-cacodylate buffer (pH 7.2) for 2 h at 4°C.
The fixed materials were stained en bloc in 0.5% aqueous uranyl acetate at 4°C overnight. They were then dehydrated using graded ethanol. The samples were immersed in propylene oxide and then placed in a 1:1 dilution of Epon 812 (Electron Microscope Science) in propylene oxide for 2 h. Next, they were placed in pure resin and were put into flat molds of fresh resin to polymerize overnight at 70°C.
Ultrathin sections were obtained with a MT-X ultratome (RMC, Tucson, AZ, USA) and were double stained by using 2% uranyl acetate and 0.5% lead citrate. The ultrathin sections were studied and micrographed in a JEM-1010 transmission electron microscope (TEM) (JEOL, Tokyo, Japan).
Results
Visualization of Threadlike Structure
We examined 13 rats (11 Wistar and 2 Sprague-Dawley) for this study. Twelve of them were males with an average weight of 200 g, and one was a female Wistar rat with a weight of 150 g. The thickness of the threadlike structure varied widely, and its average was 52 ± 30 µm for the thick part and 19 ± 9 µm for the thin part. The average thickness of the lymphatic vessels was 240 ± 70 µm. Detailed data are given in Table 1.
Table 1.
Size data on 13 threadlike structures from rats
| Subject number | Strain | Sex | Weight (g) | W (mm) | D (µm) | d (µm) | N | L × ℓ (mm × mm) |
|---|---|---|---|---|---|---|---|---|
| 1 | Wist | M | 240 | 0.36 | 20 | 9 | 3 | 0.23 × 0.13 0.27 × 0.13 0.30 × 0.10 |
| 2 | Wist | M | 250 | 0.21 | 27 | 20 | 0 | |
| 3 | Wist | M | 250 | 0.19 | 62 | 14 | 1 | 0.29 × 0.14 |
| 4 | Wist | M | 250 | 0.2 | 120 | 40 | 1 | 0.80 × 0.20 |
| 5 | SpD | M | 140 | 0.21 | 80 | 28 | 0 | |
| 6 | SpD | M | 150 | 0.26 | 46 | 17 | 1 | 0.27 × 0.15 |
| 7 | Wist | M | 185 | 0.29 | 93 | 20 | 1 | 0.49 × 0.21 |
| 8 | Wist | M | 190 | 0.23 | 30 | 14 | 1 | 1.47 × 0.13 |
| 9 | Wist | M | 180 | 0.16 | 53 | 22 | 1 | 0.91 × 0.24 |
| 10 | Wist | M | 160 | 0.23 | 30 | 22 | 1 | 1.0 × 0.05 |
| 11 | Wist | M | 175 | 0.25 | 50 | 13 | 0 | |
| 12 | Wist | F | 150 | 0.15 | 30 | 10 | 3 | 0.53 × 0.29 0.42 × 0.08 0.08 × 0.06 |
| 13 | Wist | M | 170 | 0.4 | 33 | 12 | 1 | 0.22 × 0.13 |
| Average | 192 | 0.24 | 52 | 19 | 1.3 | 0.52 × 0.15 | ||
| Std | 41 | 0.07 | 30 | 9 | 0.9 | 0.39 × 0.07 |
The abbreviations are as follows: Wist, Wistar; SpD, Sprague-Dawley; W, diameter of lymphatic vessel; D and d, diameter of the thickest and the thinnest parts of a threadlike structure, respectively; N, number of observed corpuscles; L and ℓ, long and short diameters of oval-shaped corpuscles.
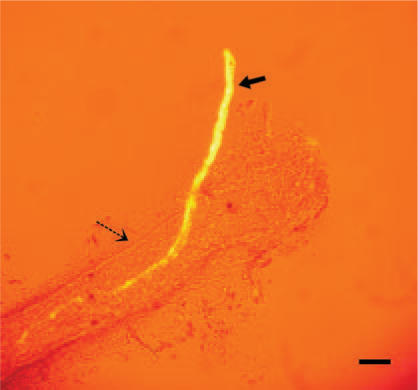
A typical view of a lymphatic vessel is so transparent that not only the intravascular valves but also the blood capillaries under the lower vessel wall are visible, but there is not even a slight hint of any threadlike structure. However, simple microscopic observations can be misleading because optically transparent tissues may not be detected at all. Here, we found a dramatic case of such a misjudgment. As Fig. 1A shows, when a magnetic field was applied to the lymphatics after nanoparticles had been injected into the lumbar lymph nodes and retained inside the vessel, a threadlike structure (solid arrows) emerged. The threadlike structure passes through the valve (open arrows) inside the lymphatic vessel near the caudal vena cava of a rat. This photograph was taken in vivo and in situ under a stereomicroscope. The movement of the lymphatic vessels due to respiration made it difficult to obtain a sharply focused picture. We put a piece of black paper under the lymphatic vessel in order to exhibit the lymphatics more clearly. Figure 1B is an illustration.
