Abstract
Inflammatory bowel disease (IBD) is a chronic condition of the intestine with unknown etiology involving multiple immune, genetic and environmental factors. We were interested to examine the effect of total extract from Zataria multiflora Boiss, a folk medicinal plant on prevention and treatment of experimental IBD. Z. multiflora was administered (400, 600, 900 p.p.m.) through drinking water to IBD mice induced by intrarectal administration of acetic acid. Prednisolone was used as the standard drug for comparison. Biochemical, macroscopic and microscopic examinations of colon were performed. Biochemical evaluation of inflamed colon was done using assay of myeloperoxidase (MPO) activity and thiobarbituric acid reactive substances (TBARS) concentration as indicators of free radical activity and cell lipid peroxidation. The activity of MPO and lipid peroxidation products (TBARS) increased in acetic acid-treated groups while recovered by pretreatment of animals with Z. multiflora (400–900 p.p.m.) and prednisolone. Z. multiflora (600 and 900 p.p.m.) and prednisolone-treated groups showed significantly lower score values of macroscopic and microscopic characters when compared with the acetic acid-treated group. The beneficial effect of Z. multiflora (900 p.p.m.) was comparable with that of prednisolone. The antioxidant, antimicrobial and anti-inflammatory potentials of Z. multiflora might be the mechanisms by which this herbal extract protects animals against experimentally induced IBD. Proper clinical investigation should be carried out to confirm the activity in human.
Keywords: inflammatory bowel disease, antioxidant, cells lipid peroxidation, myeloperoxidase, rat, Zataria
Introduction
Inflammatory bowel disease (IBD) comprises those conditions characterized by a tendency for chronic or relapsing immune activation and inflammation within the gastrointestinal tract. The intestinal inflammation is histologically characterized by infiltration of polymorphonuclear leukocytes, monocytes and macrophages. They are activated by various mediators including prostaglandins, leukotrienes, platelet-activating factor and cytokines to synthesize and liberate reactive oxygen metabolites (1,2).
The balance between the production of free radical and antioxidant defense in the body has important health implications: if there are too many free radicals or too few antioxidant for protection, a condition of oxidative stress develops, which may cause chronic and pretreatment damage (3). A favorable correlation between the activity of free radicals (O2, H2O2, OH) in the intestine and the clinical disease activity has been exhibited indicating the significance of these substances in the inflammatory process (4). Effectiveness of various therapies in IBD has been suggested to be related to antioxidant action (5–7). The previous studies showed the existence of oxidative stress in biological fluids such as saliva (8) and plasma (9) of patients with IBD. One of the markers of oxidative stress is increased lipid peroxidation of the cells that is determined by measuring thiobarbituric acid reactive substances (TBARS) (3). Myeloperoxidase (MPO) is an enzyme found in neutrophils and its activity in the colon is linearly related to infiltration of neutrophils. The assessment of MPO activity is well established for quantification of intestinal inflammation (10). In case of inflammatory conditions like IBD, the levels of neutrophils in inflamed tissues, and consequently MPO enzyme, increase. Acetic acid-induced colitis is an easily inducible model of IBD, and the similarity of the inflammatory mediators profile to IBD mentioned that the inflammatory phase bears some resemblance to human intestinal inflammation (11).
Many herbal concoctions are said to be effective in chronic inflammatory conditions. Labiatae are generally known for their various effects such as analgesic and anti-inflammatory activity (12), antioxidant (13), hepatoprotective (14) and hypoglycemic action (15). Zataria multiflora is a plant owned by the Labiatae family that is distributed only in Iran, Pakistan and Afghanistan. It is greatly used for medicinal and condimental purposes in these countries. This plant with the vernacular name of Avishan Shirazi in Iran has several traditional uses such as antiseptic, anesthetic and antispasmodic (16). From these reported activities for this plant and regarding above-mentioned points about IBD, we hypothesized that Z. multiflora may have an effect on IBD. In this study, we attempted to demonstrate the status of oxidative stress by investigating bowel TBARS and MPO activity accompanied by bowel macroscopic and microscopic findings in experimental model of IBD (acetic acid-induced colitis) in mice.
Materials and Methods
Chemicals
2-Thiobarbituric acid (TBA), 1,1,3,3,-tetraethoxypropan, trichloroacetic acid (TCA), n-butanol, hexadecyl trimethyl ammonium bromide (HETAB), EDTA, O-dianisidine hydrochloride, hydrogen peroxide, acetic acid, prednisolone, phosphate buffer, paraffin, hematoxylin and eosin from Merck Chemical Co. (Germany) were used in this study.
Plant Material
Samples of Z. multiflora were collected from Shiraz, Iran, on May 22, 2005. The leaves of the plant were dried in shadow and stored in the Department of Botany of the Research Institute of Forests and Ranglands (TARI), Tehran. A voucher specimen (No. 58416) has been deposited at the Herbarium of TARI.
Preparation of Total Extract
Amount of 86.4 kg of plant powder was wet with a solvent (methanol) in a closed plastic container; then the wet powder put in a percolator and was macerated in 10 liters methanol (100% v/v) for 24 h and, subsequently, the solution was filtered and concentrated in a percolator by 100 drops per min. This procedure was repeated twice and three times with 10 liters methanol (100 and 80% v/v), respectively. The extract was then concentrated under reduced pressure and appropriate temperature and the solvent was distilled in vacuum, and finally, 1.5 kg solid solvent was produced.
