Abstract
The space within the great pyramid and its smaller replicas is believed to have an antistress effect. Research has shown that the energy field within the pyramid can protect the hippocampal neurons of mice from stress-induced atrophy and also reduce neuroendocrine stress, oxidative stress and increase antioxidant defence in rats. In this study, we have, for the first time, attempted to study the antistress effects of pyramid exposure on the status of cortisol level, oxidative damage and antioxidant status in rats during chronic restraint stress. Adult female Wistar rats were divided into four groups as follows: normal controls (NC) housed in home cage and left in the laboratory; restrained rats (with three subgroups) subject to chronic restraint stress by placing in a wire mesh restrainer for 6 h per day for 14 days, the restrained controls (RC) having their restrainers kept in the laboratory; restrained pyramid rats (RP) being kept in the pyramid; and restrained square box rats (RS) in the square box during the period of restraint stress everyday. Erythrocyte malondialdehyde (MDA) and plasma cortisol levels were significantly increased and erythrocyte-reduced glutathione (GSH) levels, erythrocyte glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activities were significantly decreased in RC and RS rats as compared to NC. However, these parameters were maintained to near normal levels in RP rats which showed significantly decreased erythrocyte MDA and plasma cortisol and significantly increased erythrocyte GSH levels, erythrocyte GSH-Px and SOD activities when compared with RS rats. The results showed that housing in pyramid counteracts neuroendocrine and oxidative stress caused by chronic restraint in rats.
Keywords: chronic restraint, glutathione, glutathione peroxidase, pyramid model, superoxide dismutase, TBARS
Introduction
Pyramid research to date reveals some evidence that the space within the great pyramid and its smaller replicas enhances, intensifies and/or generates energy of the electromagnetic spectrum and other forms or degrees of the so-called universal energy (1). The effect of this ‘pyramid energy’ has been studied on solids, liquids, plants, animals and even human volunteers. Some of the findings of such studies include rapid growth of plants, faster healing of bruises and burns, longer preservation of milk, and increased vitalization and better relaxation in human subjects (2). Claims of ‘pyramid energy’ promoting relaxation gain a lot of significance in this age of civilization and modernization which have made stressful life inevitable. In the competitive world of modern technology, mental and emotional stress has become an unavoidable part of life and is known to have deleterious effects on physical and mental well-being.
It is well known that stress causes an increase in glucocorticoid levels. High levels of glucocorticoids are known to decrease blood reduced glutathione (GSH) and erythrocyte superoxide dismutase (SOD) activity in rats (3). Emotional stress is known to increase the plasma levels of malondialdehyde (MDA), and the plasma levels of MDA have been reported to directly correlate with the severity of emotional stress in human beings (4). MDA is a product of reactive oxygen species (ROS)-mediated damage to the polyunsaturated fatty acids. ROS are known to cause oxidative damage to macromolecules (5). Chemical compounds and reactions capable of generating potential toxic oxygen species/free radicals are referred to as prooxidants. On the other hand, compounds and reactions disposing off these species, scavenging them, suppressing their formation or opposing their actions are called as antioxidants. Some of the important antioxidants include GSH, glutathione peroxidase (GSH-Px) and SOD. In a normal cell, there is an appropriate prooxidant: antioxidant balance. However, this balance can be shifted towards the prooxidant when production of ROS is increased or when levels of antioxidants are diminished. This state is called oxidative stress and can result in serious cell damage if the stress is massive or prolonged. Oxidative stress is implicated in the etiopathogenesis of a variety of human diseases (6–9).
Although claims based on observation and experience are many to support the role of pyramid exposure in coping with stress, scientific studies confirming the same are few. Research has shown that the energy field within the pyramid can act as antistressor and thus protect the hippocampal neurons from stress-induced atrophy (10). Our earlier studies have reported that exposure of adult female Wistar rats to pyramid environment reduces stress, oxidative stress and increases antioxidant defence in them (11). In this study, we have for the first time attempted to study the antistress effects of pyramid exposure on the status of cortisol level, oxidative damage and antioxidant status in adult female Wistar rats during chronic restraint stress.
Materials and Methods
Rats
Adult female Wistar albino rats (aged 3–4 months) weighing 130–220 g were used for the studies. Standard pelleted food and water was provided ad libitum to all the groups. Proper ventilation was provided. Standard hygienic conditions were maintained in the animal house and the rats were exposed to proper light and dark cycle (12 h each of light and darkness). The experimental protocol was approved by the Institutional Animal Ethics Committee.
Experimental Design
Rats were divided into four groups as follows.
Normal Control Group (NC)
This group of rats was maintained under standard laboratory conditions in their home cages for 14 days.
Restrained Control Group (RC)
This group of rats was subject to chronic restraint stress by placing in the wire mesh restrainer for 6 h per day for 14 days. The restrainer was maintained under normal laboratory conditions.
Restrained Rats kept in Pyramid (RP)
This group of rats was subject to chronic restraint stress by placing in a wire mesh restrainer for 6 h per day for 14 days and simultaneously housed in the wooden pyramid.
