Abstract
The aim of this study was to characterize scratching behavior elicited by central administration of morphine or bombesin in rats, and to determine the role of opioid receptors in scratching induced by both pruritogenic agents. Central administration included intracisternal (i.c.), intrathecal (i.t.), and intracerebroventricular (i.c.v.) routes. Scratching events made with hind paws were counted by observers blinded to treatment conditions. Intracisternal morphine (0.01–0.1 μg) produced dose-dependent increases in scratching; the maximum response to i.c. morphine 0.1 μg was approximately 500 scratches within a 1-hour period. Neither i.t. nor i.c.v. morphine significantly increased scratching. Bombesin (0.01 – 0.32 μg) elicited robust scratching following i.c. administration. The maximum response to i.c. bombesin 0.32 μg was approximately 4000 scratches within a 1-hour period. Both i.t. and i.c.v. bombesin produced profound scratching at similar doses. Antagonist studies confirmed that mu-opioid receptors selectively mediate i.c. morphine-induced scratching. However, selective mu-, kappa-, and delta-opioid antagonists did not attenuate i.c. bombesin-induced scratching. These results demonstrate that morphine and bombesin elicit scratching through different receptor mechanisms, at different central sites, and to different degrees.
Keywords: pruritus, itch sensation, mu-opioid receptors, bombesin, scratching, rat
Introduction
A significant breakthrough in pain management during the past two decades has been administration of opioids into the spinal cord. Intrathecal (i.t.) administration of morphine is one of the most frequently used methods of analgesia following cesarean section. The most common side-effect of spinal morphine is pruritus (i.e. itch sensation), which is sometimes severe and may lessen the value of spinal opioids for pain relief (Cousins and Mather, 1984; Ballantyne et al., 1988; Chaney, 1995; Kam and Tan, 1996). The mechanism of this opioid action is poorly understood, slowing the development of appropriate antipruritics. Therefore, it is important to develop experimental itch models in animals to study the mechanisms of itch and to identify potential antipruritics.
Although several studies have demonstrated that activation of central mu-opioid receptors produces scratching behavior in rodents (Thomas and Hammond, 1995; Tohda et al., 1997; Kuraishi et al., 2000), and that this scratching behavior can be blocked by opioid antagonists in mice and rats (Tohda et al., 1997; Yamaguchi et al., 1998; Ko et al., 1999), the dose- and time-dependent aspects of this response have not been well characterized in rats. To date, many studies have investigated the antinociceptive effects of spinal mu-opioid receptors in rodents (e.g. Yaksh and Rudy, 1976, 1977; Wegert et al., 1997; Przewlocka et al., 1999). However, characterization of i.t. morphine-induced scratching has not been evaluated systemically in rats.
A similar pattern of scratching induced by another drug has been better characterized in rodents. Intracerebroventricular (i.c.v.) administration of bombesin, a tetra-decapeptide originally isolated from frog skin (Anastasi et al., 1971), causes dose-dependent excessive grooming in mice and rats (Katz, 1980; Gmerek and Cowan, 1983a). The grooming consists of vigorous scratching of the head and body with the hind paws (Gmerek and Cowan, 1983a, b; Cowan et al., 1985). This profound scratching behavior appears to be initiated in the CNS because centrally effective doses, when given intravenously, do not elicit similar behavioral effects in rats (Gmerek and Cowan, 1983a). In addition, the distribution of bombesin-like peptides has been identified in the mammalian spinal cord (O’Donohue et al., 1984; Namba et al., 1985). The mechanism of bombesin-induced scratching is not well understood. Van Wimersma Greidanus et al. 1985 reported that the non-selective opioid antagonist naloxone altered the profile of the effects of bombesin, reducing drug-induced scratching. Gmerek and Cowan (1988), on the other hand, found that bombesin-induced scratching was not altered by substantial doses of naloxone, but that the effect was reduced by administration of kappa-opioid, but not mu- or delta-opioid receptor agonists. The interactive relationship between morphine- and bombesin-induced scratching is not clear. In particular, there has been no study directly comparing the magnitude and duration of scratching responses induced by both morphine and bombesin following central administration.
The aim of this study was to characterize and compare the ability of morphine and bombesin to induce scratching, following central administration by the intracisternal (i.c.), i.t., and i.c.v. routes in rats. In addition, antagonist studies using selective opioid receptor antagonists were conducted, to verify the role of mu-, kappa-, and delta-opioid receptors in mediating i.c. morphine- and bombesin-induced scratching.
Methods
Animals
Adult male Wistar rats, approximately 300 – 350 g (Harlan, Indianapolis, Indiana, USA), were maintained on a 12-h light/dark cycle with free access to food and water in a temperature-controlled (23 ± 1°C) room. Each animal was used only once. Animals were maintained in accordance with the University Committee on the Use and Care of Animals in the University of Michigan, and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, USA, 1996).
Procedure
Central administration of morphine and bombesin included intracisternal (i.c.), intrathecal (i.t.), and intracerebroventricular (i.c.v.) injection techniques. For i.c. administration, the rat was lightly anesthetized with halothane and placed in a stereotaxic device. The head of the rat was held perpendicular to the body axis as a needle (26G, ½″) was inserted in the cisterna magna to a depth of 5mm. The position of the needle was confirmed by a flow of clean cerebrospinal fluid. A solution of 100 μl was infused over 20 s and the needle was held in place for an additional 10 s before being withdrawn (Paronis et al., 1993; Shannon and Lutz, 2002).
For i.t. administration, the rat was implanted with a spinal catheter (see details in Miyamoto et al., 1991) at least 7 days before the behavioral study. A solution of 10 μl was infused followed by an additional 15 μl of saline for flushing the catheter over a 30-s period. For i.c.v. administration, the rat was implanted with a stainless-steel cannula in the lateral cerebral ventricle, as previously described (Tortella et al., 1981; Miyamoto et al., 1991). After surgery, rats were housed individually and allowed 7 days for recovery. A solution of 10 μl was used for delivery of morphine or bombesin. Both i.t. catheter and i.c.v. cannula placements were verified after each experiment, by injecting methylene blue and checking for distribution within the cerebroventricular or spinal subarachnoid space.