Animals
NMRI albino mice weighing between 20 and 30 g were used for the study. Mice were maintained under standard conditions of temperature (23 ± 1°C), relative humidity (55 ± 10%) and 12 h/12 h light/dark cycle, and fed with a standard pellet diet with water ad libitum. They were housed in standard polypropylene cages with wire mesh top. All studies were carried out using six mice in each group. All ethical considerations using animals were considered carefully and the experimental protocol was approved by the Ethics Committee of TUMS.
Induction of Colitis and Treatments
The protocol of the study including doses and duration of treatment, and groups were all designed according to previous studies (10,17). The study comprised of six different groups as follows: normal or untreated mice which did not receive any treatment; control animals, which received 0.1 ml of 6% acetic acid solution (once, intrarectally); Z. multiflora-treated animals, which received 7 days pretreatment with Z. multiflora (400, 600, 900 p.p.m. in drinking water) and 0.1 ml of 6% acetic acid solution; prednisolone-treated group, which received prednisolone (1.14 mg kg−1, for 3 days) and 0.1 ml of 6% solution. Acetic acid was administered intrarectally on 8th day. Drug treatment was continued until 10th day. Prednisolone as standard drug was started on the day of acetic acid treatment and given orally as suspension containing 0.5% of sodium CMC.
For induction of colitis, overnight fasted mice were anesthetized using pentobarbital sodium (55 mg kg−1, i.p.) and then 0.1 ml of 6% acetic acid solution was instilled into the rectum of the mouse. Animals were allowed to hang in air by holding their tails for 1–2 min. This prevented spillage of the solution from the rectum. After 48 h mice were sacrificed by cervical dislocation and dissected open to remove colon. Five centimeters long piece of colon was flushed gently with saline, cut open and scored for inflammation based on the macroscopic features. Tissues were fixed in 10% formalin saline and examined histopathologically. Biochemical evaluation of colon inflammation was done using assay of MPO activity and TBARS concentration.
Assay of Colon Macroscopic Characters
The colon (5 cm long) was scored for macroscopic features using scoring pattern (10,17) given in the Table 1.
Table 1.
Scoring pattern
| Percentage area affected | Score |
|---|---|
| 0 | 0 |
| 1–5 | 1 |
| 5–10 | 2 |
| 10–25 | 3 |
| 25–50 | 4 |
| 50–75 | 5 |
| 75–100 | 6 |
Assay of Colon Microscopic (Histological) Characters
To process for microscopic studies, 5 μm thick paraffin sections were stained in hematoxylin and eosin. The stained sections were examined for any inflammatory changes like edema, granulated tissues, ulceration or thickening of mucosa, inflammation, existence of polymorphonuclears and mononuclears, necrosis, disrupted architecture of the crypt, and narrow lumen. The sections were all recorded by a histopathologist and a sign score between 0 and 3 for each sign and a total of 24 were used to determine the severity of colon inflammation (10,17).
Assay of Colon MPO Activity
To measure MPO activity, colonic samples were minced on ice and homogenized in 10 ml of ice-cold 50 mM potassium phosphate buffer (pH 6.0), containing 0.5% HETAB and 10 mM EDTA. The homogenates were then sonicated and centrifuged for 20 min at 12 000 g. MPO activity was measured spectrophotometrically as follows: 0.1 ml of supernatant was combined with 2.9 ml of 50 mM phosphate buffer containing 0.167 mg ml−1 O-dianisidine hydrochloride and 0.0005% H2O2. The change in absorbance was measured spectrophotometrically (Shimadzu 160A UV-VIS spectrophotometer) at 460 nm. One unit of MPO activity is defined as the change in absorbance per minute at room temperature, in the final reaction. MPO activity (U g−1) = X/weight of the piece of tissue taken, where X = 10 × change in absorbance per minute/volume of supernatant taken in the final reaction (10).
Assay of Colon TBARS Concentration
Malondialdehyde (MDA) is the main end product of the oxidation of polyunsaturated fatty acids and its concentration in the medium is an established measure of lipid peroxidation extent. In this test, the reaction of TBA with lipid peroxide products makes a complex which is determined spectrophotometrically and lipid peroxidation in samples are assessed in terms of TBARS produced. Briefly, the colonic samples were homogenized in buffered saline (1:5) and then 800 μl of TCA (28% w/v) was added to 400 μl of this mixture and centrifuged in 3000 g for 30 min. Then, 600 μl of the supernatant was added to 150 μl of TBA (1% w/v). Then the mixture was incubated for 15 min in a boiling water bath and then 4 ml n-butanol was added, the solution was centrifuged, cooled and absorption of the supernatant was recorded in 532 nm by UV-160-A Shimadzu double beam spectrophotometer (Japan). The calibration curve of a 1,1,3,3-tetraethoxypropan standard solutions was used to determine the concentrations of TBARS that presented as μmol g−1 of colon (18).
Statistical analysis
Values are reported as mean ± SEM. Statistical significance between groups was computed by analysis of variance (ANOVA) and Tukey multiple comparison post hoc tests. P-values <0.05 were considered significant.
Results
Significant Macroscopic and Microscopic Changes in the Colon
Colon macroscopic and microscopic changes in control and Z. multiflora-treated groups are shown in Tables 2 and 3, respectively. Intrarectal instillation of acetic acid caused significant inflammatory reactions as indicated by macroscopic and microscopic changes (Tables 2 and 3). Z. multiflora-treated (600 and 900 p.p.m.) and prednisolone-treated groups showed significantly lower score values of macroscopic and microscopic characters when compared with the control group. Positive effects of Z. multiflora (900 p.p.m.) in acetic acid-induced colon macroscopic and microscopic alterations were comparable with that of prednisolone. Histology photographs of the colon sections from different treated groups are shown in Figs 1–6 that match well with macroscopic and microscopic scores (Tables 2 and 3).