Figure 1.
A stereomicroscopic image of the lymphatic vessel around the caudal vena cava of a rat. The photograph (A) and its illustration (B) show the novel threadlike structure (solid arrow) that passes throw the lymphatic valve (open arrow). The photograph was taken in vivo and in situ, and a piece of black paper was put under the lymphatic vessel to exhibit the target clearly. The scale bar is 100 μm.
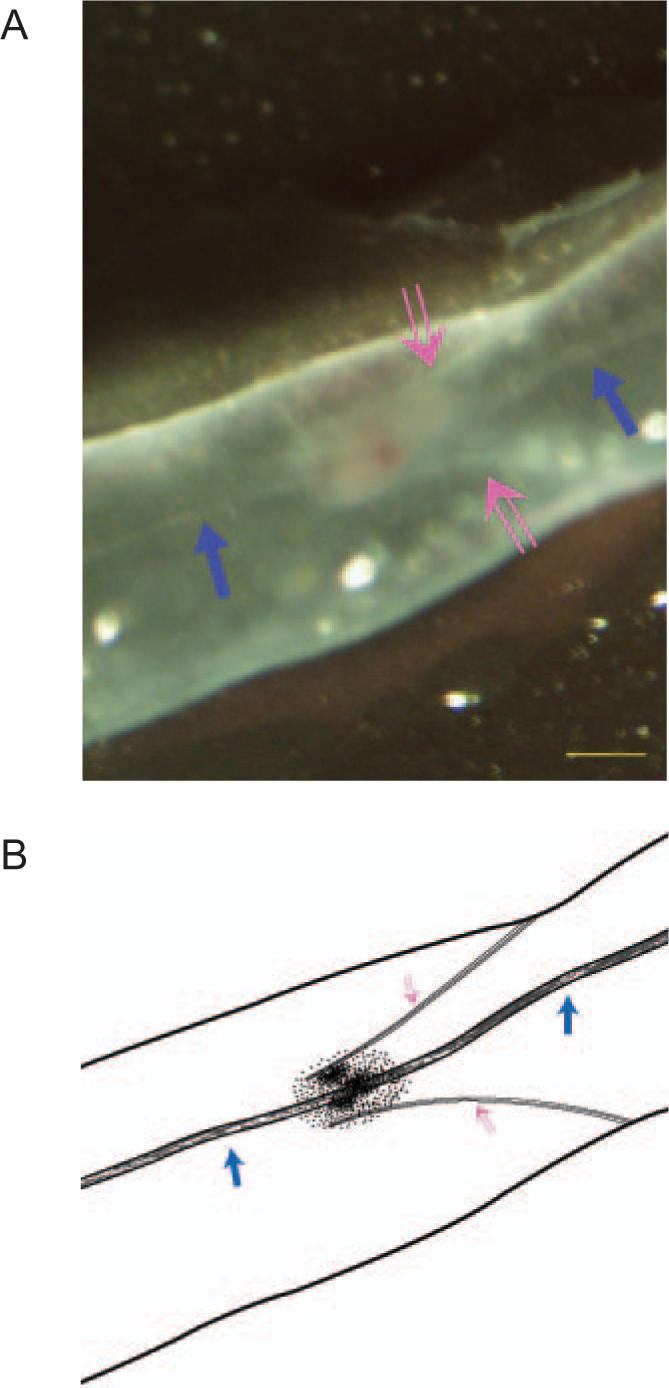
The emergence of the nearly invisible threadlike structure was due to a taking-up of the fluorescent nanoparticles. We cut a piece of a lymphatic vessel, fixed it with neutral-buffered formalin (NBF 10%), and examined it under an inverted microscope. One end of the specimen was torn off using a sharp needle, and its fluorescence image is shown in Fig. 2; the threadlike structure (solid arrow) is vividly bright due to the fluorescence of the uptaken nanoparticles and clearly exhibits its existence even after fixing with NBF.