Restrained Rats kept in Square Box (RS)
This group of rats was subject to restraint stress by placing in a wire mesh restrainer for 6 h per day for 14 days and simultaneously housed in the wooden square box.
Blood samples were collected from the rats on the 15th day between 9.00 and 10.00 a.m. The blood obtained was centrifuged to separate the plasma and cells. Packed red cells were washed three times in ice-cold phosphate-buffered saline (PBS-phosphate buffer 0.01 M, pH 7.4, containing 0.15 M NaCl) and used for the estimation of thiobarbituric acid reactive substances (TBARS) and reduced GSH levels. An equal volume suspension of erythrocytes in PBS was prepared and used for assay of GSH-Px activity and SOD activity. Plasma was used for the estimation of cortisol.
Pyramid and Square Box Model Design
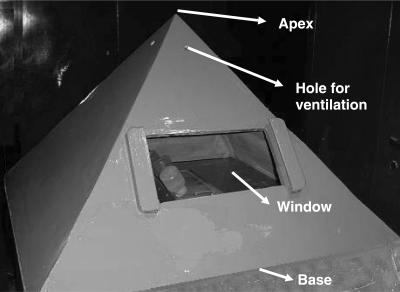
Since conducting materials like metals are believed to block the electromagnetic forces owing to their tendency to absorb the energy (12), wood was chosen as the pyramid material. A wooden pyramid model of 30 in. height, 45 in. base and 41.5 in. side (13) fabricated locally was used for the study. Holes were provided for ventilation. A small glass window was provided for observation. The four triangular sides of the pyramid angled upwards at nearly 51° to the base and met at the apex of the pyramid. The pyramid rested on a wooden base (Fig. 1).
Figure 1.
Pyramid model housing stressed rats.
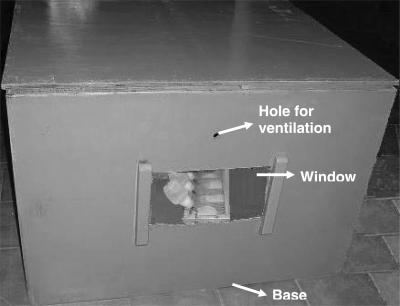
A wooden square box of 30 in. height and 45 in. base fabricated locally, with all other provisions as in the case of pyramid mentioned above was used as a shape control, to ensure that the beneficial effect of pyramid exposure, if seen, was due to its shape and not mere enclosure (Fig. 2).
Figure 2.
Square box model housing stressed rats.
Procedure of Exposure of the Rats Within the Pyramid/Square Box
The pyramid/square box was aligned on the four cardinal points—north, east, south and west as specified by Schul and Pettit (12) using a standard magnetic compass. Only one polypropylene cage each, with three to four rats, was placed in the pyramid and square box at any given time. The cage was placed such that its long axis was in the magnetic north–south with the food and water in the north. Maximum effect of the pyramid is believed to be exerted at one-third the height of the pyramid from its base. Therefore, a wooden stool each, of 10 in. height, was placed in the pyramid and the square box and the cage was kept on this stool.
Dimensions of the Wire Mesh Restrainer and Procedure of Restraint Stress Induction
A wire mesh restrainer was fabricated locally with a PVC box having four individual compartments, one for each rat. Each compartment had a length of 15 cm, breadth of 7 cm and height of 7 cm (Fig. 3) (14). The compartments had a common sliding wire mesh roof. The wire mesh roof had four holes, one corresponding to each compartment, through which water was provided to the rats at all times. Each compartment had a bedding of as much paddy husk as was required to restrict the movement of the rat within its compartment. Food was also provided to the rats at all times on the bedding on the same side that contained water. Although most researchers deprive the rats of food and water during the stress period, we provided the same even during restraint stress because food deprivation is known to exacerbate oxidative stress in rat liver mitochondria (15). Food provision during restraint stress has been adopted by Harris et al. (16) who have reported that food intake was not inhibited during restraint in rats fed during the day. Interestingly, in our study also, it was seen that rats were freely accessing food and water even during restraint stress.
Figure 3.
Rats under stress in a restrainer.
The home cage of NC rats and the restrainer with the RC group of rats was left in the open in the procedure room while the restrainer containing the restrained pyramid (RP) and restrained square box (RS) group of rats were respectively placed in the pyramid and square box such that the side containing water and food was in the magnetic north. This ensured that the long axis of each compartment was aligned in magnetic north–south. At the end of 6 h every day, the RC, RP and RS group of rats were relieved from restraint stress by removing from the restrainer and housing in their respective home cages.
Estimation of Plasma Cortisol Levels
Since it is well known that stress mediates its actions through the HPA axis and is consequently marked by a general increase in cortisol secretion, this parameter was estimated as a marker of neuroendocrine stress. Plasma (1 ml) was mixed with 7.5 ml methylene dichloride and centrifuged to separate plasma and methylene dichloride layer. The lower methylene dichloride layer was used for cortisol estimation (17). A blank containing 1 ml water and a standard containing 1 ml of cortisol were included in the assay. Five milliliters of the extract was then mixed with 2.5 ml fluorescence reagent containing 70% concentrated H2SO4 and 30% absolute ethanol. The fluorescence of the lower layer of acid extract was measured at 530 nm at exactly 12 min after adding the fluorescence reagent, with excitation wavelength 470 nm. Cortisol concentration was expressed as nmol l−1 of plasma.