Table 2.
Evaluation based on macroscopic features in acetic acid-induced colitis
| Group | Mean of macroscopic scores ± SEM |
|---|---|
| Control | 4.46 ± 0.57 |
| Prednisolone | 1.12 ± 0.14* |
| Z 400 | 4.23 ± 0.62 |
| Z 600 | 2.34 ± 0.49* |
| Z 900 | 1.11 ± 0.15*,† |
Observations were recorded by histopathologist and scored as described in Methods section. Each value represents mean of macroscopic scores ± SEM of six animals in each group.
*Significant (P < 0.01) decrease in macroscopic score values compared to control.
†The difference in macroscopic score values of Z. multiflora (Z)-treated and prednisolone-treated groups in not significant.
Table 3.
Evaluation based on microscopic features in acetic acid-induced colitis
| Group | Mean of microscopic scores ± SEM |
|---|---|
| Control | 23.72 ± 0.69 |
| Prednisolone | 5.53 ± 0.40* |
| Z 400 | 22.12 ± 0.58 |
| Z 600 | 12.1 ± 0.70* |
| Z 900 | 5.85 ± 0.39*,† |
The sections were all recorded by a histopathologist and a sign score between 0 and 3 for each sign and a total of 24 were used to determine the severity of colon inflammation. The signs are mentioned in the Methods section (10,17). Data are mean ± SEM of six observations.
*Significant (P < 0.01) decrease in microscopic score values compared to control.
†The difference in microscopic score values of Z. multiflora (Z)-treated and prednisolone-treated groups in not significant.
Figure 1.
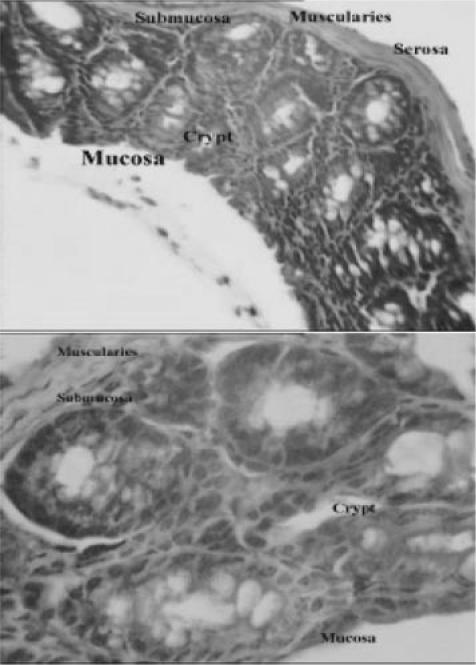
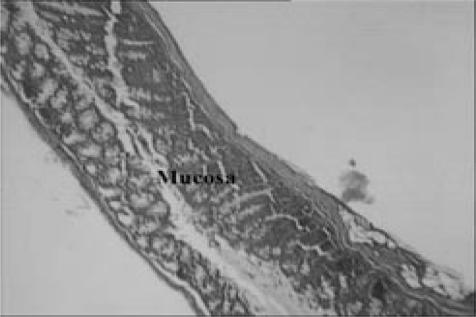
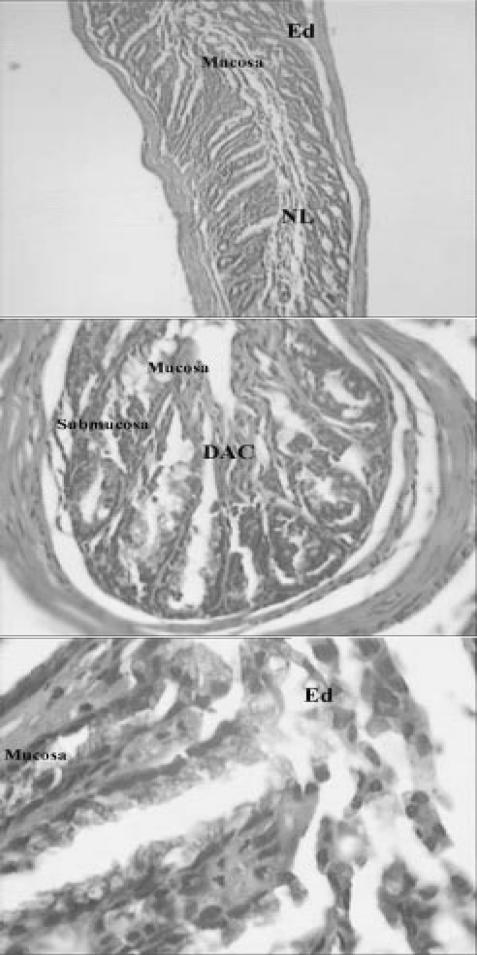
Light micrograph of bowel tissue from normal or untreated animals which did not receive any treatment. No major histological changes are apparent in micrograph. (H & E, ×40 and ×100).
Figure 2.
