Abstract
Heavy metal pollution of soil is a significant environmental problem and has its negative impact on human health and agriculture. Rhizosphere, as an important interface of soil and plant, plays a significant role in phytoremediation of contaminated soil by heavy metals, in which, microbial populations are known to affect heavy metal mobility and availability to the plant through release of chelating agents, acidification, phosphate solubilization and redox changes, and therefore, have potential to enhance phytoremediation processes. Phytoremediation strategies with appropriate heavy metal-adapted rhizobacteria have received more and more attention. This article paper reviews some recent advances in effect and significance of rhizobacteria in phytoremediation of heavy metal contaminated soils. There is also a need to improve our understanding of the mechanisms involved in the transfer and mobilization of heavy metals by rhizobacteria and to conduct research on the selection of microbial isolates from rhizosphere of plants growing on heavy metal contaminated soils for specific restoration programmes.
Keywords: Rhizobacteria, Phytoremediation, Heavy metals, Rhizosphere
INTRODUCTION
Soil pollution by heavy metals
Heavy metals are conventionally defined as elements with metallic properties (ductility, conductivity, stability as cations, ligand specificity, etc.) and an atomic number >20. The most common heavy metal contaminants are Cd, Cr, Cu, Hg, Pb and Ni. Metals are natural components in soil with a number of heavy metals being required by plants as micronutrients. However, pollution of biosphere by toxic metals has accelerated dramatically since the beginning of the industrial revolution. As a result of human activities such as mining and smelting of metals, electroplating, gas exhaust, energy and fuel production, fertilizer, sewage and pesticide application, municipal waste generation, etc. (Kabata-Pendias and Pendias, 1989), metal pollution has become one of the most severe environmental problems today.
Excessive accumulation of heavy metals is toxic to most plants. Heavy metals ions, when present at an elevated level in the environment, are excessively absorbed by roots and translocated to shoot, leading to impaired metabolism and reduced growth (Bingham et al., 1986; Foy et al., 1978). Heavy metal contamination to water and soil poses a major environmental and human health problem. In addition, excessive metal concentrations in contaminated soils result in decreased soil microbial activity and soil fertility, and yield losses (McGrath et al., 1995). Cadmium, as a non-essential, toxic heavy metal to plants, which may well demonstrate the problem, can inhibit root and shoot growth, affect nutrient uptake and homeostasis, and is frequently accumulated by agriculturally important crops (Sanità di Toppi and Gabrielli, 1999). Thus, when Cd-enriched crop products are consumed by animals and humans, it can cause diseases. On condition that soil Cd pollution is cumulative with levels increasing over time, the soil may eventually become unusable for crop production. Similarly, contamination of soil with Cd can negatively affect biodiversity and the activity of soil microbial communities (McGrath, 1994). Burd et al.(1998)’s experiments revealed that canola seeds developed normally in the presence of up to 1 mmol/L nickel chloride, but that plant root and shoot elongation were inhibited at higher levels.
Remediation technologies
Heavy metals cannot be destroyed biologically (no “degradation”, change in the nuclear structure of the element, occurs) but are only transformed from one oxidation state or organic complex to another (Garbisu and Alkorta, 2001), remediation of heavy metal contamination in soils is more difficult.
Until now, methods used for their remediation such as excavation and land fill, thermal treatment, acid leaching and electroreclamation are not suitable for practical applications, because of their high cost, low efficiency, large destruction of soil structure and fertility and high dependence on the contaminants of concern, soil properties, site conditions, and so on. Thus, the development of phytoremediation strategies for heavy metals contaminated soils is necessary (Chaney et al., 2000; Cheng et al., 2002; Lasat, 2002).