Estimation of Erythrocyte Membrane Lipid Peroxidation
Increased levels of ROS during oxidative stress attack the polyunsaturated fatty acids of membranes to release cytotoxic aldehydes like MDA, which hence serves as a good indicator of the oxidative stress status. Packed red cells (0.2 ml) were used for the estimation of MDA as TBARS (18). The absorbance at 600 nm was subtracted from absorbance at 532 nm. TBARS was expressed as nmol g−1 hemoglobin. Hemoglobin concentration was measured by cyanmethemoglobin method of Drabkin (19).
Estimation of Reduced GSH
Reduced GSH, which is the major cellular antioxidant playing a central role in coordinating body's antioxidant defense processes, was measured in rat erythrocytes by the method of Beutler et al. (20). Packed red cells (0.2 ml) were used in the assay. The GSH was made to react with 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) which reacts with sulfhydryl groups, to develop a stable color. The absorbance was measured at 412 nm. GSH content was expressed as mg g−1 hemoglobin.
Assay of GSH-Px Activity
GSH-Px is an antioxidant enzyme found in cytosol as well as mitochondria of mammalian cells and is effective in scavenging free radicals and detoxifying H2O2. It was estimated by the method of Paglia and Valentine (21). The erythrocyte suspension in PBS was used for the assay. The erythrocytes were hemolysed with water and all the hemoglobin in the lysate was converted to stable cyanmethemoglobin by adding Drabkin's reagent. This hemolysate was used for the assay (21). The rate of oxidation of GSH by H2O2, catalyzed by GSH-Px was measured. The rate of formation of oxidized glutathione (GSSG) was measured by following the decrease in absorbance of the reaction mixture at 340 nm as NADPH is converted to NADP+. Non-enzymatic oxidation of GSH was measured in a simultaneous assay system, without the hemolysate. The difference between the two systems gave the enzyme activity, which was expressed as units g−1 of hemoglobin where units represented the number of micromoles of NADPH oxidized per minute in the reaction mixture.
Assay of SOD Activity
SOD is an antioxidant enzyme that scavenges one of the ROS, i.e. superoxide radicals, by converting them to hydrogen peroxide which is then detoxified by GSH-Px. Inhibition of the reduction of nitro blue tetrazolium (NBT) by superoxide radicals, generated by the illumination of riboflavin in the presence of oxygen and the electron donor methionine, was used as the basis for the assay of SOD (22). A chloroform ethanol extract was prepared from the hemolysates and the clear supernatant obtained was used for the assay. The reaction mixture was illuminated for 10 min. The absorbance was then read at 560 nm. Controls with and without NBT were always included in the assay. One unit of SOD activity was taken as that producing 50% inhibition of NBT reduction. Values were expressed as units of enzyme activity per gram hemoglobin.
Statistical Analysis
All statistical analysis were done using one-way analysis of variance (ANOVA) in the Graph Pad Instat (GPIS) package.
Results
The results for the various parameters are shown in Figs 4–8.
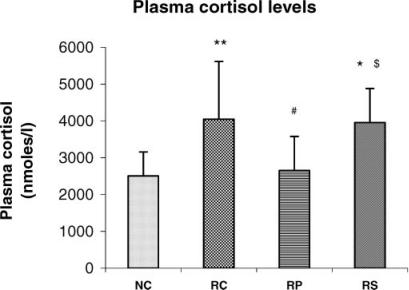
Figure 4.
Antistress effect of pyramid exposure on plasma cortisol levels in adult female Wistar rats in comparison with controls. NC, normal control (n = 13); RC, restrained control (n = 13); RP, restrained in pyramid (n = 13); RS, restrained in square box (n = 13). Each bar represents mean + SD. **P < 0.01, *P < 0.05 versus NC; #P < 0.05 versus RC; $P < 0.05 versus RP (ANOVA).
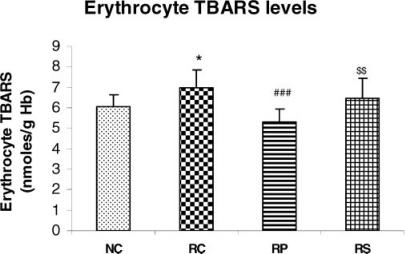
Figure 5.
Antistress effect of pyramid exposure on erythrocyte TBARS levels in adult female Wistar rats in comparison with controls. NC, normal control (n = 13); RC, restrained control (n = 13); RP, restrained in pyramid (n = 13); RS, restrained in square box (n = 13). Each bar represents mean + SD. *P < 0.05 versus NC; ###P < 0.001 versus RC; $$P < 0.01 versus RP (ANOVA).