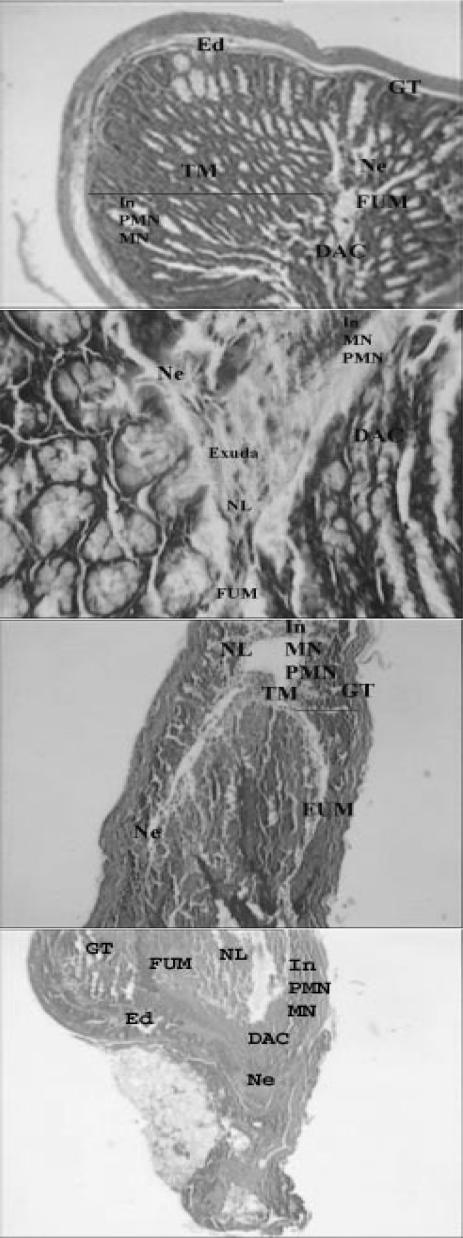
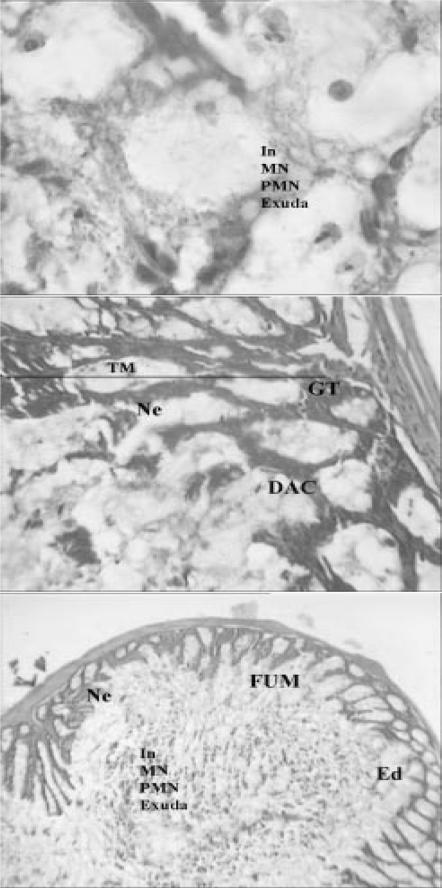
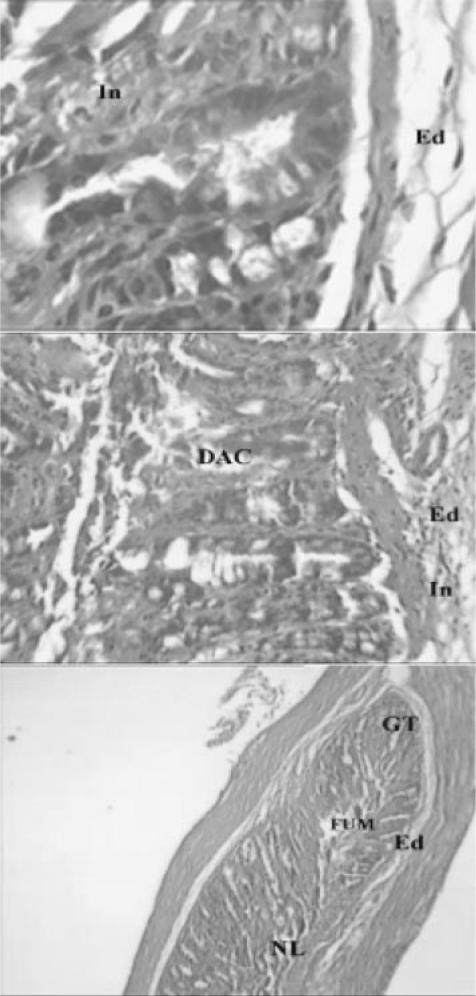
Light micrographs of bowel tissues from control mice received 0.1 ml of 6% acetic acid solution (once, intrarectally). Major histological changes are apparent in micrograph. Ed, edema; TM, thickened mucosa; GT, granulated tissue; In, inflammation; NL, narrowed lumen; Ne, necrosis; DAC, disrupted architecture of the crypt; PMN, polymorphonuclears; MN, mononuclear; FUM, focal ulceration of mucosa (H & E, ×& E, ×40 and ×100).
Figure 3.
Light micrographs of bowel tissues from prednisolone-treated group, which received prednisolone (1.14 mg kg−1 for 3 days) and acetic acid (0.1 ml, 6% solution, once, intrarectally). Minor histological changes are apparent (H & E, ×40 and ×100).
Figure 4.
Light micrograph of bowel tissue from Z. multiflora-treated animals, which received 7 days pretreatment with extract (400 p.p.m. in drinking water) and 0.1 ml of 6% acetic acid solution. Major histological changes are apparent in micrograph. Ed, edema; GT, granulated tissue; In, inflammation; Ne, necrosis; DAC, disrupted architecture of the crypt; PMN, polymorphonuclears; MN, mononuclear; TM, thickened mucosa (H & E, ×40 and ×100).
Figure 5.
Light micrograph of bowel tissue from Z. multiflora-treated animals, which received 7 days pretreatment with extract (600 p.p.m. in drinking water) and 0.1 ml of 6% acetic acid solution. Some histological changes are apparent. Ed, edema; In, inflammation; DAC, disrupted architecture of the crypt; NL, narrowed lumen; FUM, focal ulceration of mucosa; GT, granulated tissue (H & E, ×40 and ×100).