Phytoremediation assisted by soil rhizobacteria
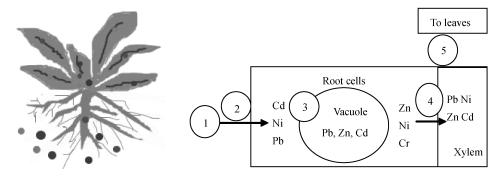
Phytoremediation, the use of plants to extract, sequester, and/or detoxify pollutants through physical, chemical, and biological processes (Cunningham and Ow, 1996; Saxena et al., 1999; Wenzel et al., 1999), has been reported to be an effective, in situ, non-intrusive, low-cost, aesthetically pleasing, ecologically benign, socially accepted technology to remediate polluted soils (Alkorta and Garbisu, 2001; Garbisu et al., 2002; Weber et al., 2001). It also helps prevent landscape destruction and enhances activity and diversity of soil microorganisms to maintain healthy ecosystems, which is consequently considered to be a more attractive alternative than traditional methods to the approaches that are currently in use for dealing with heavy metal contamination (Bogardt and Hemmingsen, 1992; Cunningham and Berti, 1993; Cunningham and Ow, 1996; Cunningham et al., 1995; Salt et al., 1995). Fig.1 plots the process of metal uptake and accumulation in plants.
Fig. 1.
Metal transfer in plants
1: A metal iron is sorbed at root surface; 2: Bioavailable metal moves across cellular membrane into root cells; 3: A fraction of the metal absorbed into roots is immobilized in the vacuole; 4: Intracellular mobile metal crosses cellular membranes into root vascular tissue (xylem); 5: Metal is translocated from the root to aerial tissues (stems and leaves)
Phytoremediation of heavy metals may take one of several forms: phytoextraction, rhizofiltration, phytostabilization, and phytovolatilization. Phytoextraction refers to processes in which plants are used to concentrate metals from the soil into the roots and shoots of the plant; rhizofiltration is the use of plant roots to absorb, concentrate or precipitate metals from effluents; and phytostabilization is the use of plants to reduce the mobility of heavy metals through absorption and precipitation by plants, thus reducing their bioavailability; phytovolatilization is the uptake and release into the atmosphere of volatile materials such as mercury- or arsenic-containing compounds.
The ideal plant for phytoextraction should grow rapidly, produce a high amount of biomass, and be able to tolerate and accumulate high concentrations of metals in shoots. Most of the commonly known heavy metal accumulators belong to the Brassicaceae family (Kumar et al., 1995). Although hyperaccumulator plants have exceptionally high metal accumulating capacity, most of these have a slow growth rate and often produce limited amounts of biomass when the concentration of available metal in the contaminated soil is very high. An alternative is to use species with a lower metal accumulating capacity but higher growth rates, such as Indian mustard (Brassica juncea); another alternative is to provide them with an associated plant growth-promoting rhizobacteria, which also is considered to be an important component of phytoremediation technology (Wenzel et al., 1999; Glick, 2003). Obviously, the rhizosphere contains a large microbial population with high metabolic activity compared to bulk soil (Anderson et al., 1993). Microbial populations are known to affect heavy metals mobility and availability to the plant through release of chelating agents, acidification, phosphate solubilization, and redox changes (Abou-Shanab et al., 2003a; Smith and Read, 1997). Especially, some plant growth-promoting bacteria associated with plant roots also may exert some beneficial effects on plant growth and nutrition through a number of mechanisms such as N2 fixation, production of phytohormones and siderophores, and transformation of nutrient elements when they are either applied to seeds or incorporated into the soil (Kloepper et al., 1989; Glick, 1995; Glick et al., 1999). The use of rhizobacteria in combination with plants is expected to provide high efficiency for phytoremediation (Abou-Shanab et al., 2003a; Whiting et al., 2001). Therefore, the potential and the exact mechanism of rhizobacteria to enhance phytoremediation of soil heavy metals pollution have recently received some attention (de Souza et al., 1999a; Whiting et al., 2001). For example, Burd et al.(1998) observed that both the number of Indian mustard seeds that germinated in a nickel-contaminated soil, and the attainable plant size increased by 50%~100% by the addition of K. ascorbata SUD165/26, an associated plant growth-promoting rhizobacteria, to the soil in preliminary field trials, and de Souza et al.(1999b) investigated phytoremediation of Se and Hg in constructed wetlands and found that accumulation of Se and Hg were enhanced by rhziobacteria in wetland plant tissues.