The behavioral measurement was the numbers of scratching and grooming events, scored by individuals who did not know the experimental conditions. Experiments took place between 10.00 and 16.00 hours. Rats were placed singly in Plexiglas observation boxes (55 cm long × 30 cm wide × 26 cm high) and allowed to habituate for at least 15min. Due to a slow onset of morphine following central administration (Thomas and Hammond, 1995; our preliminary data), behavioral observation for the morphine groups started 20 min after drug administration. Centrally administered bombesin has a fast onset of action (Gmerek and Cowan, 1983a, b; Gmerek et al., 1983). Therefore, observation for the bombesin groups started 2 min after drug administration. In order to distinguish scratching from grooming, scratching events were counted as those made by the hind paws (Kuraishi et al., 1995; Thomas and Hammond, 1995; Tohda et al., 1997); movements with the forepaws were counted as grooming events. A scratch was defined as one short-duration episode of scraping contact by the hind paw on the skin surface of other body parts.
Experimental design
The first part of this study characterized the time course and total scratching and grooming responses following central administration of morphine or bombesin. All animals were randomly assigned to different experimental conditions and were only used once. The numbers of scratching and grooming events were counted simultaneously in 10min bins for 1 hour. Each person only observed the behaviors of one rat at any time. The range of morphine (0.0032–10μg) or bombesin (0.0032–0.32μg) doses was chosen based on previous studies showing active doses after central administration (Gmerek and Cowan, 1983a; Gmerek et al., 1983; Thomas and Hammond, 1995; Tohda et al., 1997). As noted, we found that 1 μg of i.c.v. bombesin produced similar effects as 0.32 μg (Gmerek and Cowan, 1983a; Gmerek et al., 1983) and that 3.2 μg of i.c.v. bombesin produced tremors in rats from our pilot study; therefore, we only characterized the effects of bombesin up to 0.32 μg in this study.
The second part of the study determined the effects of opioid receptor antagonists on i.c. morphine- and bombesin-induced scratching responses. Initial experiments were conducted to compare the effect of nalmefene, a long-acting opioid antagonist (Gal and DiFazio, 1986), on the dose–response curves of i.c. morphine and bombesin on scratching. Several studies have shown that s.c. administration of nalmefene 0.032 mg/kg could antagonize mu-opioid receptor-mediated effects (France and Gerak, 1994; Ko and Naughton, 2000). Thus, s.c. nalmefene 0.032 mg/kg with 30-min pretreatment time was used to study its antagonist effect.
In addition, selective opioid antagonists were used to verify the role of each opioid receptor type in mediating both i.c. morphine- and bombesin-induced scratching. Naltrexone (NTX), nor-binaltorphimine (nor-BNI), and naltrindole (NTI) are antagonists selective for mu-, kappa-, and delta-opioid receptors, respectively. The dose and pretreatment time (PT) for these antagonists (i.e. NTX 0.1 mg/kg with 30-min PT; nor-BNI 10 mg/kg with 24-h PT; NTI 3 mg/kg with 30-min PT) were chosen based on previous studies (Takemori et al., 1988; Endoh et al., 1992; Chang et al., 1993; Walker et al., 1994). The doses of i.c. morphine (0.1 μg) and bombesin (0.32 μg) were doses that produced maximum scratching events in this study.
Data analysis
Mean values (mean ± SEM) were calculated from individual values for all behavioral endpoints. Data for the time course and total responses were analyzed by a two-way analysis of variance (ANOVA) followed by the Newman–Keuls test for multiple (post-hoc) comparisons. ED50 values for i.c. morphine and bombesin on scratching were calculated by least-squares regression with the portion of the dose–response curves that spanned the 50% level of responding. The 95% confidence limits (CL) were also determined (P > 0.05). Mean ED50 values were considered to be significantly different when their 95% CL did not overlap.
The antagonist effect of nalmefene was determined by in vivo apparent pKB value: pKB = − log[B/(dose ratio − 1)], in which B equals the antagonist dose in moles per kilogram. Data for the interaction of the selective opioid antagonists with i.c. morphine and bombesin were analyzed by one-way ANOVA followed by the Newman–Keuls test for multiple (post-hoc) comparisons.
Drugs
Morphine sulfate (Mallinckrodt, St. Louis, Missouri, USA) and bombesin (Peninsula, San Carlos, California, USA) were dissolved in saline. Nalmefene, NTX, NTI (National Institute on Drug Abuse, Bethesda, Maryland, USA), and nor-BNI (Sigma, St. Louis, Missouri, USA) were all dissolved in sterile water. For systemic administration, all compounds were administered s.c. in the back at a volume of 1 ml/kg.
Results
Figure 1 illustrates the degree and time course of i.c. morphine-induced scratching and grooming events. I.c. administration of morphine produced dose-dependent increases in scratching events [F(5,40) = 4.0; P < 0.05]; scratching peaked 10 – 20 min into the observation period, 30 – 40 min following drug administration, and declined gradually over the course of the one-hour observation period. Morphine given intracisternally i.c. did not greatly increase grooming, and there was no dose-dependent effect of morphine on grooming [F(5,40) = 3.1; NS].
Fig. 1.