Figure 6.
Light micrograph of bowel tissue from Z. multiflora-treated animals, which received 7 days pretreatment with extract (900 p.p.m. in drinking water) and 0.1 ml of 6% acetic acid solution. Ed, edema; NL, narrowed lumen; DAC, disrupted architecture of the crypt. Minor histological changes are apparent (H & E, ×40 and ×100).
Lipid Peroxidation Revealed Significant Changes
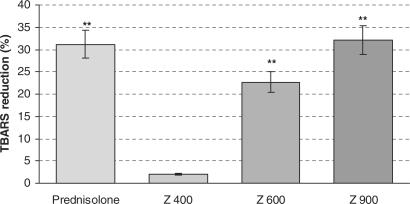
The changes in the extent of lipid peroxidation in bowel homogenate of treated animals is shown in Fig. 7. The TBARS level significantly increased in control group in comparison to normal group (3.14 versus 5.11 μmol g−1 colon). The TBARS level significantly decreased in Z. multiflora-treated (600 and 900 p.p.m.) and prednisolone-treated groups. The mean percentage of decreases of TBARS in Z. multiflora-treated (600 and 900 p.p.m.) and prednisolone-treated groups were 22.71, 32.14 and 31.2, respectively.
Figure 7.
Effects of Z. multiflora (Z) and prednisolone on lipid peroxidation in acetic acid-induced colitis. Each value represents mean ± SEM percentage of reduction of acetic acid-induced TBARS elevation by treated compounds. The mean ± SEM of TBARS level in normal and control groups were 3.14 ± 0.31 and 5.11 ± 0.50 (μmol g−1 colon), respectively.
Colon Myeloperoxidase Activity Changes
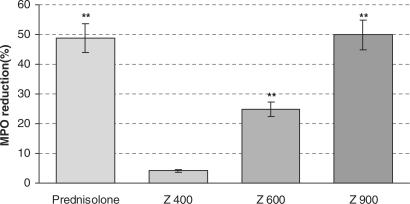
The changes in the activity of MPO in bowel homogenates of treated animals is shown in Fig. 8. The MPO activity of control group showed significant increase in comparison to normal group (2.89 versus 4.76 U g−1 colon). The MPO activity decreased in Z. multiflora-treated (600 and 900 p.p.m.) and prednisolone-treated groups compared to control group. The mean percentages of decreases of MPO activity in Z. multiflora-treated (600 and 900 p.p.m.) and prednisolone-treated groups were 24.9, 50, and 48.81, respectively.
Figure 8.
Effects of Z. multiflora (Z) and prednisolone on MPO activity in acetic acid-induced colitis. Each value represents mean ± SEM percentage of reduction of acetic acid-induced MPO elevation by treated compounds. The mean ± SEM of MPO activity in normal and control groups were 2.89 ± 0.17 and 4.76 ± 0.15 (U g−1 colon), respectively.
Discussion
The results showed that Z. multiflora has a good potential to suppress colitis in mice as indicated by the macroscopic, microscopic and biochemical evaluations. Biochemical assays well indicated that administration of Z. multiflora reduces MPO activity and TBARS concentrations, both of which are established indicators of oxidative stress and markers of colitis (8,11,19). Interestingly, Z. multiflora specially at dose of 900 p.p.m. was comparable with prednisolone which showed significant protection against acetic acid-induced colitis proved by biochemical, macroscopic and microscopic examinations. Acetic acid-induced colitis model in mice is similar to human ulcerative colitis in terms of histological features. It affects the distal colon portion and induces non-transmural inflammation, massive necrosis of mucosal and submucosal layers, mucosal edema, neutrophil infiltration of the mucosa and submucosal ulceration. The protonated form of the acid liberates protons within the intracellular space and causes a massive intracellular acidification resulting in massive epithelial damage. Inflammation is the pathogenesis of IBD and several pathways are associated with inflammatory response in IBD.
The inflammatory response initiated by acetic acid includes activation of cyclooxygenase and lipooxygenase pathways (20,22). Prednisolone as an anti-inflammatory agent decreases the recruitment of macrophages in the affected area and suppresses the synthesis of many inflammatory mediators, e.g. production of IL-1 from monocytes, production of IL-2 and tumor necrosis factor from lymphocytes. Prednisolone also inhibit the enzyme phospholipase A2 and thus decreases availability of prostaglandins and leukotrienes (23). Therefore, the first concept that may come to mind is that Z. multiflora possesses inhibitory effects on the synthesis or release of these inflammatory mediators. Supporting this idea, antinociceptive and anti-inflammatoty effects of the aqueous infusion, ethanolic maceration extract (24,25), the hydroalcoholic extract and the essential oil (26) of the aerial parts of Z. multiflora have been shown and are possibly due to the inhibition of cyclooxygenase enzymes (24). The composition of the essential oil of Z. multiflora was studied by GLC, column chromatography (CC), NMR and GLC/MS (27–30).
Zatarinal, β-sitosterol, stigmasterol, oleanolic acid, betulic acid, hexadecanoic, luteolin, α-tocophorolquinone and rosmarinic acid (RA) were reported as the composition of Z. multiflora essential oil. On the other hand, phytochemical screening of ethanolic extract of the plant supported the presence of flavonoids in Z. multiflora (31,32), mainly carvacrol, p-cymene, thymol, linalool and γ-terpinene (29). The luteolin, one of the compositions of Z. multiflora has shown inhibitory effects on TNF-α-induced IL-8 production in the intestinal epithelial cells. Interleukin IL-8 plays a central role in the initiation and maintenance of inflammatory responses in the IBD (33).