INTERACTIONS IN RHIZOSPHERE
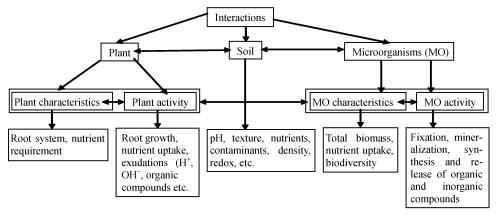
Potential for phytoremediation depends upon the interactions among soil, heavy metals, bacteria, and plants. As shown schematically in Fig.2, these complex interactions are affected by a variety of factors, such as characteristics and activity of plant and rhizobateria, climatic conditions, soil properties, etc.
Fig. 2.
Plant-soil-microbial interactions in the rhizosphere
Plant-bacteria interactions
The roots of plants interact with a large number of different microorganisms, with these interactions being major determinants of the extent of phytoremediation (Glick, 1995). The functioning of associative plant-bacterial symbioses in heavy-metal-polluted soil can be affected from the side of both the micropartner (plant-associated bacteria) and the host plant. Soil microbes play significant roles in recycling of plant nutrients, maintenance of soil structure, detoxification of noxious chemicals, and control of plant pests and plant growth (Elsgaard et al., 2001; Filip, 2002; Giller et al., 1998). Thus, bacteria can augment the remediation capacity of plants or reduce the phytotoxicity of the contaminated soil. In addition, plants and bacteria can form specific associations in which the plant provides the bacteria with a specific carbon source that induces the bacteria to reduce the phytotoxicity of the contaminated soil. Alternatively, plants and bacteria can form nonspecific associations in which normal plant processes stimulate the microbial community, which in the course of normal metabolic activity degrades contaminants in soil. Plants roots can provide root exudate, as well as increase ion solubility. These biochemical mechanisms increase the remediation activity of bacteria associated with plant roots. To sum up, the adaptation capabilities of both partners of the associative symbiosis as well as the bioremediation potential of the microsymbiont are of importance in minimizing the detrimental effect of heavy-metal pollution.
Heavy metal-bacteria interactions
Rhizobacteria have been shown to possess several traits that can alter heavy metals bioavailability (Lasat, 2002; McGrath et al., 2001; Whiting et al., 2001) through the release of chelating substances, acidification of the microenvironment, and by influencing changes in redox potential (Smith and Read, 1997). For example, Abou-Shanab et al.(2003a) reported that the addition of Sphingomonas macrogoltabidus, Microbacterium liquefaciens, and Microbacterium arabinogalactanolyticum to Alyssum murale grown in serpentine soil significantly increased the plant uptake of Ni when compared with the un-inoculated controls as a result of soil pH reduction.
However, heavy metals are known to be toxic to plants and most organisms when present in soils in excessive concentrations. Giller et al.(1998) reported that there was a detrimental effect to soil microbial diversity and microbial activities (indexes of microbial metabolism and of soil fertility) in metal-polluted environments.
Plant-bacteria-soil interactions
The specificity of the plant-bacteria interaction is dependent upon soil conditions, which can alter contaminant bioavailability, root exudate composition, and nutrient levels. In addition, the metabolic requirements for heavy metals remediation may also dictate the form of the plant-bacteria interaction i.e., specific or nonspecific.
Along with metal toxicity, there are often additional factors that limit plant growth in contaminated soils including arid conditions, lack of soil structure, low water supply and nutrient deficiency.