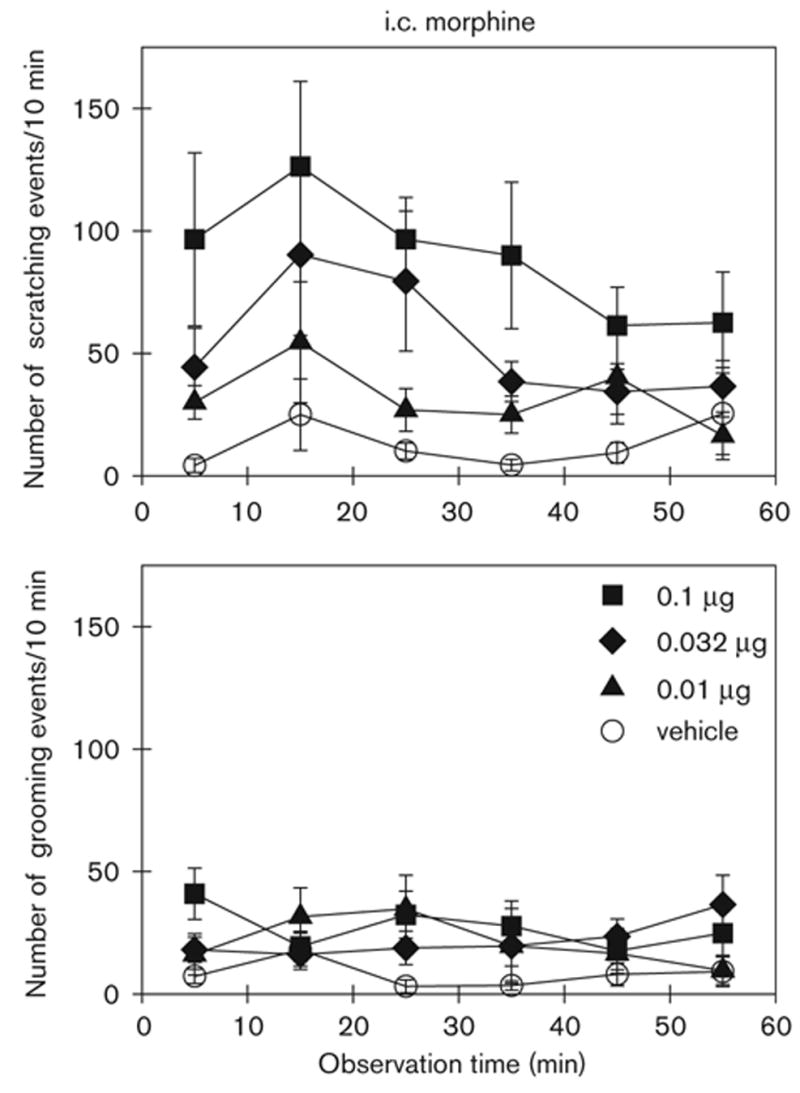
Time course of i.c. morphine-induced scratching and grooming events in rats. Morphine (μg) was given intracisternally 20 min before observation. Each value represents the mean ± SEM (n = 9–12). Not all of the data are shown for the sake of clarity. See Fig. 3 for the complete dose–response of i.c. morphine.
Figure 2 illustrates the degree and time course of i.c. bombesin-induced scratching and grooming events. I.c. administration of bombesin produced significant increases in both scratching [F(5,40) = 76.8; P < 0.05] and grooming [F(5,40) = 10.7; P < 0.05] events in a dose-dependent manner. The peak response occurred at the first observation period, 10 min after drug administration, and declined only slightly throughout the 1-hour observation period.
Fig. 2.
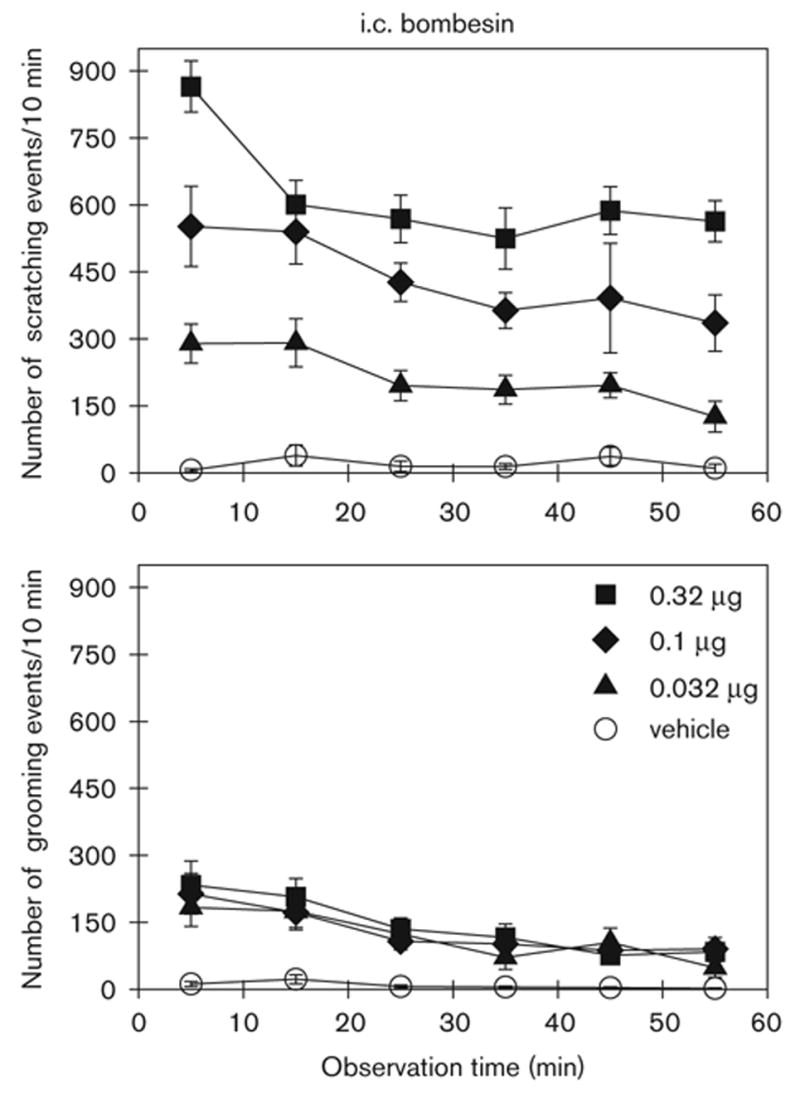
Time course of i.c. bombesin-induced scratching and grooming events in rats. Bombesin (μg) was given intracisternally 2 min before observation. Each value represents the mean ± SEM (n = 9–10). See Fig. 3 for the complete dose–response of i.c. bombesin.