Figure 6.
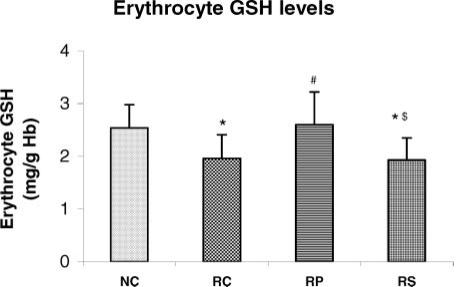
Antistress effect of pyramid exposure on erythrocyte GSH levels in adult female Wistar rats in comparison with controls. NC, normal control (n = 13); RC, restrained control (n = 13); RP, restrained in pyramid (n = 13); RS, restrained in square box (n = 13). Each bar represents mean + SD. *P < 0.05 versus NC; #P < 0.05 versus RC; $P < 0.05 versus RP (ANOVA).
Figure 7.
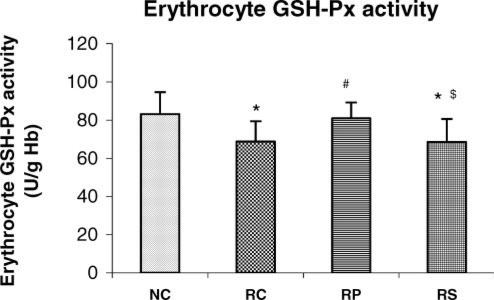
Antistress effect of pyramid exposure on erythrocyte GSH-Px activity in adult female Wistar rats in comparison with controls. NC, normal control (n = 13); RC, restrained control (n = 13); RP, restrained in pyramid (n = 13); RS, restrained in square box (n = 13). Each bar represents mean + SD. *P < 0.05 versus NC; #P < 0.05 versus RC; $P < 0.05 versus RP (ANOVA).
Figure 8.
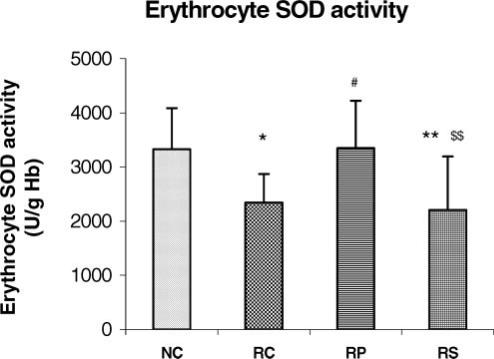
Antistress effect of pyramid exposure on erythrocyte SOD activity in adult female Wistar rats in comparison with controls. NC, normal control (n = 13); RC, restrained control (n = 13); RP, restrained in pyramid (n = 13); RS, restrained in square box (n = 13). Each bar represents mean + SD. **P < 0.01, *P < 0.05 versus NC; #P < 0.05 versus RC; $$P < 0.01 versus RP (ANOVA).
Restraint-induced increase in plasma cortisol levels is counteracted by pyramid exposure
Chronic restraint stress significantly increased plasma cortisol level in comparison with NC (P < 0.01 for RC versus NC, P < 0.05 for RS versus NC). However, the mean value of the RP rats for plasma cortisol was very close to that of NC group in magnitude and was significantly lower than that of the RC group (P < 0.05). The RS rats had significantly higher plasma cortisol when compared with RP rats (P < 0.05) and the mean was almost equal to that of the RC rats. Correlation was not statistically significant between plasma cortisol and parameters of oxidative stress in any of the groups.
Erythrocyte membrane lipid peroxidation increases during chronic restraint but not in the pyramid
Erythrocyte TBARS levels were higher in the RC rats (P < 0.05) and RS rats as compared to NC rats indicating that chronic restraint stress significantly increased lipid peroxidation and this could not be reversed by square box exposure. The mean values for erythrocyte TBARS in RP rats and NC rats were not significantly different but the RS rats had significantly higher erythrocyte TBARS when compared with RP rats (P < 0.01). The RP rats had significantly lower erythrocyte TBARS than the RC rats (P < 0.001).
Decrease in Erythrocyte Antioxidants due to Chronic Restraint is Counteracted by Pyramid Exposure
When compared with NC rats, the RC and RS rats had significantly lower erythrocyte GSH level and GSH-Px as well as SOD activities (P < 0.05 for all parameters in both groups, P < 0.01 in RS rats for SOD). There was no significant difference in these three parameters between NC and RP rats but a significant increase was seen in RP rats when compared with RC rats (P < 0.05 for GSH, P < 0.05 for both enzyme activities) suggesting that pyramid exposure maintained the erythrocyte antioxidant defence status during chronic restraint stress. Erythrocyte GSH level (P < 0.05) as well as erythrocyte GSH-Px (P < 0.05) and SOD (P < 0.01) activities were significantly lower in RS group as compared to RP group and were almost similar to the RC group.