EFFECT OF RHIZOBACTERIA ON PHYTOREMEDIATION
Plant-growth
Rhizosphere microorganisms, which are closely associated with roots, have been termed plant growth promoting rhizobacteria (PGPR) (Glick, 1995). Plant growth-promoting rhizobacteria include a diverse group of free-living soil bacteria that can improve host plant growth and development in heavy metal contaminated soils by mitigating toxic effects of heavy metals on the plants (Belimov et al., 2004). Table 1 shows effects of variations of PGPR on plant. It is well known that heavy metals can even be toxic for metal-accumulating and metal-tolerant plants, if the concentration of metals in the environment is sufficiently high. This is partly attributable to iron deficiency in a range of different plant species (Mishra and Kar, 1974; Ma and Nomoto, 1993; Römheld and Marschner, 1986; Wallace et al., 1992) in heavy metal contamination soil. Furthermore, the low iron content of plants that are grown in the presence of high levels of heavy metals generally results in these plants becoming chlorotic, since iron deficiency inhibits both chloroplast development and chlorophyll biosynthesis (Imsande, 1998). However, microbial iron-siderophore complexes can be taken up by plants, and thereby serve as an iron source for plants (Bar-Ness et al., 1991; Reid et al., 1986; Wang et al., 1993). It was therefore reasoned that the best way to prevent plants from becoming chlorotic in the presence of high levels of heavy metals was to provide them with an associated siderophore-producing bacterium. This suggests that some plant growth-promoting bacteria can significantly increase the growth of plants in the presence of heavy metals including nickel, lead and zinc (Burd et al., 1998; 2000), thus allowing plants to develop longer roots and get better established during early stages of growth (Glick et al., 1998). Once the seedling is established, the bacterium can also help the plant acquire sufficient iron for optimal plant growth. Similarly, chromium-resistant pseudomonads, isolated from paint industry effluents, were able to stimulate seed germination and growth of Triticum aestivus in the presence of potassium bichromate (Hasnain and Sabri, 1996). In this case, the bacterial enhancement of seedling growth was associated with reduced chromium uptake. The effect of adding K. ascorbata SUD165, a plant growth-promoting bacterium, to canola or tomato seeds before the seeds germinate, was also examined in the presence of inhibitory concentrations of Ni2+. The results of these experiments showed that at all concentrations of nickel tested (1 to 6 mmol/L Ni2+), using both a low-level and a high-level bacterial cell treatment (cell suspension absorbance of 0.025 or 0.50, respectively), with both canola and tomato plants, with both roots and shoots, and in both pouches and pots, the addition of K. ascorbata SUD165 significantly decreased the toxicity of the added nickel (Burd et al., 1998).
Table 1.
Effects of PGPR on plant
| PGPR | Plant | Effects | References |
| Delftia tsuruhatensis, strain HR4, Pseudomonas corrugata 13 or Pseudomonas aureofaciens 63±28 | Rice, cucumber | Suppressant to plant pathogens | Han et al., 2005; Kamnev et al., 2005 |
| Bacillus licheniformis CECT5106, B. pumilus CECT5105, Bacillus licheniformis, Serratia proteamaculans 1-102, Serratia liquefaciens 2-68, Pseudomonas sp. strain GRP3, Pseudomonas alcaligenes PsA15, P. denitrificans PsD6, Bacillus polymyxa BcP26, Mycobacterium phlei MbP18 | Pinus pinea, European alder, corn, mung bean, cotton and pea | Promotion of growth and nutrient uptake of plant | Probanza et al.,2002; Ramos et al., 2003; Pan et al., 1999; Sharma et al., 2003; Egamberdiyeva and Höflich, 2004 |
| Kluyvera ascorbata SUD165 and SUD165/26, Pseudomonas tolaasii RP23 and Pseudomonas fluorescens RS9, Variovorax paradoxus, Rhodococcus sp. and Flavobacterium sp. | Tomoto, canola, perennial grasses (Graminaceae), and Indian mustard (Brassica juncea L. Czern.) | Resistant to Cd, Zn, Cu, Ni, Co, Cr, Pb and stimulation to root elongation of plant seedlings | Burd et al.,1998; 2000; Dell’Amico et al., 2005; Belimov et al., 2005 |
| Achromobacter piechaudii ARV8 | Tomatoes and peppers | Resistant to water and salt stress | Mayak et al.,2004a; 2004b |
Bacteria in the rhizosphere are involved in the accumulation of potentially toxic trace elements into plant tissues. de Souza et al.(1999b) found that axenic saltmarsh bulrush plants supplied with different rhizosphere bacteria accumulated (70±80)% higher Se concentrations in their roots and (40±60)% higher Se concentrations in shoots than plants grown under axenic conditions. Four out of the six bacterial strains tested significantly enhanced Se accumulation in roots and shoots of axenic plants when they were added as pure cultures (P<0.05; n=3). A mixture of the six bacterial strains tested also enhanced Se accumulation in axenic bulrush plants. However, there were some opposite viewpoints that the presence of ectomycorrhizal or vesicular-arbuscular fungi on the roots of plants decreased the uptake of metals by the plants and thereby increased plant biomass (Bradley et al., 1982; Brown and Wilkins, 1985; Dueck et al., 1986; Heggo et al., 1990; Killham and Firestone, 1983; Tam, 1995). The reason might be that some plants involve the use of plant growth-promoting bacteria or mycorrhizal fungi to lessen the deleterious effects of heavy metals.