Figure 3 illustrates the total scratching and grooming events produced by i.c. morphine and bombesin. Post-hoc comparisons indicated that both i.c. morphine (0.032 – 0.32 μg) and bombesin (0.01–0.32 μg) significantly increased scratching compared with the vehicle-treated group (P < 0.05). Only i.c. bombesin from 0.032 to 0.32 μg significantly increased grooming (P < 0.05). Bombesin evoked more profound scratching than did morphine under these conditions. The peak responses produced by i.c. morphine 0.1 μg were approximately 534 ± 94 scratches (mean ± SEM). In contrast, the peak responses produced by i.c. bombesin were approximately 3710 ± 208 scratches.
Fig. 3.
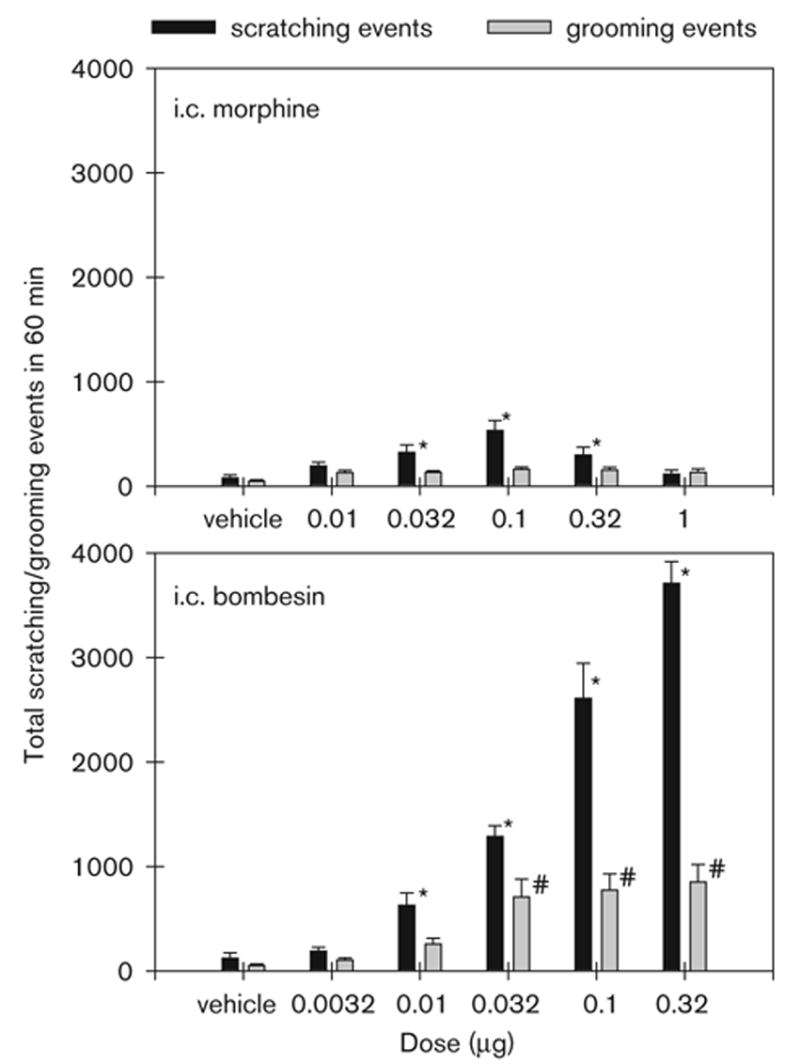
Effects of i.c. administration of morphine and bombesin on scratching and grooming behavior. Vertical axes: total number of scratching or grooming events over a 1-hour period. Horizontal axes: doses (μg) of i.c. morphine or bombesin. Each value represents the mean ± SEM (n = 9–12). The asterisk represents a significant difference from the vehicle condition in scratches (*, P < 0.05). The sign, #, represents a significant difference from the vehicle condition in grooming events (P < 0.05).
Figure 4 illustrates the degree and time course of the effects of i.t. and i.c.v. morphine and bombesin on scratching events. ANOVA analysis indicated that neither i.t. nor i.c.v. morphine produced increases in scratching (P > 0.05). However, both i.t. and i.c.v. bombesin significantly increased scratching in a dose-dependent manner {i.t.: [F(5,35) = 63.2; P < 0.05]; i.c.v.: [F(5,35) = 28.5; P < 0.05]}. Figure 5 illustrates the total scratching and grooming events produced by i.t. and i.c.v. morphine or bombesin. Post-hoc comparisons indicated that i.t. bombesin (0.01–0.32 μg) and i.c.v. bombesin (0.032–0.32 μg) significantly evoked scratching compared with the vehicle-treated group (P < 0.05). Only two doses of i.t. or i.c.v. bombesin (0.1 and 0.32 μg) significantly evoked grooming (P < 0.05). The peak responses of i.t. and i.c.v. bombesin 0.32 μg were 4491 ± 208 and 3944 ± 544 scratches, respectively.
Fig. 4.