Discussion
Reversal of Stress-Induced Increase in Plasma Cortisol Levels Inside the Pyramid
It has been suggested that chronic stress exerts a stimulatory influence at the hypothalamic level, by increasing the corticotropin releasing factor (CRF) mRNA levels in the paraventricular nucleus of the hypothalamus (23). Increased CRF release contributes to the dysregulation of corticosterone secretion (24). Chronic stress also causes an increase in the ACTH secreting mechanism (25) and hence an increase in plasma cortisol. Although it is expected that corticosteroid feedback reduces CRF mRNA levels, Lightman and Harbuz (26) have opined that this occurs in a dose-dependent manner, that both physical and psychological stressors produce a robust and readily reproducible increase in CRF mRNA and that these responses cannot be prevented by changes in circulating corticosteroids. It can be contended that chronic restraint stress in this study activated the HPA axis, thus resulting in increased plasma cortisol and possibly by mechanisms mentioned by the above researchers, plasma cortisol remained elevated even on the 15th day. Pyramid exposure during chronic restraint stress prevented stress-induced hypercortisolemia. This prevention was not achieved by square box enclosure, the RS rats having plasma cortisol levels comparable with those of RC rats. These results indicate that the shape of the pyramid acts as an antistressor and reverses the effect of chronic restraint stress on the hormonal level.
Research has shown that the energy field within the pyramid can act as antistressor and thus protect the hippocampal neurons from stress-induced atrophy (10). Our earlier studies have reported that exposure of adult female Wistar rats to pyramid environment reduces stress, oxidative stress and increases antioxidant defence in them (11). Pyramid exposure is believed to put the mind into an alpha state (27). EEG from subjects meditating in pyramids has shown higher frequency and higher amplitude alpha waves (28). A number of volunteers have stated that meditating inside the pyramid was easier than meditating outside as they felt more peaceful, more relaxed and less distracted (29,30). Thus, the pyramid, by promoting relaxation and fostering non-reactivity of the mind, probably by putting the mind into an alpha state, decreased the subjective experience of stress in RP rats.
Stress-Induced Increase in Erythrocyte Membrane Lipid Peroxidation Minimized Inside the Pyramid
Chronic stress is known to increase lipid peroxidation in the plasma, hippocampus and intestinal mucosa of rats (31–33) and in the brain of mice (34). Previous workers have reported an increased ROS production in the CNS during chronic restraint stress (35–40). While some workers have attributed this to the increase in glucocorticoids (35–37,39), others have explained it as an effect of increased glutamate release caused by chronic restraint. Thus, in this study, chronic restraint stress could have increased extracellular glutamate leading to increased oxidative stress in the CNS of RC rats. Associated with these changes, the oxidative stress in RBCs was increased, the circulating red cells being mobile free radical scavengers (41). Thus, the RC group of rats had significantly higher erythrocyte TBARS as compared to NC.
The lack of correlation between plasma cortisol and parameters of oxidative stress may suggest that the above process may be cortisol independent. Like plasma cortisol, erythrocyte TBARS of RP rats matched that of the NC suggesting that even though these rats were under chronic restraint stress, housing in the pyramid protected them from its deleterious effects. Although stress is known to increase lipid peroxidation, pyramid exposure checked this increase by preventing the increase of plasma cortisol and probably attenuating the glucocorticoid-mediated increase in glutamate and its excitotoxicity, which is known to occur during stress. By thus probably attenuating stress-induced oxidative stress in the CNS, it may also have attenuated oxidative stress in the RBCs, as reflected by the significantly decreased erythrocyte TBARS in the pyramid-exposed rats when compared with RC and RS rats.
Decrease in Erythrocyte Antioxidants due to Chronic Restraint Counteracted by Pyramid Exposure
May et al. (42) have reported that erythrocytes may contribute to the antioxidant capacity of blood by regenerating ascorbate from dehydroascorbate primarily by using GSH. So, it can be said that the increased usage of GSH to prevent lipid peroxidation and to regenerate the reduced forms of vitamins C and E which are oxidized during their effort to terminate lipid peroxidation may lead to lower levels of this compound in the erythrocytes of RC rats. GSH-Px, an important part of the cellular antioxidant defence system that detoxifies H2O2 and also organic hydroperoxides, has been reported to be decreased in the hippocampus, cerebral cortex, cerebellum and liver tissues in glucocorticoid-treated rats (43). Decreased GSH-Px activity due to immobilization stress has been reported in the interstitial compartment of rat testicular tissue (44). Thus, immobilization caused by chronic restraint stress and also the increased plasma cortisol in the RC rats of our study may have decreased the GSH-Px activity in them.
Chronic restraint stress significantly decreased erythrocyte SOD activity when compared with NC. Although previous workers have reported an increase in erythrocyte SOD activity in repeated restraint stress (45) and in immobilization stress (46), abundant literature is available to say that chronic stress causes a decrease in SOD activity in various tissues. Singh et al. (47) have reported that chronic swim stress caused a decrease in the brain SOD activity in mice. Long-term restraint stress decreased the activities of both manganese SOD and Cu, Zn-SOD isoforms in the dentate gyrus of rats (48). It is well known that Cu, Zn-SOD is inactivated by H2O2 and subsequently undergoes proteolytic fragmentation (49). Thus, it can be said that inefficient removal of H2O2 in the RC rats, due to decreased GSH level and GSH-Px activity, may be causing inactivation of SOD in erythrocytes of chronically stressed rats. The pyramid exposure-mediated decrease in hypercortisolemia and hence lipid peroxidation, on the other hand, could have resulted in decreased use of reduced GSH and the reduced forms of vitamin C and vitamin E to terminate lipid peroxidation.