Figure 2.
Fluorescence image of a piece of the lymphatic vessel (dotted arrow) fixed by NBF. The threadlike structure (solid arrow) is vividly bright due to fluorescence of the nanoparticles. We intentionally tore one side of the vessel off to exhibit the threadlike structure more clearly. The scale bar is 50 μm.
Uptake of Nanoparticles by Threadlike Structure (Confocal Image)
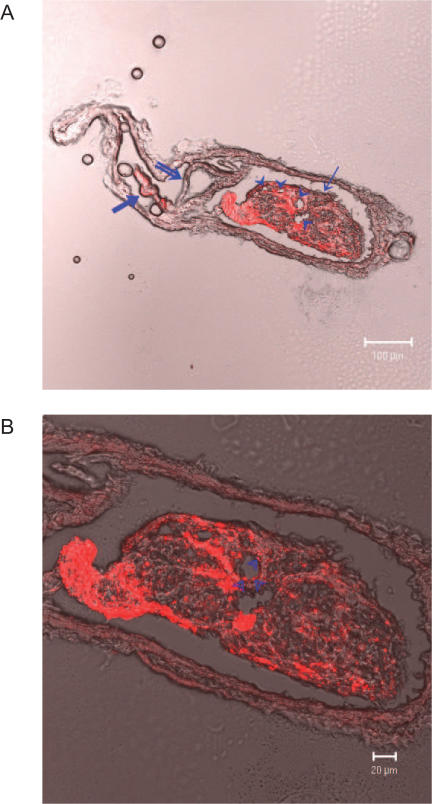
Confocal laser scanning microscope images of cryosections of a threadlike structure and a corpuscle inside a lymphatic vessel with a valve are shown in Fig. 3. The existence of corpuscles along the threadlike structure is one of the essential features to confirm Bong Han Kim's theory (10). Indeed, Fig. 1 shows a corpuscle along the threadlike structure at the valve area. The coexistence of a valve and a corpuscle was not a typical situation, so it was a good opportunity to consider both of them simultaneously (14). In the figure, the corpuscle is to the right in the lymphatic duct, and the entwined valve is in the middle. To the left side, a part of a threadlike structure can be seen. Figure 3A shows that the threadlike structure and the corpuscle are far brighter than the lymphatic valve and the lymphatic walls, which implies that the nanoparticles are taken up more strongly by the novel structures. In the magnified views of the corpuscles in Fig. 3B, two large holes with spherical bodies inside are clearly seen. These are thought to be sinuses through which some liquid and granules flow and to be deeply involved in the physiological functions of the novel structure.
Figure 3.
Confocal laser scanning microscope images of cryosectional threadlike and corpuscular structures. The orange color is due to a nanoparticle (rhodamine). (A) The valve (double line arrow) is in the middle of the lymphatic vessel. The corpuscle (arrow) is on the right, and the threadlike structure (thick arrow) is on the left. There are four sinuses (arrow heads) in the corpuscle. (B) A magnified image of (A). There are granules in the two large sinuses.