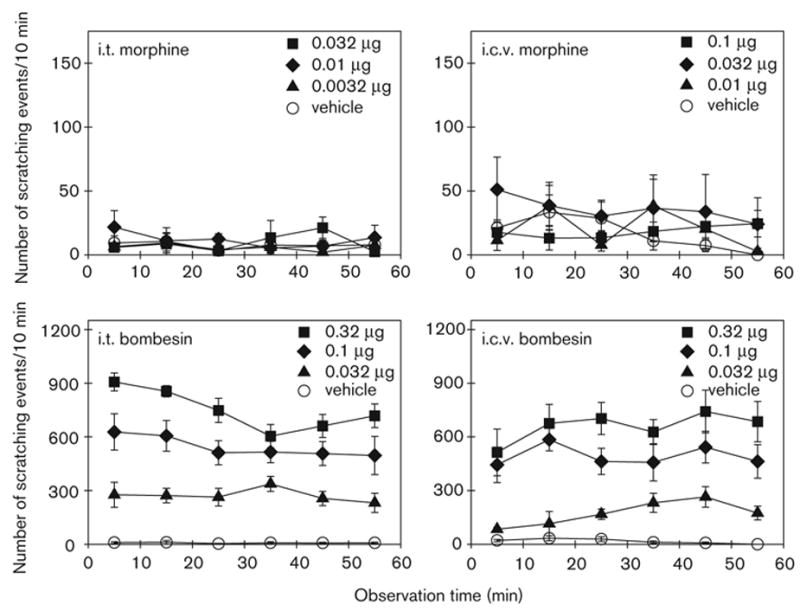
Time course of i.t. and i.c.v. administration of morphine- or bombesin-induced scratching events. I.t. or i.c.v. morphine and bombesin (μg) were administered 20 min and 2 min before observation, respectively. Each value represents the mean ± SEM (n = 8–9). Not all of the data are shown for the sake of clarity. See Fig. 5 for the complete dose–response of i.t or i.c.v. morphine and bombesin.
Fig. 5.
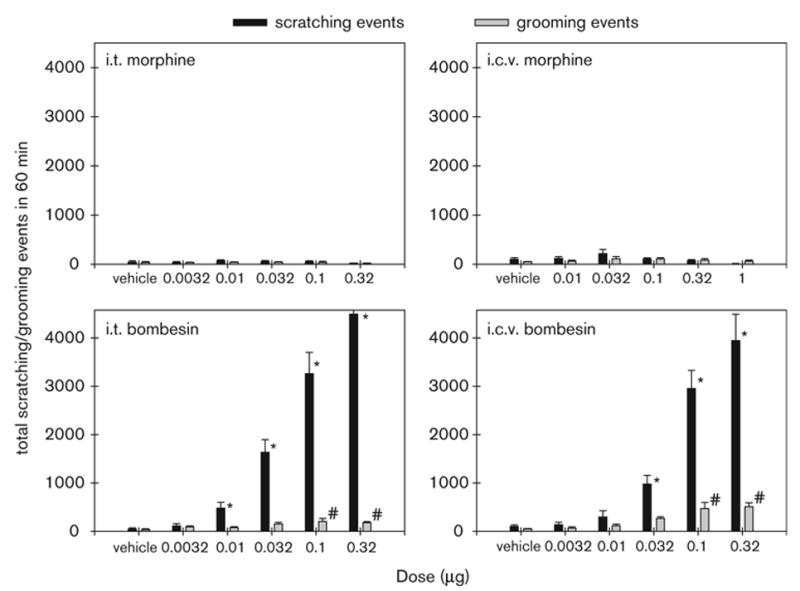
Effects of i.t. or i.c.v. administration of morphine and bombesin on scratching and grooming behavior. Vertical axes: total number of scratching or grooming events over a 1-hour period. Horizontal axes: doses (μg) of morphine or bombesin. Each value represents the mean ± SEM (n = 8–9). The asterisk represents a significant difference from the vehicle condition in scratches (*, P < 0.05). The sign, #, represents a significant difference from the vehicle condition in grooming events (P < 0.05).
Figure 6 illustrates the antagonist effect of nalmefene (0.032 mg/kg, s.c.) on the dose–response curves of i.c. morphine- and bombesin-induced scratching. The ED50 value for the ascending limb of i.c. morphine dose–response curve is approximately 0.028 μg (95% CL: 0.0083 – 0.091 μg). Pretreatment with s.c. nalmefene 0.032 mg/kg produced a rightward shift of the i.c. morphine dose–response curve for scratching (ED50: 0.32 μg; 95% CL: 0.11–0.96 μg). The nalmefene pKB value is 8.0 under these conditions. In contrast, the ED50 value for the i.c. bombesin dose–response curve for scratching is approximately 0.049 μg (95% CL: 0.029–0.085 μg). Pretreatment with nalmefene did not significantly shift the i.c. bombesin dose–response curve (ED50: 0.045 μg; 95% CL: 0.023–0.093 μg).
Fig. 6.
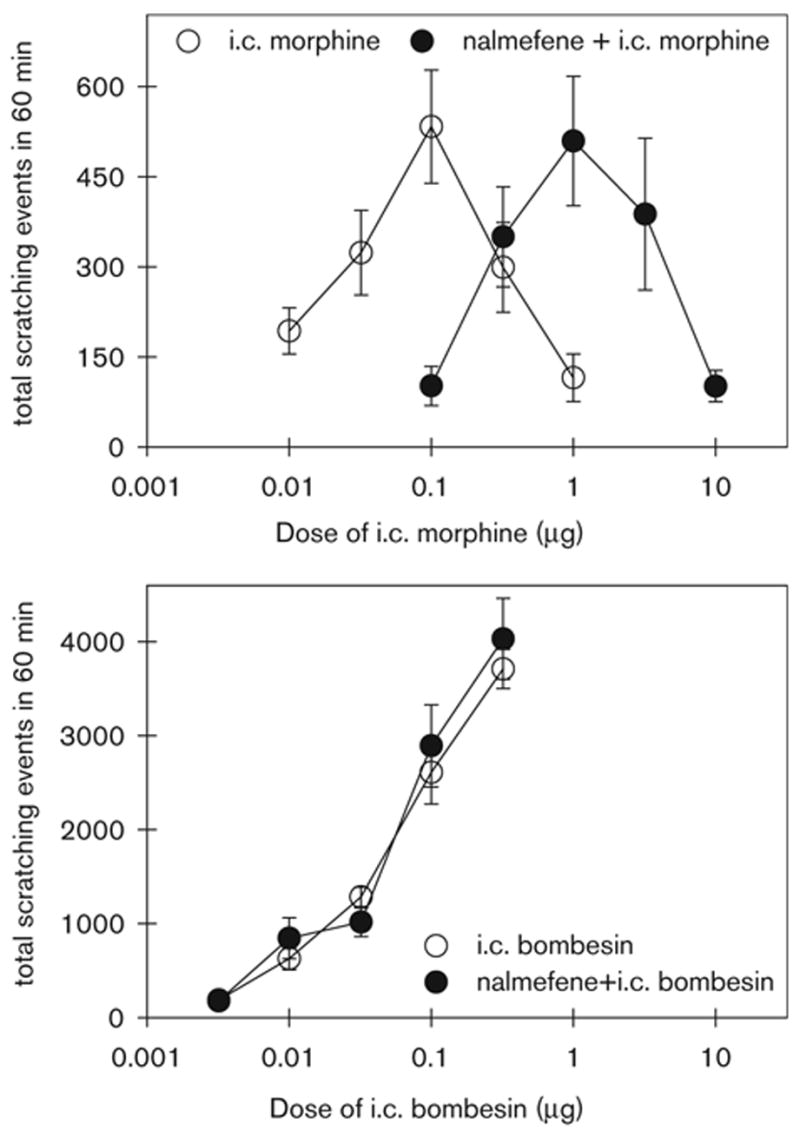
The antagonist effect of nalmefene on i.c. morphine- and bombesin-induced scratching events. Nalmefene (0.032 mg/kg) was given subcutaneously 30 min before i.c. administration of morphine or bombesin. Each value represents the mean ± SEM (n = 9–12). Complete dose–response curves for scratching events are shown with or without nalmefene pretreatment.