The abundant pool of GSH levels in the erythrocytes of pyramid-exposed rats could have maintained erythrocyte GSH-Px activity as reflected by the significantly higher activity of the enzyme in RP rats in comparison with RC rats, because previous workers have reported a positive correlation between GSH levels and GSH-Px activity in epidermis of normal rats (50) and in erythrocytes of rats and human beings (51). The abundant pool of erythrocyte GSH and the improved activity of GSH-Px in the RP rats indicate a pyramid exposure-induced enhancement in the ability of the erythrocytes to detoxify the reactive peroxides that are generated due to chronic restraint stress. This in turn has decreased the susceptibility of erythrocyte membrane lipids to peroxidative damage. Our observations are similar to the effects of bioresonance therapy that normalized the activities of SOD and GSH-Px in blood lymphocytes of patients with rheumatoid arthritis (52), thus suggesting the presence of a magnetic field in the pyramid, similar to that used in bioresonance therapy, as claimed by pyramidologists (53).
This field could have normalized the erythrocyte GSH-Px and SOD activity in RP rats, and hence a decrease in these enzyme activities caused by chronic stress was counteracted by pyramid exposure. By maintaining a better erythrocyte SOD activity in RP rats, the pyramid has ensured prompt conversion of superoxide radicals generated in erythrocytes by chronic restraint stress to H2O2, which was then efficiently removed by the improved activity of erythrocyte GSH-Px in these rats. Therefore, by maintaining the levels of GSH and the activities of GSH-Px and SOD in the erythrocytes of RP rats, pyramid exposure during chronic restraint stress resulted in a better functioning of the antioxidant system for removing toxic peroxides; thus, decreasing the oxidative stress to levels similar to those seen in NC rats. It can be said that the shape of the pyramid, and not mere enclosure, was responsible for the above beneficial effects because a similar reversal of effects of chronic restraint on oxidative stress was not seen in the square box-exposed RS rats.
In conclusion, chronic restraint stress in adult female rats induces neuroendocrine and oxidative stress, but these effects are counteracted by the shape of the pyramid. This suggests that the shape of the housing has its effects and pyramid shape appears to have beneficial effects in terms of stress management. Thus, sitting inside a pyramid-shaped structure can be used as an effective technique for stress management and for non-invasive treatment of diseases in which the role of free radicals and ROS has been implicated. Pyramid-shaped rooms can be built in residences, hospitals and recreation centers. This study is a scientific backing to the editorial view of Cooper (54) whose ‘new view of an ancient structure points to an essential quality of scientific research, especially in the varied world of CAM’. However, further studies are required to determine the nature of and quantitate the energy/magnetic field that is claimed to be present within the pyramid.
References
- 1.Schul B, Pettit E. The Secret Power of Pyramids. New York: Fawcett Gold Medal; 1975. The historical enigma; pp. 24–41. [Google Scholar]
- 2.Schul B, Pettit E. The Secret Power of Pyramids. New York: Fawcett Gold Medal; 1975. The pyramid: ancient and new miracle worker; pp. 11–23. [Google Scholar]
- 3.Orzechowski A, Ostaszewski P, Brodnicka A, Wilczak J, Jank M, Balasinska B, et al. Excess of glucocorticoids impairs whole-body antioxidant status in young rats-relation to the effect of dexamethasone in soleus muscle and spleen. Horm Metab Res. 2000;32:174–80. doi: 10.1055/s-2007-978617. [DOI] [PubMed] [Google Scholar]
- 4.Aleksandrovskii IA, Poiurovskii MV, Neznamov GG, Seredeniia SB, Krasova EA. Lipid peroxidation in emotional stress and neurotic disorders. Zh Nevropatol Psikhiatr Im S S Korsakova. 1988;88:95–101. (in Russian) [PubMed] [Google Scholar]
- 5.Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, et al. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–45. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 6.Frei B. Reactive oxygen species and antioxidant vitamins: mechanism of action. Am J Med. 1994;97:5s–13s. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 7.Peterhans E. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J Nutr. 1997;127:962s–5s. doi: 10.1093/jn/127.5.962S. [DOI] [PubMed] [Google Scholar]