Nanoparticles were Captured in ECM Fibers (TEM Image)
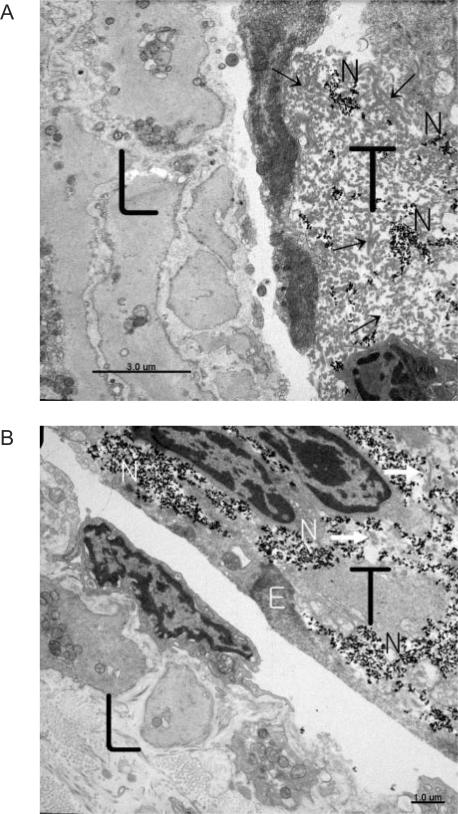
The nanoparticles were taken up by the threadlike structure, but not by the walls of the lymphatic vessel. This is an essential contribution to the threadlike structures' visibility in vivo. We further confirmed the threadlike structure's preference for nanoparticle absorption by using TEM studies of the sample. In Fig. 4A, a lymphatic vessel wall (the left-hand side) and a threadlike structure (the right-hand side) are seen to exist together very closely. On the left side of Fig. 4A, which is the lymphatic wall, there are no nanoparticles at all, whereas on the right side there are scattered black points, which are the nanoparticles. The nuclei in the surrounding wall of the threadlike structure did not take up the nanoparticles, which is in agreement with a previous work (21). The nanoparticles were captured in an extracellular matrix composed of mostly longitudinally and transversally running reticular fibers (arrows) in this particular region.
Figure 4.
TEM images of a lymphatic vessel wall and the threadlike structure which were closely located. The scattered black dots are nanoparticles. (A) On the left-hand side is the lymphatic wall in which no nanoparticles are seen. On the right-hand side is the threadlike structure in which nanoparticles (black dots) are scattered around in the exterior cellular matrix of reticular fibers. (B) Abundant nanoparticles are captured in the extracellular matrix of the threadlike structure in the upper right region. In the lower left region is the lymphatic wall where no nanoparticles are found. (Arrows are inside collagen fibrils, and L stand for lymphatic vessel; T, threadlike structure; N, nanoparticle; and E, cytoplasmic membrane of the surrounding cell of the threadlike structure.)
In the lower part of Fig. 4B, which shows another region of the same sample, one can see the wall of the lymphatic vessel, and there are no nanoparticles. On the other hand, in the upper part of the same figure, there are many nanoparticles inside the wall surrounding the threadlike structure. The reason the nanoparticles were taken up preferentially by the threadlike structure seems to the fenestrated surface revealed in the scanning electron microscopic study.
The position and the thickness of the threadlike structure varied widely from one sample to another. The specimens of Figs 3 and 4 were from different animals. The closeness of the threadlike structure to the lymphatic wall in Fig. 4 is not a typical situation; such threadlike structures were chosen to contrast the distribution of nanoparticles.
Discussion
Biomedical imaging or visualizing applications of nanoparticles are usually done on cellular or subcellular scales. In this article, we reported a novel application for visualizing a nearly invisible threadlike structure inside transparent lymphatic vessels of rats. This work provides a rare example of nanobiotechnology on a macroscale to find a new anatomical structure by combining magnetism and fluorescence in relation to nanoparticles.
The existence of the threadlike structure inside lymphatic vessels was first claimed to have been observed in the early 1960s by Kim (10), but he neither presented any photographic evidence nor described methods to detect it. As far as we know, for the first time, its existence is explicitly shown and its microscopic morphology is investigated in this and a previous study, which was almost simultaneously done and used a conventional staining dye (14). The staining method used Janus Green B as a dye, which turned out to stain the threadlike structure more strongly than the lymphatic walls. The current study used a new method employing the modern technology of multifunctional nanoparticles. The corroborating result is reassuring evidence for the existence of a novel threadlike structure that is a completely new object in Western anatomy.
Several levels of investigations are needed to fully elucidate the details of this intralymphatic threadlike structure as follows: (i) imaging and anatomy, (ii) histology, (iii) electron microscopy for ultrastructural analysis, (iv) immunohistochemistry and (v) functions, especially circulation and signal propagation, in connection with acupuncture. The current work is mainly concerned with the first stage (imaging and anatomy), but its significance may extend beyond this introductory level because the promising technology of nanoparticles will provide a unique tool for studying the functions of the threadlike structure by using a magnetically controllable means. Still, one must admit that using a visualizing technique to observe the anatomical structure is a very important step toward full exploration of this novel structure.