Rhizospheric microorganism
The rhizobacteria of metal accumulating and hyperaccumulating plants and their role in the tolerance to and uptake of heavy metals by the plants have been studied. Research has also shown that many rhizobacteria are tolerant to heavy metals and play important roles in mobilization or immobilization of heavy metals (Gadd, 1990).
It is now well-clarified that the population of rhizobateria is several orders of magnitude greater than that in the bulk soil with the elevated levels of heavy metals in these soils have significant impacts on microorganism population size, community structure, and overall activity of the soil microbial communities. Experiments showed that the number of bacteria in the rhizosphere of D. fusca reached 1.0×107 CFU/g. This relatively low bacterial count can be attributed to the presence of heavy metals in high concentrations (39 mg Co/kg, 3 mg Cd/kg, 79 mg Ni/kg, 30 mg Cu/kg, 4834 mg Zn/kg, 123 mg Cr/kg and 114 mg Pb/kg dry soil) (Abou-Shanab et al., 2005). Chaudri et al.(1992) also found that rhizobium populations were reduced at concentrations >7 mg/kg soil in their Cd treatments. Field studies of metal contaminated soils have similarly demonstrated that elevated metal loadings can result in decreased microbial community size (Brookes and McGrath, 1984; Chander and Brookes, 1991; Jordan and LeChevalier, 1975; Konopka et al., 1999).
Besides, the microorganism community structure of the rhizosphere population is important in the context of plant growth. This is largely attributed to the finding that microbial populations often establish some sort of positive cooperation with the host plant system. For example, soil pollution with heavy metals could lead to the appearance of heavy-metal resistant rhizobateria in the soil of industrial regions (Aleem et al., 2003). It was revealed that a high proportion of metal resistant bacteria persist in the rhizosphere of the hyperaccumulators Thalaspi caerulescens (Delorme et al., 2001) and Alyssum bertolonii (Mengoni et al., 2001) or Alyssum murale (Abou-Shanab et al., 2003a) grown in soil contaminated with Zn and Ni or Ni, respectively. The presence of rhizobacteria increased concentrations of Zn (Whiting et al., 2001), Ni (Abou-Shanab et al., 2003b) and Se (de Souza et al., 1999a) in T. caerulescens, A. murale and B. juncea, respectively.
Multiple metal-resistance (MMR) in bacteria seems to be the rule rather than the exception. Abou-Shanab et al.(2005) tested the patterns of tolerance of the heavy metals in the 107 rhizobacterial isolates at 1 mmol/L concentrations and found all the rhizobacterial strains to be tolerant to multiple metal ions. Strains with hexa-, penta-, tetra-, and tri-metal ions tolerant, respectively, were found more frequent than those with hepta-, double and mono-tolerance. Notably cadmium, copper, lead, and nickel resistance seemed to be restricted to those strains which were resistant to six metals or more. Similar observations have been previously reported by Sabry et al.(1997).
High levels of heavy metals could decrease rhizobateria metabolic activity, biomass and diversity (Gremion et al., 2004; Sandaa et al., 1999). The activities of the large population of bacteria inhabiting the rhizosphere can also be expected to influence heavy metals uptake by plants. It is reported that under non-sterile soil system, plants showed no iron-deficiency symptoms and have fairly high iron level in roots in contrast to plants grown in sterile system. This can attribute to rhizospheric microbial activity, which plays an important role in iron acquisition (Masalha et al., 2000).