Table 1 illustrates the effects of s.c. opioid receptor antagonists on i.c. morphine- and bombesin-induced scratching. Post-hoc comparisons indicated that only pretreatment with NTX 0.1 mg/kg significantly blocked i.c. morphine (0.1 μg)-induced scratching (P < 0.05). Neither nor-BNI nor NTI antagonized i.c. morphine-induced scratching (P > 0.05). In addition, i.c. bombesin-induced scratching could not be blocked by NTX, nor-BNI, or NTI (P > 0.05).
Table 1.
Effects of opioid receptor antagonists selective for each opioid receptor type on i.c. morphine- and bombesin-induced scratching events
| Number of scratching events (mean ± SEM)a |
||
|---|---|---|
| Pretreatment conditionsb | i.c. morphine 0.1 μg | i.c. bombesin 0.32 μg |
| Vehicle (1 ml/kg) | 473 ± 73 | 3750 ± 210 |
| Naltrexone (0.1 mg/kg) | 75 ± 19*c | 3960 ± 163 |
| Nor-binaltorphimine (10 mg/kg) | 509 ± 149 | 3650 ± 223 |
| Naltrindole (3 mg/kg) | 539 ± 119 | 4100 ± 266 |
Each value represents the mean ± SEM (n = 9–10).
Naltrexone, naltrindole, or vehicle (sterile water) was administered subcutaneously 30 min before intracisternal (i.c.) injection. The pretreatment time with nor-binaltorphimine was 24 hours before i.c. injection.
The asterisk represents a significant difference from the vehicle condition (P < 0.05).
Discussion
The present study demonstrated that morphine given intracisternally, but not intrathecally or intracerebroventricularly, produces scratching in the rat. The doses of i.c. morphine that produce scratching are smaller than doses of i.c. morphine necessary to produce antinociceptive responses against acute or inflammatory pain in rats (Ko et al., 1999; Shannon and Lutz, 2002). Large doses of i.t. morphine produce antinociception against a variety of noxious stimuli, indicating that i.t. morphine activates spinal mu-opioid receptors (e.g. Yaksh and Rudy, 1976, 1977; Wegert et al., 1997; Przewlocka et al., 1999). We had tested multiple doses of i.t. morphine from 1 pg to 30 μg in the pilot study and did not observe significant scratching activity. Although higher doses (i.e. 90–150 μg) of i.t. morphine produce allodynia-like behavior, these effects are not reversed by opioid-receptor antagonists (Yaksh and Harty, 1988). To date, there is no report of i.t. or i.c.v. morphine-induced scratching in rats. These findings may suggest that spinal mu-opioid receptors do not mediate scratching responses in rats.
This is different from the profile of morphine-induced effects observed in primates. In both humans and rhesus monkeys, i.t. morphine produces scratching as well as antinociception and these effects occur at the same doses (Palmer et al., 1999; Ko and Naughton, 2000). Although morphine has a slow onset of action, scratching was observed 20 min after i.c. administration. We noticed large individual differences of i.c. morphine-induced scratching in rats. This is similar to other animal studies (Thomas and Hammond, 1995; Ko and Naughton, 2000) and is consistent with the clinical observation that the incidence of pruritus varies widely, from 0 to 100%, in patients receiving spinal opioids (Chaney, 1995; Kam and Tan, 1996). Small doses of i.c. morphine (0.01–0.1 μg) dose-dependently increase scratching in rats. However, larger doses of morphine (0.32–1 μg) start to inhibit this scratching. This inverted U-shape curve of i.c. morphine-induced scratching was also observed in mouse studies (Tohda et al., 1997; Yamaguchi et al., 1998). It might be due to general suppression of behaviors by high doses of morphine.
Pretreatment with a single dose of nalmefene (0.032 mg/kg) produces an 11-fold rightward shift of the i.c. morphine dose–response curve for scratching. The nalmefene pKB value is 8.0, similar to the in vivo nalmefene pA2/pKB values (7.8–8.4) for mu-opioid agonists in rats and monkeys using other behavioral endpoints (France and Gerak, 1994; Ko et al., 1999; Ko and Naughton, 2000). This supports the notion that morphine is acting on mu-opioid receptors to produce scratching after i.c. administration. Additional antagonist studies demonstrate that a small dose of NTX, 0.1 mg/kg, which is selective for the mu-opioid receptor antagonism (Walker et al., 1994), significantly blocks i.c. morphine-induced scratching. Neither the kappa-selective antagonist, nor-BNI, nor the delta-selective antagonist, NTI, attenuates the effects of i.c. morphine on scratching. These results support previous studies indicating that mu-opioid receptors, rather than kappa- or delta-opioid receptors, mediate scratching induced by centrally administered morphine (Thomas et al., 1992; Tohda et al., 1997; Ko and Naughton, 2000; Kuraishi et al., 2000).