Histological and structural studies of these threadlike structures are being performed using various techniques. Mallory's triple staining and Verhoeff's elastic staining, in addition to the basic hematoxylin and eosin staining, were performed (22), and Gomori's silver staining to reveal reticular fibers in the intravascular threadlike structures was done. Further examination by immunohistochemical analysis identified endothelial cells at the sinus boundaries and a fibrous extracellular matrix between the sinuses in the threadlike structures. These data are supported by TEM images. A distinguishing feature of the threadlike tissues is its bundle structure of multiple ductules, which was verified by using a light microscope image after Feulgen reaction staining (13), a scanning electron microscope picture and an X-ray microscope picture.
After the early confirmation by Fujiwara and Yu (23), the threadlike structures inside blood vessels were observed in animals such as rabbits (24), rats (25) and pigs (26). Those on the surfaces of the internal organs of rabbits and rats were also observed (13,27). Recently, ultrasonic imaging of acupoints has revealed structures in agreement with these results (28). In this series of development the current work using nanoparticles is significant in the sense that it provides a direct visual demonstration of the threadlike structure. In addition, this technology has promising potential for tracing the new circulatory system when relevant techniques become more developed because the movement of nanoparticles can be easily controlled by a magnetic field. Once the new circulatory system has been traced, the information can be applied for drug delivery to internal organs through acupuncture meridians with traceable and controllable means.
Finally, we should consider any possible effects of the magnetic field on the structural and/or functional features of the threadlike structure because numerous reports provide evidence that magnetic fields affect crucial dynamics both at the cellular level and at the level of intact organisms (29,30). Although our data on sizes might have been affected by the magnetic field, we consider our visualization of the threadlike structure to be reliable because non-magnetic methods gave similar results. In addition, other parameters of magnetic fields such as intensity, distribution and direction can affect the cellular absorption mechanism of the nanoparticles and the efficiency of the visualization, which may become a subject for future study in magnetobiology.
Acknowledgments
This work was supported in part by the Korea Science and Engineering Foundation (NRL, M1-0302-00-0007) and by the Ministry of Science and Technology through the Cavendish-Korean Advanced Institute of Science and Technology Cooperative Research Program.
References
- 1.Lee JD, Park HJ, Chae Y, Lim S. An overview of bee venom acupuncture in the treatment of arthritis. Evid Based Complement Alternat Med. 2005;2:79–84. doi: 10.1093/ecam/neh070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim YS, Jun H, Chae Y, Park HJ, Kim BH, Chang IM, et al. The practice of Korean medicine: an overview of clinical trials in acupuncture. Evid Based Complement Alternat Med. 2005;2:325–52. doi: 10.1093/ecam/neh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao JCI. CAM for pediatric pain: what is state-of-the-research? Evid Based Complement Alternat Med. 2006;3:143–4. doi: 10.1093/ecam/nek003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johng HM, Cho JH, Shin HS, Soh KS, Koo TH, Choi SY, et al. Frequency dependance of impedances at the acupuncture point quze (PC3) IEEE Eng Med Biol. 2002;21:33–6. doi: 10.1109/memb.2002.1000183. [DOI] [PubMed] [Google Scholar]
- 5.Ji ZP. Studies on propagated sensation along channels. J Tradit Chin Med. 1981;1:3–6. [PubMed] [Google Scholar]
- 6.Darras JC, de Vernejoul P, Albarede P. Nuclear medicine and acupuncture: a study on the migration of radioactive tracers after injection at acupoints. Am J Acupunct. 1992;20:245–56. [Google Scholar]