Some rhizobateria can exude a class of rhizobateria secretion, such as antibiotics (including the antifungals), phosphate solubilization, hydrocyanic acid, indoleacetic acid (IAA), siderophores, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase which increase bioavailability and facilitate root absorption of heavy metals, such as Fe (Crowley et al., 1991) and Mn (Barber and Lee, 1974), as well as nonessential metals, such as Cd (Salt et al., 1995), enhance tolerance of host plants by improving the P absorption (Davies et al., 2001; Liu et al., 2000) and promote plant growth (Budzikiewicz, 1997; Duffy and Défago, 1999; Burd et al., 2000; Ellis et al., 2000; Meyer, 2000). Abou-Shanab et al.(2005) investigated the correlation between metal resistance and metal mobilization abilities of rhizobacteria under heavy metals stress. The highest incidence of the biochemical activity of isolates and metal resistance was recorded for: phosphate solubilizers with Cr, Zn and Pb (92.5%, 82.2% and 68.2%), respectively; then for siderophore producers with Cr, Zn and Pb (78.5%, 71.02% and 61.6%), respectively, and finally for acid producers with Cr, Zn and Pb (63.5%, 53.3% and 42.9%), respectively. This implies that phosphate solubilization is not only the mechanism adopted by bacteria towards metals in soil; but that siderophores and acid production are involved in mobilizing metals.
It should be mentioned that IAA production by rhizobacteria is believed to play an important role in plant-bacterial interactions (Lambrecht et al., 2000). Therefore, any direct influence on IAA production by bacteria may in turn affect their phytostimulating efficiency. It has been well documented that the biosynthesis of auxins with their excretion into soil makes a major contribution to the bacterial plant-growth-promoting effect (Lambrecht et al., 2000; Kamnev, 2003; Steenhoudt and Vanderleyden, 2000). It has been found that Cu2+ and Cd2+ significantly suppressed the production of IAA (auxin) by non-endophytic and facultatively endophytic strains of A. brasilense (Sp7 and Sp245, respectively), which can directly affect the plant-growth-stimulating efficiency of associative plant-bacterial symbioses in heavy-metal-polluted soils (Kamnev et al., 2005). Kamnev et al.(2005) similarly discovered that both Cu2+ and Cd2+ ions significantly decreased the level of IAA production for strain Sp7, whereas the bacterial growth rate was virtually not affected.
In addition, some plant growth-promoting bacteria i.e., free-living soil bacteria that are involved in a beneficial association with plants, contain the enzyme ACC deaminase (Glick, 1995; Glick et al., 1995; Jacobson et al., 1994), which can cleave the plant ethylene precursor ACC and lower the level of ethylene in a developing or stressed plant. Plant growth-promoting bacteria that contain ACC deaminase may act to insure that the ethylene level does not impair root growth (Glick et al., 1998), and that by facilitating the formation of longer roots, these bacteria may enhance seedling survival and plant root growth.
Other properties of this bacterial strain, in addition to ACC deaminase activity, may contribute to this result: various N2-fixing and auxin-producing PGPR, siderophores, and antibiotics, all of which may stimulate plant growth in the presence of toxic metals concentrations. For example, Masalha et al.(2000) reported that plants grown under non-sterile soil systems were better in terms of iron nutrition to those grown under sterile condition. Their data emphasize the role of microbial community on the iron nutrition of plants. In fact, there is evidence that at least part of the toxic effects of some heavy metals in plants results from an induced iron deficiency, and since bacterial siderophores can provide iron to various plants (Bar-Ness et al., 1991; Reid et al., 1986; Wang et al., 1993), siderophores produced by rhizobacteria may reduce nickel toxicity by supplying the plant with iron and hence reducing the severity of nickel toxicity (Bingham et al., 1986; Bollard, 1983; Foy et al., 1978; Yang et al., 1996).
Bioavailability of toxic heavy metals
Soil rhizobateria can also directly influence metal solubility by changing heavy metal speciation in the rhizosphere. Study of the roles of mycorrhiza in metal speciation in the rhizosphere and the impact on increasing host plant tolerance against excessive heavy metals in soil showed that speciations of Cu, Zn and Pb changed significantly in the rhizosphere of AM (arbuscular mycorrhiza) infected and non-infected maize in comparison to bulk soil; The greatest change was exchangeable Cu that increased by 26% and 43% in non-infected and AM-infected rhizosphere, respectively, than in bulk soil. With the exception of organic bound Cu in AM, other speciations were stable in the rhizosphere of AM and non-AM treatments. It is understandable that Cu was activated by inducing rhizobacteria (Huang et al., 2005). The organic bound Zn and Pb increased significantly in the rhizosphere in comparison to those in the bulked soil. In contrast, carbonate and Fe-Mn oxides of Zn and Pb did not exhibit significant changes. The results might indicate that mycorrhiza could protect its host plants from the phytotoxicity of excessive copper, zinc and lead by changing the speciation from bioavailable to the non-bioavailable form. The fact that copper and zinc accumulation in the roots and shoots of mycorrhiza infected plants were significantly lower than those in the non-infected plants might also suggest that mycorrhiza efficiently restricted excessive copper and zinc absorptions into the host plants (Huang et al., 2005).