The RA another component of Z. multiflora essential oil has been reported to have antibacterial, antiviral, antioxidant and anti-inflammatory potentials (34–36). RA inhibits the adhesion molecule ICAM-1, which is primarily involved in interactions with β2 integrins during inflammatory responses, and inhibits the synthesis of eicosanoids, and oxidative DNA injury (36,37). Oxidative stress is believed to play a key role in the pathogenesis of IBD-related intestinal damage (8,9,38–42). As a matter of fact, intestinal mucosal damage in the IBD, including Crohn's disease and ulcerative colitis, is related to both increased free radical production and to a low concentration of endogenous antioxidant defense (43,44). Supporting this idea, the activity of MPO, which is a major enzyme in the formation of reactive oxygen species leading to tissue damage increased in this colitis model. Together with the membranous NADPH oxidase, MPO is involved in the formation of reactive oxygen species and oxidation of biological material. It produces not only oxidative equivalents, but contributes also to the regulation in general response to invading microorganisms (19). Increased TBARS as a marker of lipid peroxidation observed in association with higher MPO activity in this experimental colitis model again confirms the role of free radicals in IBD.
Flavonoids as the main constituent of Z. multiflora extract are a class of plant phenolics with significant antioxidant and chelating properties. Their positive effects come from their ability to inhibit lipid peroxidation, chelate redox-active metals and attenuating other procedure involving reactive oxygen species. From this point of view, there is a great interest in interactions between polyphenolic substance and the intestinal mucosa, including their possible role as modulators of proliferation and apoptosis in the rapidly proliferating cells of the gastrointestinal epithelia (45,46). Recent studies have shown that flavonoids exert a beneficial effect in experimental colon inflammation (47,48). In addition, the anti-inflammatory effects of flavonoids seem to be related to a decrease of neutrophils infiltration, absence of upregulation of IL-1β and decrease of prostaglandin production in colon mucosa (49). In supporting this idea, there is evidence that flavonoids have anti-phosphodiesterase activity and thus could elevate intracellular levels of cyclic nucleotides (50). Recent studies well indicate that both cAMP and cGMP can diminish oxidative stress in many biological systems and diseases (51–56). Therefore, the beneficial effects of Z. multiflora in IBD mostly back to its strong antioxidant potential.
On the other hand, GC-MS in the absolute of fresh plant showed that thymol is the major constituent while carvacrol is the major constituent in the absolute of dried plant (57). The Z. multiflora oil exhibited strong antimicrobial activity against Escherichia coli, Staphylococcus aureus and Salmonella typhimurium (58–60). In addition, both thymol and carvacrol in Z. multiflora essential oil exerted antibacterial and antifungal effects (61). Thus, antimicrobial activity of Z. multiflora can be another mechanism of action for its beneficial effects in IBD.
Conclusion
Taking collectively, the data provide very good evidence on benefit of Z. multiflora in experimental model of IBD. This effect supports the antioxidant, antimicrobial and anti-inflammatory potentials of Z. multiflora. Regarding the resemblance of acetic acid-induced colitis to human UC (62), it can be concluded that Z. multiflora extract may be useful in treating UC in humans. Proper clinical investigations should also be carried out to confirm the same activity in human disease. It should not be forgotten that there are a lot of herbal formulas in the Asia and Middle East regions that are used traditionally. However, little effort has been made so far to scientifically clarify the nature of such effects. Scientists from these regions of the world are responsible for confirming the pharmacological effects of these herbals by suitable animal tests. Many of them are capable entering clinical trials and deserve to be used as a drug in humans with minor adverse effects in comparison to synthetic compounds (63,64).
Acknowledgments
This study was carried out with financial support from Research Council of Tehran University of Medical Sciences.
References
- 1.Simmonds NJ, Rampton DS. Inflammatory bowel disease—a radical view. Gut. 1993;34:865–8. doi: 10.1136/gut.34.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 3.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaiee A. Pesticides and oxidative stress: a review. Med Sci Monit. 2004;10:RA144–7. [PubMed] [Google Scholar]
- 4.Nielsen OH, Ahnfelt-Ronne I. Involvement of oxygen-derived free radicals in the pathogenesis of chronic inflammatory bowel disease. Klin Wochenschr. 1991;69:995–1000. doi: 10.1007/BF01645145. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa DS, Cecchini REl, Kadri MZ, Rodriguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition. 2003;19:837–42. doi: 10.1016/s0899-9007(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 6.Hirayama A, Nagase S, Ueda A, Ishizu T, Taru Y, Yoh K, et al. Oxidative stress during leukocyte absorption apheresis. J Clin Apher. 2003;18:61–6. doi: 10.1002/jca.10054. [DOI] [PubMed] [Google Scholar]