It is not clear why only i.c. morphine produces scratching responses that are not seen with either i.t. or i.c.v. morphine. Although we used a larger volume (i.e. 100 μl) for i.c. administration, our pilot studies confirm that 0.1 μg of morphine in 10 μl produces a similar peak response of scratching in rats (data not shown) and a difference between injection volumes does not contribute to the scratching observed in this study. It is possible that the site of action of mu-receptor-mediated scratching is more localized and anatomically close to the cisternal injection of morphine. The finding that microinjection of morphine into the medullary dorsal horn in rats produces scratching responses (Thomas and Hammond, 1995) seems to support our notion.
In contrast to the finding that only i.c. morphine induces scratching, all three central routes of bombesin administration resulted in marked scratching. Scratching increased monotonically as dose of bombesin was increased. Grooming likely reached a plateau with larger doses of bombesin. The potency, effectiveness and duration of action of bombesin are the same regardless of the route of administration. Pretreatment with nalmefene did not modify the i.c. bombesin dose–response curve. These results indicate that mu-opioid receptors do not mediate scratching behavior evoked by i.c. bombesin. This supports earlier findings by Gmerek and Cowen (1988) but not those of Van Wimersma Greidanus et al. 1985 who found that the mu-opioid antagonist, naloxone, was able to block the ability of bombesin to increase scratching. NTX, nor-BNI, and NTI also failed to attenuate i.c. bombesin-induced scratching. This finding agrees with a previous study that centrally administered bombesin-induced scratching is independent from classical opioid receptors (Gmerek and Cowan, 1988).
Although several bombesin receptor antagonists have been developed (de Castiglione and Gozzini, 1996), none has been found to be effective in attenuating i.c.v. bombesin-induced scratching (e.g. Cowan et al., 1985). A phyllolitorin analogue, [desTrp3,Leu8]phyllolitorin, was effective in attenuating i.c.v. bombesin-induced scratching, but this peptide has no binding affinity for the bombesin receptor site (Johnson et al., 1999). Nevertheless, central bombesin-induced scratching is experimentally useful for detecting potential antipruritics. For example, several kappa-opioid agonists are selectively active in attenuating i.c.v. bombesin-induced scratching (Gmerek and Cowan, 1984; Cowan and Gmerek, 1986), which indicates that kappa-opioid agonists may be effective for treating refractory pruritus. As noted, kappa-opioid agonists have been shown to inhibit scratching behavior induced by morphine in monkeys (Ko et al., 2003). It will be worthwhile to determine whether kappa-opioid agonists can attenuate scratching produced by centrally administered bombesin in non-human primates.
Taken together, the data demonstrate that i.c. morphine and bombesin elicit scratching behavior through different receptor mechanisms in rats. In particular, centrally administered bombesin induces profound scratching that is not attenuated by mu-, kappa-, or delta-opioid receptor antagonists. Centrally administered morphine induces a milder pattern of scratching that is attenuated by muopioid receptor antagonists. Compared with the non-human primate model, the rodent model is inexpensive and less labor intensive. The differences in the ability of i.t. morphine to produce scratching in the two species is interesting and needs to be investigated in order to determine if the rodent model is limited by its insensitivity to i.t. opioid pruritic effects. Several experimental itch models have been established in rodents by using a variety of pruritogenic agents (e.g. Gmerek and Cowan, 1983b; Kuraishi et al., 1995; Tohda et al., 1997; Andoh et al., 1998). These models are useful for evaluating the effectiveness of proposed antipruritics. They will facilitate itch/pruritus research and allow the identification of potential antipruritics.
Acknowledgments
The authors thank Dr Gail Winger for her assistance with the editing of manuscript. In addition, the authors thank Kurt Willmont, Kevin Choo, Michael Song, Denise Chang, Dana Begnoche, Chun-Yan Dou, and Shekyla Scott for excellent technical assistance.
Footnotes
Sponsorship: This study was supported by US Public Health Service Grant DA-13685 to M.C.H.K.