Soil properties
Evidence has been shown that the chemical conditions of the rhizosphere differ from those of the bulk soil, as a consequence of various processes that are induced by plant roots and/or by the rhizobacteria (Hinsinger, 2001; Marschner, 1995). Plant-bacteria interactions could stimulate the production of compounds that could alter soil chemical properties in rhziosphere and enhance heavy metals accumulation in plants. For example, Delorme et al.(2001) found that soil acidification in the rhizosphere of Thalaspi caerulescens facilitates metal ion uptake by increasing metal ion mobility around the roots. de Souza et al.(1999b) also reported that the accumulation of Hg increases when the pH of the culture solution is lowered and hypothesized that rhziobacteria of the plants reduced the pH in the rhizosphere, thereby increasing Hg uptake into plants. A further study of the influence of hydrogen and aluminum ions on the growth of the associative nitrogen-fixing and growth-promoting bacteria Azospirillum lipoferum 137, Arthrobacter mysorens 7, Agrobacterium radiobacter 10, and Flavobacterium sp. L30 showed that the response of plants to the inoculation strongly varied from positive to negative with the soil pH (Belimov et al., 1998). In addition, Microorganisms can remove a number of metals from the environment by reducing them to a lower redox state (Lovley, 1995). Many of the microorganisms that catalyze such reactions use the metals as terminal electron acceptors in anaerobic respiration. Such microorganisms, known as dissimilatory metal-reducing bacteria, are phylogenetically (Lonergan et al., 1996) and physiologically (Lovley et al., 1997) diverse; although, most share the ability to use Fe3+ and S0 as terminal electron acceptors (Lovley et al., 1997). The microbial reduction of Cr6+ to Cr3+ has been one of the most widely studied forms of metal bioremediation (Lovley, 1995; Wang and Shen, 1995). A wide diversity of heterotrophic organisms are known to carry out this reaction which, depending upon the organism, can take place anaerobically or aerobically (Wang and Shen, 1995; Lovley, 1993).
Plant pathogens
Plant growth-promoting bacterial could induce resistance in plants against fungal, bacterial and viral diseases (Maurhofer et al., 1998), and insect (Zehnder et al., 1997) and nematode pests (Sikora, 1992). The induction of systemic resistance by rhizobacteria is referred to as ISR. In recent years, the use of PGPR as an inducer of systemic resistance in crop plants against different pathogens has been demonstrated under field conditions (Wei et al., 1991; 1996; Vidhyasekaran and Muthamilan, 1999; Viswanathan and Samiyappan, 1999). Nie et al.(2002) reported that antibiotic-secreting plant growth-promoting bacterial strains can inhibit the proliferation and subsequent invasion of phytopathogens, hence protecting plants from further damage in the presence of arsenate. Experiments showed that seed treated with P. fluorescens strain 97 protected beans against halo blight disease caused by Pseudomonas syringae pv. phaseolicola (Alstrom, 1991). Reports on PGPR-mediated ISR against insects are restricted to very few crops. Induction of systemic resistance by PGPR strains, viz., P. putida strain 89B-27, S. marcescens strain 90-166, Flavomonas oryzihabitans strain INR-5 and Bacillus pumilus strain INR-7 have significantly reduced populations of the striped cucumber beetle, Acalyma vittatum and the spotted cucumber beetle, Diabrotica undecimpunctata howardi on cucumber (Zehnder et al., 1997). PGPR also induces systemic resistance against nematode pests (Oostendorp and Sikora, 1990; Sikora, 1992; Sikora and Hoffmann-Hergarten, 1992). P. fluorescens has induced systemic resistance and inhibited early root penetration of Heterodera schachtii, the cyst nematode in sugar beet (Oostendorp and Sikora, 1989; 1990).