- 8.Beck MM, Levander OA. Dietary oxidative stress and the potentiation of viral infection. Annu Rev Nutr. 1998;18:93–116. doi: 10.1146/annurev.nutr.18.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Irshad M, Chaudhuri PS. Oxidant–antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–9. [PubMed] [Google Scholar]
- 10.Bharathi H. PhD thesis. Manipal: Manipal Academy of Higher Education; 2002. Effect of energy within a pyramid model on learning, memory and stress—a behavioral and morphological study in mice. [Google Scholar]
- 11.Bhat S, Rao G, Murthy KD, Bhat PG. Effect of housing rats within a pyramid on stress parameters. Indian J Exp Biol. 2003;41:1289–93. [PubMed] [Google Scholar]
- 12.Schul B, Pettit E. The Secret Power of Pyramids. New York: Fawcett Gold Medal; 1975. Home experiments; pp. 192–201. [Google Scholar]
- 13.Toth M, Nielsen G. Pyramid Power. Vermont, USA: Inner Traditions India; 1985. Model pyramid construction; pp. 151–6. [Google Scholar]
- 14.Sunanda. 1997. Stress induced changes in hippocampus—a morphological, neurochemical, behavioral and in vitro study; pp. 44–79. Thesis submitted to NIMHANS (Deemed University) Bangalore, India. [Google Scholar]
- 15.Domenicali M, Caraceni P, Vendemiale G, Grattagliano I, Nardo B, Dall'Agata M, et al. Food deprivation exacerbates mitochondrial oxidative stress in rat liver exposed to ischemia reperfusion injury. J Nutr. 2001;131:105–10. doi: 10.1093/jn/131.1.105. [DOI] [PubMed] [Google Scholar]
- 16.Harris RB, Zhou J, Mitchell T, Hebert S, Ryan DH. Rats fed only during the light period are resistant to stress induced weight loss. Physiol Behav. 2002;76:543–50. doi: 10.1016/s0031-9384(02)00754-0. [DOI] [PubMed] [Google Scholar]
- 17.Gowenlock AH, McMurray JR, McLauchlan DM. Varley's Practical Clinical Biochemistry. 6th edn. London: Heinemann; 1988. Measurement of steroids in body fluids—fluorimetric assays; pp. 814–6. [Google Scholar]
- 18.Jain SK, Mc Vie R, Duett J, Herbest JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–43. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 19.Tentori L, Salvati AM. Hemoglobinometry in human blood. Methods Enzymol. 1981;76:707–15. doi: 10.1016/0076-6879(81)76152-4. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 22.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 23.Marti O, Harbuz MS, Andres R, Lightman SL, Armario A. Activation of the hypothalamic pituitary axis in adrenalectomised rats: potentiation by chronic stress. Brain Res. 1999;821:1–7. doi: 10.1016/s0006-8993(98)01212-8. [DOI] [PubMed] [Google Scholar]
- 24.Daniels WM, Richter L, Stein DJ. The effects of repeated intra-amygdala CRF injections on rat behaviour and HPA axis after stress. Metab Brain Dis. 2004;19:15–23. doi: 10.1023/b:mebr.0000027413.42946.61. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto K, Suemaru S, Takao T, Sugawara M, Makino S, Ota Z. Corticotropin releasing hormone and pituitary adrenocortical responses in chronically stressed rats. Regul Pept. 1988;23:117–26. doi: 10.1016/0167-0115(88)90019-5. [DOI] [PubMed] [Google Scholar]
- 26.Lightman SL, Harbuz MS. Expression of corticotrophin releasing factor mRNA in response to stress. Ciba Found Symp. 1993;172:173–87. doi: 10.1002/9780470514368.ch9. [DOI] [PubMed] [Google Scholar]
- 27.Effects/experiments with pyramid/orgone energy. Available at: www.geocities.com/undergsci/pyramideffects.html. Assessed on 2005.
- 28.Stark NH. The First Practical Pyramid Book. KS: Sheed Andrews and McMeel Inc., Kansas City; 1977. What can a pyramid do? p. 29. [Google Scholar]
- 29.Schul B, Pettit E. The Secret Power of Pyramids. New York: Fawcett Gold Medal; 1975. The pyramid and altered states of consciousness; pp. 61–177. [Google Scholar]
- 30.Toth M, Nielsen G. Pyramid Power. Vermont, USA: Inner Traditions India; 1985. Transform yourself with pyramid energy; pp. 124–38. [Google Scholar]
- 31.Zaidi SM, Al-Qirim TM, Hoda N, Banu N. Modulation of restraint stress induced oxidative changes by antioxidant vitamins. J Nutr Biochem. 2003;14:633–6. doi: 10.1016/s0955-2863(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 32.Abidin I, Yargicoglu P, Agar A, Gumuslu S, Aydin S, Ozturk O, et al. The effect of chronic restraint stress on spatial learning and memory: relation to oxidant stress. Int J Neurosci. 2004;114:683–99. doi: 10.1080/00207450490430543. [DOI] [PubMed] [Google Scholar]