- 7.Ahnfelt-Ronne I, Nielsen OH. The antiinflammatory moiety of sulfasalazine, 5-aminosalicylic acid, is a radical scavenger. Agents Actions. 1987;21:191–4. doi: 10.1007/BF01974941. [DOI] [PubMed] [Google Scholar]
- 8.Jahanshahi G, Motavasel V, Rezaie A, Hashtroudi AA, Daryani NE, Abdollahi M. Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases. Dig Dis Sci. 2004;49:1752–7. doi: 10.1007/s10620-004-9564-5. [DOI] [PubMed] [Google Scholar]
- 9.D'Odorico A, Bortolan S, Cardin R, D'Inca' R, Martines D, Ferronato A, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol. 2001;36:1289–94. doi: 10.1080/003655201317097146. [DOI] [PubMed] [Google Scholar]
- 10.Krawisz JE, Sharon P, Stenson WF. Qualitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 11.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Perez M, Rabanal RM, de la Torre MC, Rodriguez B. Analgesic, anti-inflammatory, antipyretic and haematological effects of aethiopinone, an o-naphthoquinone diterpenoid from Salvia aethiopis roots and two hemisynthetic derivatives. Planta Med. 1995;61:505–9. doi: 10.1055/s-2006-959358. [DOI] [PubMed] [Google Scholar]
- 13.Cuppett SL, Hall CA., 3rd Antioxidant activity of the Labiatae. Adv Food Nutr Res. 1998;42:245–71. doi: 10.1016/s1043-4526(08)60097-2. [DOI] [PubMed] [Google Scholar]
- 14.Wasser S, Ho JM, Ang HK, Tan CE. Salvia miltiorrhiza reduces experimentally-induced hepatic fibrosis in rats. J Hepatol. 1998;29:760–71. doi: 10.1016/s0168-8278(98)80257-2. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Haddad Khodaparast MH, Shokohizadeh H. Antihyperglycemic effect of Saliva leriifolia leaf and seed extract in mice. Irn J Med Sci. 1998;23:78–80. [Google Scholar]
- 16.Zargari A. Medicinal Plants. 4th edn. Tehran: Tehran University Press; 1990. pp. 1–57. [Google Scholar]
- 17.Jagtap AG, Shirke SS, Phadke AS. Effect of polyherbal formulation on experimental models of inflammatory bowel diseases. J Ethnopharmacol. 2004;90:195–204. doi: 10.1016/j.jep.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Satho K. Serum lipid peroxidation in cerebrovascular disorders determined by a new colorimetric method. Clin Chem Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 19.Arnhold J. Properties, functions, and secretion of human myeloperoxidase. Biochemistry (Mosc) 2004;69:4–9. doi: 10.1023/b:biry.0000016344.59411.ee. [DOI] [PubMed] [Google Scholar]
- 20.Sharon P, Stenson WF. Metabolism of arachidonic acid in acetic acid colitis in rats. Gastroenteroloogy. 1985;88:55–63. doi: 10.1016/s0016-5085(85)80132-3. [DOI] [PubMed] [Google Scholar]
- 21.Elson CO, Cong Y, Brandwein S, Weaver CT, McCabe RP, Mahler M, et al. Experimental models to study molecular mechanisms underlying intestinal inflammation. Ann N Y Acad Sci. 1998;859:85–95. doi: 10.1111/j.1749-6632.1998.tb11113.x. [DOI] [PubMed] [Google Scholar]
- 22.MacPherson B, Pfeiffer CJ. Experimental colitis. Digestion. 1976;14:424–52. doi: 10.1159/000197966. [DOI] [PubMed] [Google Scholar]
- 23.Katzung BG. Basic & Clinical Pharmacology. 8th edn. New York. Appleton & Lange; 2000. [Google Scholar]
- 24.Hosseinzadeh H, Ramezani M, Salmani GA. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73:379–85. doi: 10.1016/s0378-8741(00)00238-5. [DOI] [PubMed] [Google Scholar]
- 25.Ramezani M, Hosseinzadeh H, Samizadeh S. Antinociceptive effects of Zataria multiflora Boiss fractions in mice. J Ethnopharmacol. 2004;91:167–70. doi: 10.1016/j.jep.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Jaffary F, Ghannadi A, Siahpoush A. Antinociceptive effects of hydroalcoholic extract and essential oil of Zataria multiflora. Fitoterapia. 2004;75:217–20. doi: 10.1016/j.fitote.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Shafiee A, Javidnia K. Composition of essential oil of Zataria multiflora. Planta Med. 1997;63:371–2. doi: 10.1055/s-2006-957707. [DOI] [PubMed] [Google Scholar]
- 28.Ebrahimzadeh H, Yamini Y, Sefidkon F, Chaloosi M, Pourmortazavi SM. Chemical composition of the essential oil and supercritical CO2 extracts of Zataria multiflora Boiss. Food Chem. 2003;83:357–61. [Google Scholar]
- 29.Mohagheghzadeh A, Shams-Ardakani M, Ghannadi A. Volatile constituents of callus and flower-bearing tops of Zataria multiflora Boiss (Lamiaceae) Flavour Fragr J. 2000;15:373–6. [Google Scholar]
- 30.Shaiq Ali M, Jahangir M, Saleem M, Uddin Ahmad V. Chemical constituents of Pulicaria gnaphalodes. Nat Prod Sci. 1999;5:134–7. [Google Scholar]
- 31.Martinez-Vazquez M, Ramirez Apan TO, Aguilar H, Bye R. Analgesic and antipyretic activities of an aqueous extract and of the flavone linarin of Buddleia cordata. Planta Med. 1996;62:137–40. doi: 10.1055/s-2006-957836. [DOI] [PubMed] [Google Scholar]
- 32.Ramesh M, Rao YN, Rao AV, Prabhakar MC, Rao CS, Muralidhar N, et al. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol. 1998;62:63–6. doi: 10.1016/s0378-8741(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 33.Kim JA, Kim DK, Kang OH, Choi YA, Park HJ, Choi SC, et al. Inhibitory effect of luteolin on TNF-alpha-induced IL-8 production in human colon epithelial cells. Int Immunopharmacol. 2005;5:209–17. doi: 10.1016/j.intimp.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Parnham MJ, Kesselring K. Rosmarinic acid. Drugs Future. 1985;10:756–7. [Google Scholar]