- 7.Zhang K, Dang R, Guan L, Fang T, Guo S, Yao Y, et al. A morphological study on the receptors of acupuncture points. J Tradit Chin Med. 1982;2:251. [PubMed] [Google Scholar]
- 8.Heine H. Anatomical structure of acupoints. J Tradit Chin Med. 1988;8:207. [PubMed] [Google Scholar]
- 9.Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec. 2002;269B:257–65. doi: 10.1002/ar.10185. [DOI] [PubMed] [Google Scholar]
- 10.Kim BH. On the kyungrak system. J Acad Medi Sci. 1963;10:1–41. [Google Scholar]
- 11.Kellner G. Bau und function der haut. Deutsche Zeitschrift fur Akupunktur. 1966;15:1–31. [Google Scholar]
- 12.Kroger WS. Acupuncture analgesia: its explanation by conditioning theory, autogenic training and hypnosis. Am J Psychiatry. 1973;130:855–60. doi: 10.1176/ajp.130.8.855. [DOI] [PubMed] [Google Scholar]
- 13.Shin HS, Johng HM, Lee BC, Cho SI, Soh KS, Baik KY, et al. Feulgen reaction study of novel threadlike structures (Bonghan ducts) on the surfaces of mammalian organs. Anat Rec. 2005;284B:35–40. doi: 10.1002/ar.b.20061. [DOI] [PubMed] [Google Scholar]
- 14.Lee BC, Yoo JS, Baik KY, Kim KW, Soh KS. Novel threadlike structures (Bonghan ducts) inside lymphatic vessels of rabbits visualized with a Janus Green B staining method. Anat Rec. 2005;286B:1–7. doi: 10.1002/ar.b.20076. [DOI] [PubMed] [Google Scholar]
- 15.Wu EX, Tang H, Wong KK, Wang J. Mapping cyclic change of regional myocardial blood volume using steady-state susceptibility effect of iron oxide nanoparticles. J Magn Reson Imaging. 2004;19:50–8. doi: 10.1002/jmri.10426. [DOI] [PubMed] [Google Scholar]
- 16.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–9. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 17.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277:1078–81. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 18.Perez JM, Josephson L, Weissleder R. Use of magnetic nanoparticles as nanosensors to probe for molecular interactions. Chembiochem. 2004;5:261–4. doi: 10.1002/cbic.200300730. [DOI] [PubMed] [Google Scholar]
- 19.Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. Washington, D.C. 21.: National Academy Press; 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 21.Yoon TJ, Kim JS, Kim BG, Yu KN, Cho MH, Lee JK. Multifunctional nanoparticles possessing a “magnetic motor effect” for drug or gene delivery. Angew Chem Int Ed Engl. 2005;44:1068–71. doi: 10.1002/anie.200461910. [DOI] [PubMed] [Google Scholar]
- 22.Baik KY, Park ES, Lee BC, Shin HS, Choi CH, Yi SH, et al. Histological aspect of threadlike structure inside blood vessel. J Int Soc Life Inf Sci. 2004;22:473–6. [Google Scholar]
- 23.Fujiwara S, Yu SB. ‘Bonghan theory’ morphological studies. Igaku no Ayumi. 1967;60:567–77. [Google Scholar]
- 24.Lee BC, Baik KY, Johng HM, Nam TJ, Lee J, Sung B, et al. Acridine orange staining method to reveal characteristic features of intravascular threadlike structure. Anat Rec. 2004;278B:27–30. doi: 10.1002/ar.b.20018. [DOI] [PubMed] [Google Scholar]
- 25.Baik KY, Lee JW, Lee BC, Johng HM, Nam TG, Sung BK, et al. Acupuncture meridian and intravascular Bonghan duct. Key Eng Mater. 2005;277:125–9. [Google Scholar]
- 26.Cho SJ, Kim BS, Park YS. Thread-like structures in the aorta and coronary artery of swine. J Int Soc Life Inf Sci. 2004;22:609–11. [Google Scholar]
- 27.Lee KJ, Kim S, Jung TE, Jin D, Kim DH, Kim HW. Unique duct system and the corpuscle-like structures found on the surface of the liver. J Int Soc Life Inf Sci. 2004;22:460–2. [Google Scholar]
- 28.Jones JP, Bae YK. Ultrasonic visualization and stimulation of classical oriental acupuncture points. Med Acupunct. 2004;15:24–6. [Google Scholar]
- 29.Taniguchi N, Kanai S, Kawamoto M, Endo H, Higashino H. Study on application of static magnetic field for adjuvant arthritis in rats. Evid Based Complement Alternat Med. 2004;1:187–91. doi: 10.1093/ecam/neh024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ventura C. CAM and cell fate targeting: molecular and energetic insights into cell growth and differentiation. Evid Based Complement Alternat Med. 2005;2:277–83. doi: 10.1093/ecam/neh100. [DOI] [PMC free article] [PubMed] [Google Scholar]