- 33.Bagchi D, Carryl OR, Tran MX, Bagchi M, Garg A, Milnes MM, et al. Acute and chronic stress induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. Mol Cell Biochem. 1999;196:109–16. [PubMed] [Google Scholar]
- 34.Singh A, Garg V, Gupta S, Kulkarni SK. Role of antioxidants in chronic fatigue syndrome in mice. Indian J Exp Biol. 2002;40:1240–4. [PubMed] [Google Scholar]
- 35.McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–6. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- 36.Eid YZ, Ohtsuka A, Hayashi K. Tea polyphenols reduce glucocorticoid-induced growth inhibition and oxidative stress in broiler chickens. Br Poult Sci. 2003;44:127–32. doi: 10.1080/0007166031000085427. [DOI] [PubMed] [Google Scholar]
- 37.Ohtsuka A, Kojima H, Ohtani T, Hayashi K. Vitamin E reduces glucocorticoid-induced oxidative stress in rat skeletal muscle. J Nutr Sci Vitaminol (Tokyo) 1998;44:779–86. doi: 10.3177/jnsv.44.779. [DOI] [PubMed] [Google Scholar]
- 38.Itoh T, Saito T, Fujimura M, Watanabe S, Saito K. Restraint stress induced changes in endogenous zinc release from the rat hippocampus. Brain Res. 1993;618:318–22. doi: 10.1016/0006-8993(93)91283-x. [DOI] [PubMed] [Google Scholar]
- 39.Elissa SE, Elizabeth HB, Jue L, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avshalumov MV, Chen BT, Marshall SP, Pena DM, Rice ME. Glutamate dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci. 2003;23:2744–50. doi: 10.1523/JNEUROSCI.23-07-02744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siems WG, Sommerburg O, Grune T. Erythrocyte free radical and energy metabolism. Clin Nephrol. 2000;53(1 Suppl):59–17. [PubMed] [Google Scholar]
- 42.May JM, Quip JC, Whitesell RR, Cobb CE. Ascorbate recycling in human erythrocytes: role of GSH in reducing dehydroascorbate. Free Radic Biol Med. 1996;20:543–51. doi: 10.1016/0891-5849(95)02130-2. [DOI] [PubMed] [Google Scholar]
- 43.McIntosh LJ, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant capacity in the brain: baseline studies. Brain Res. 1998;791:209–14. doi: 10.1016/s0006-8993(98)00115-2. [DOI] [PubMed] [Google Scholar]
- 44.Kostic TS, Andric SA, Maric D, Kovacevic RZ. Inhibitory effects of stress-activated nitric oxide on antioxidant enzymes and testicular steroidogenesis. J Steroid Biochem Mol Biol. 2000;75:299–306. doi: 10.1016/s0960-0760(00)00185-0. [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Luo C, Richardson JS, Li XM. Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics J. 2004;4:322–31. doi: 10.1038/sj.tpj.6500265. [DOI] [PubMed] [Google Scholar]
- 46.Oishi K, Yokoi M, Maekawa S, Sodeyama C, Shiraisi T, Kondo R, et al. Oxidative stress and haematological changes in immobilized rats. Acta Physiol Scand. 1999;165:65–9. doi: 10.1046/j.1365-201x.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- 47.Singh A, Garg V, Gupta S, Kulkarni SK. Role of antioxidants in chronic fatigue syndrome in mice. Indian J Exp Biol. 2002;40:1240–4. [PubMed] [Google Scholar]
- 48.Grillo CA, Piroli GG, Rosell DR, Hoskin EK, Mcewen BS, Reagan LP. Region specific increases in oxidative stress and superoxide dismutase in the hippocampus of diabetic rats subjected to stress. Neuroscience. 2003;121:133–40. doi: 10.1016/s0306-4522(03)00343-9. [DOI] [PubMed] [Google Scholar]
- 49.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJA. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–27. [PubMed] [Google Scholar]
- 50.Romeu M, Mulero M, Giralt M, Folch J, Nogues MR, Torres A, et al. Parameters related to oxygen free radicals in erythrocytes, plasma and epidermis of the hairless rat. Life Sci. 2002;71:1739–49. doi: 10.1016/s0024-3205(02)01946-x. [DOI] [PubMed] [Google Scholar]
- 51.Godin DV, Garnett ME. Species related variations in tissue antioxidant status-I, differences in antioxidant enzyme profiles. Comp Biochem Physiol B. 1992;103:737–42. doi: 10.1016/0305-0491(92)90399-c. [DOI] [PubMed] [Google Scholar]
- 52.Islamov BI, Balabanova RM, Funtikov VA, Gotovskii YV, Melzerov EE. Effect of bioresonance therapy on antioxidant system in lymphocytes in patients with rheumatoid arthritis. Bull Exp Biol Med. 2002;134:248–50. doi: 10.1023/a:1021599216581. [DOI] [PubMed] [Google Scholar]
- 53.Schul B, Pettit E. The Secret Power of Pyramids. New York: Fawcett Gold Medal; 1975. Healing powers; pp. 113–30. [Google Scholar]
- 54.Cooper EL. Editorial on CAM, eCAM, bioprospecting: the 21st century pyramid. Evid Based Complement Alternat Med. 2005;2:125–7. doi: 10.1093/ecam/neh094. [DOI] [PMC free article] [PubMed] [Google Scholar]