References
- Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971;27:166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–1145. [PubMed] [Google Scholar]
- Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33:149–160. doi: 10.1016/0304-3959(88)90085-1. [DOI] [PubMed] [Google Scholar]
- Chaney MA. Side effects of intrathecal and epidural opioids. Can J Anaesth. 1995;42:891–903. doi: 10.1007/BF03011037. [DOI] [PubMed] [Google Scholar]
- Chang KJ, Rigdon GC, Howard JL, McNutt RW. A novel, potent and selective nonpeptidic delta opioid receptor agonist BW373U86. J Pharmacol Exp Ther. 1993;267:852–857. [PubMed] [Google Scholar]
- Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- Cowan A, Gmerek DE. In-vivo studies on kappa opioid receptors. Trends Pharmacol Sci. 1986;7:69–72. [Google Scholar]
- Cowan A, Khunawat P, Zhu XZ, Gmerek DE. Effects of bombesin on behavior. Life Sci. 1985;37:135–145. doi: 10.1016/0024-3205(85)90416-3. [DOI] [PubMed] [Google Scholar]
- de Castiglione R, Gozzini L. Bombesin receptor antagonists. Crit Rev Oncol Hematol. 1996;24:117–151. doi: 10.1016/1040-8428(96)00220-x. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: A potent and selective κ-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn. 1992;16:30–42. [PubMed] [Google Scholar]
- France CP, Gerak LR. Behavioral effects of 6-methylene naltrexone (nalmefene) in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:992–999. [PubMed] [Google Scholar]
- Gal TJ, DiFazio CA. Prolonged antagonism of opioid action with intravenous nalmefene in man. Anesthesiology. 1986;64:175–180. doi: 10.1097/00000542-198602000-00008. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. Studies on bombesin-induced grooming in rats. Peptides. 1983a;4:907–913. doi: 10.1016/0196-9781(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. An animal model for preclinical screening of systemic antipruritic agents. J Pharmacol Methods. 1983b;10:107–112. doi: 10.1016/0160-5402(83)90073-6. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. In vivo evidence for benzomorphan-selective receptors in rats. J Pharmacol Exp Ther. 1984;230:110–115. [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. Role of opioid receptors in bombesin-induced grooming. Ann NY Acad Sci. 1988;525:291–300. doi: 10.1111/j.1749-6632.1988.tb38614.x. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A, Vaught JL. Intrathecal bombesin in rats: effects on behaviour and gastrointestinal transit. Eur J Pharmacol. 1983;94:141–143. doi: 10.1016/0014-2999(83)90451-x. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Ko MC, Choo KS, Traynor JR, Mosberg HI, Naughton NN, et al. The effects of the phyllolitorin analogue [desTrp(3), Leu(8)]phyllolitorin on scratching induced by bombesin and related peptides in rats. Brain Res. 1999;839:194–198. doi: 10.1016/s0006-8993(99)01708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam PCA, Tan KH. Pruritus – itching for a cause and relief? Anaesthesia. 1996;51:1133–1138. doi: 10.1111/j.1365-2044.1996.tb15050.x. [DOI] [PubMed] [Google Scholar]
- Katz R. Grooming elicited by intracerebroventricular bombesin and eledoisin in the mouse. Neuropharmacology. 1980;19:143–146. doi: 10.1016/0028-3908(80)90181-1. [DOI] [PubMed] [Google Scholar]
- Ko MCH, Naughton NN. An experimental itch model in monkeys: Characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Choo KS, Song MS, Chang D, Naughton NN, et al. An experimental itch model in rats: Characterization of intracisternal morphine-induced scratching and antinociception. FASEB J. 1999;13:A802. doi: 10.1097/00000542-200003000-00023. 622.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MCH, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, et al. Activation of kappa opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther. 2003;305:173–179. doi: 10.1124/jpet.102.044909. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Yamaguchi T, Miyamoto T. Itch-scratch responses induced by opioids through central mu opioid receptors in mice. J Biomed Sci. 2000;7:248–252. doi: 10.1007/BF02255473. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Morita N, Kitabata Y, Yamanishi T, Kishioka S, Ozaki M, et al. Antinociceptive synergism between supraspinal and spinal sites after subcutaneous morphine evidenced by CNS morphine content. Brain Res. 1991;552:136–140. doi: 10.1016/0006-8993(91)90671-h. [DOI] [PubMed] [Google Scholar]
- Namba M, Ghatei MA, Anand P, Bloom SR. Distribution and chromatographic characterization of neuromedin B-like immunoreactivity in the human spinal cord. Brain Res. 1985;342:183–186. doi: 10.1016/0006-8993(85)91372-1. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the Care and Use of Laboratory Animals. 7. Washington DC: National Academic Press; 1996. [Google Scholar]
- O’Donohue TL, Massari VJ, Pazoles CJ, Chronwall BM, Shults CW, Quirion R, et al. A role for bombesin in sensory processing in the spinal cord. J Neurosci. 1984;4:2956–2962. doi: 10.1523/JNEUROSCI.04-12-02956.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose–response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–444. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Waddell AB, Holtzman SG. Naltrexone in vivo protects μ receptors from inactivation by β-funaltrexamine, but not κ receptors from inactivation by nor-binaltorphimine. Pharmacol Biochem Behav. 1993;46:813–817. doi: 10.1016/0091-3057(93)90206-9. [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Mika J, Labuz D, Toth G, Przewlocki R. Spinal analgesic action of endomorphins in acute, inflammatory and neuropathic pain in rats. Eur J Pharmacol. 1999;367:189–196. doi: 10.1016/s0014-2999(98)00956-x. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Lutz EA. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–261. doi: 10.1016/s0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- Thomas DA, Hammond DL. Microinjection of morphine into the rat medullary dorsal horn produces a dose-dependent increase in facial scratching. Brain Res. 1995;695:267–270. doi: 10.1016/0006-8993(95)00871-m. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Williams GM, Iwata K, Kenshalo DR, Jr, Dubner R. Effects of central administration of opioids on facial scratching in monkeys. Brain Res. 1992;585:315–317. doi: 10.1016/0006-8993(92)91227-6. [DOI] [PubMed] [Google Scholar]
- Tohda C, Yamaguchi T, Kuraishi Y. Intracisternal injection of opioids induces itch-associated response through mu-opioid receptors in mice. Jpn J Pharmacol. 1997;74:77–82. doi: 10.1254/jjp.74.77. [DOI] [PubMed] [Google Scholar]
- Tortella FC, Cowan A, Adler MW. Comparison of the anticonvulsant effects of opioid peptides and etorphine in rats after icv administration. Life Sci. 1981;29:1039–1045. doi: 10.1016/0024-3205(81)90464-1. [DOI] [PubMed] [Google Scholar]
- Van Wimersma Greidanus TJ, Donker DK, Walhof R, Van Grafhorst JC, De Vries N, Van Schaik SJ, et al. The effects of neurotensin, naloxone and haloperidol on elements of excessive grooming behavior induced by bombesin. Peptides. 1985;6:1179–1183. doi: 10.1016/0196-9781(85)90447-4. [DOI] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP, Jr, et al. Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain. 1997;71:57–64. doi: 10.1016/s0304-3959(97)03337-x. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Harty GJ. Pharmacology of the allodynia in rats evoked by high dose intrathecal morphine. J Pharmacol Exp Ther. 1988;244:501–507. [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther. 1977;202:411–428. [PubMed] [Google Scholar]
- Yamaguchi T, Kitagawa K, Kuraishi Y. Itch-associated response and antinociception induced by intracisternal endomorphins in mice. Jpn J Pharmacol. 1998;78:337–343. doi: 10.1254/jjp.78.337. [DOI] [PubMed] [Google Scholar]