- 35.Mohagheghzadeh A, Shams-Ardakani M, Ghannadi A, Minaeian M. Rosmarinic acid from Zataria multiflora tops and in vitro cultures. Fitoterapia. 2004;75:315–21. doi: 10.1016/j.fitote.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–57. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 37.Staunton DE, Marlin SD, Stratowa C, Dustin ML, Springer TA. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988;52:925–33. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- 38.Tuzun A, Erdil A, Inal V, Aydin A, Bagci S, Yesilova Z, et al. Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–72. doi: 10.1016/s0009-9120(02)00361-2. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–41. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–61. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 41.Allgayer H. Clinical relevance of oxygen radicals in inflammatory bowel disease—facts and fashion. Klin Wochenschr. 1991;69:1001–3. doi: 10.1007/BF01645146. [DOI] [PubMed] [Google Scholar]
- 42.Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- 43.Koutroubakis IE, Malliaraki N, Dimoulios PD, Karmiris K, Castanas E, Kouroumalis EA. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci. 2004;49:1433–7. doi: 10.1023/b:ddas.0000042242.22898.d9. [DOI] [PubMed] [Google Scholar]
- 44.Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, et al. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- 45.Le Marchand L. Cancer preventive effects of flavonoids—a review. Biomed Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 46.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–67S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez de Medina F, Vera B, Galvez J, Zarzuelo A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097–108. doi: 10.1016/s0024-3205(02)01568-0. [DOI] [PubMed] [Google Scholar]
- 48.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–71. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 49.Villegas I, Alarcon de la Lastra C, Orjales A, La Casa C. A new flavonoid derivative, dosmalfate, attenuates the development of dextran sulphate sodium-induced colitis in mice. Int Immunopharmacol. 2003;l3:1731–41. doi: 10.1016/j.intimp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Abdollahi M, Chan TS, Subrahmanyam V, O'Brien PJ. Effects of phosphodiesterase 3,4,5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and susceptibility to a mitochondrial toxin. Mol Cell Biochem. 2003;252:205–11. doi: 10.1023/a:1025568714217. [DOI] [PubMed] [Google Scholar]
- 51.Abdollahi M, Bahreini-Moghadam A, Emami B, Fooladian F, Zafari K. Increasing intracellular cAMP and cGMP inhibits cadmium-induced oxidative stress in rat submandibular saliva. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135:331–6. doi: 10.1016/s1532-0456(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 52.Abdollahi M, Fooladian F, Emami B, Zafari K, Bahreini-Moghadam A. Protection by sildenafil and theophylline of lead acetate-induced oxidative stress in rat submandibular gland and saliva. Hum Exp Toxicol. 2003;22:587–92. doi: 10.1191/0960327103ht399oa. [DOI] [PubMed] [Google Scholar]
- 53.Aghababaeian R, Ghazi-Khansari M, Abdi K, Taghadosinejad F, Abdollahi M. Protective effects of sildenafil and dipyridamol from lead-induced lipid peroxidation in perfused rat liver. Intl J Pharmacol. 2005;1:157–60. [Google Scholar]
- 54.Milani E, Nikfar S, Khorasani R, Zamani MJ, Abdollahi M. Reduction of diabetes-induced oxidative stress by phosphodiesterase inhibitors in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:251–5. doi: 10.1016/j.cca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Radfar M, Larijani B, Hadjibabaie M, Rajabipour B, Mojtahedi A, Abdollahi M. Effects of pentoxifylline on oxidative stress and levels of EGF and NO in blood of diabetic type-2 patients; a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother. 2005;59:302–6. doi: 10.1016/j.biopha.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Zamani MJ, Sharifzadeh M, Rezaie A, Mashayekhi F, Abdollahi M. Effects of sildenafil on rat irritable bowel syndrome. Therapy. 2005;2:237–42. [Google Scholar]
- 57.Saleem M, Nazli R, Afza N, Sami A, Ali MS. Biological significance of essential oil of Zataria multiflora boiss. Nat Prod Res. 2004;18:493–7. doi: 10.1080/14786410310001608064. [DOI] [PubMed] [Google Scholar]
- 58.Rasooli I, Rezaei MB. Bioactivity and chemical properties of essential oils from Zataria multiflora Boiss and Mentha longifolia (L.) Huds. J Essent Oil Res. 2002;14:141–6. [Google Scholar]
- 59.Basti AA, Razavilar V, Misaghi A, Radmehr B, Abbasifar R, Yazdani D, et al. Effect of Zataria multiflora Boiss. essential oil on probability of growth initiation of Staphylococcus aureus in a brain heart infusion broth. J Med Plants. 2004;3:53–60. [Google Scholar]
- 60.Basti AA, Razavilar V, Abbasifar R, Radmehr B, Misaghi A, Khalighi Sigaroodi F. Effect of Zataria multiflora Boiss essential oil on probability of growth initiation of Salmonella typhimurium in a brain heart infusion broth. J Med Plants. 2004;3:85–92. [Google Scholar]
- 61.Shafiee A, Javidnia K, Tabatabai M. Volatile constituents and antimicrobial activity of Zataria multiflora, population Iran. Irn J Chem Chem Eng. 1999;18:1–5. [Google Scholar]
- 62.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Kiyohara H, Matsumoto T, Yamada H. Combination effects of herbs in a multi-herbal formula: expression of Juzen-taiho-to's immuno-modulatory activity on the intestinal immune system. Evid Based Complement Alternat Med. 2004;1:83–91. doi: 10.1093/ecam/neh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hadjibabaie M, Rastkari N, Rezaie A, Abdollahi M. The adverse drug reaction in the gastrointestinal tract: an overview. Intl J Pharmacol. 2005;1:1–8. [Google Scholar]