MECHANISMS OF RHZIOBACTERIA INFLUENCING HEAVY METAL ACCUMULATION
Rhizobacteria secretion
As mentioned above, rhziobacteria secretion may play a major role among mechanisms of phytoremediation assisted by rhziobacteria. Indirect mechanisms include preventing phytopathogens from inhibiting plant growth and development while direct mechanisms include: nitrogen fixation; synthesis of siderophores which can solubilize and sequester iron from the soil; production of phytohormones such as auxins and cytokinins, which can enhance plant growth; and solubilization of minerals such as phosphorus (Kloepper et al., 1989; Glick, 1995; Glick et al., 1999; Patten and Glick, 1996).
Rhizobacteria produce metal-chelating agents called siderophores, which have an important role in the acquisition of several heavy metals (Leong, 1986). These organic substances have the effect of scavenging Fe3+ and significantly enhancing the bioavailability of soil bound iron (Kanazawa et al., 1994). It has also been recognized that plants grown in metal-contaminated soils are often iron deficient, the production of siderophores by plant growth-promoting bacteria may help plants obtain sufficient iron (Burd et al., 2000; Wallace et al., 1992). Microbial siderophores are used as iron chelating agents that can regulate the availability of iron in the plant rhizosphere (Bar-Ness et al., 1992; Loper and Henkels, 1999). It has been assumed that competition for iron in the rhizosphere is controlled by the affinity of the siderophore for iron and ultimately decides the rhizosphere population structure. The important factors, which participate, are concentration of various types of siderophore, kinetics of exchange, and availability of Fe-complexes to microbes as well as plants (Loper and Henkels, 1999). Interestingly, the binding affinity of phytosiderophores for iron is less than the affinity of microbial siderophores, but plants require a lower iron concentration for normal growth than do microbes (Meyer, 2000).
A number of PGPR, which stimulate root growth of different plant species including Indian mustard (Burd et al., 1998; Belimov et al., 2001), contain the enzyme ACC deaminase, which hydrolyses and decreases the amount of ACC, an ethylene precursor of the plant hormone ethylene, in plants and, as a result, to decrease ethylene biosynthesis by plants (Glick et al., 1994; 1998; Hall et al., 1996). The model which represents how a PGPR bound to either a seed or plant root lowers the ethylene concentration and thereby prevents ethylene inhibition of root elongation was previously proposed by Glick et al.(1998). In some of the plants, ACC is exuded from roots or seeds and then taken up by the bacterium and cleaved by ACC deaminase to ammonia and α-ketobutyrate (Glick et al., 1998). The bacteria utilize the ammonia evolved from ACC as a nitrogen source and thereby decrease ACC within the plant (Penrose and Glick, 2001) with the concomitant reduction of plant ethylene and promoting root elongation (Burd et al., 1998; Mayak et al., 1999; Grichko and Glick, 2001; Belimov et al., 2002). To maintain the gradient between internal and external ACC levels, the plant must exude increasing amounts of ACC. The lowering of ACC levels within the plant results in a reduction in the amount of plant ethylene and a decreased extent of ethylene inhibition of plant seedling root elongation. This model may also be invoked to explain how plant growth-promoting bacteria lower the concentration of stress ethylene in plants. Evidence for this model includes the fact that the ability of a bacterium to promote root elongation is positively correlated with both the ACC deaminase activity of the bacterium and the ACC content (measured by high-pressure liquid chromatography) of the plant tissues.
In addition, depending on the conditions, plant root growth may also be stimulated by IAA produced by PGPR bound to the seeds or roots (Patten and Glick, 2002). As a matter of fact, low levels of IAA produced by rhizobacteria promote primary root elongation, whereas high levels of IAA stimulate lateral and adventitious root formation (Glick, 1995) but inhibit primary root growth (Xie et al., 1996). Thus, plant growth-promoting bacteria can facilitate plant growth by altering the hormonal balance within the affected plant (Glick et al., 1999). Similarly, although an ethylene pulse is important in breaking seed dormancy, too much ethylene can inhibit plant seed germination (Bewley and Black, 1985; Mayer and Poljakoff-Mayber, 1989; Smalle and van der Straeten, 1997). As just described above, a significant portion of the damage to plants from infection with fungal phytopathogens may occur as a direct result of the response of the plant to the increased level of stress ethylene (van Loon, 1984). In the presence of fungal pathogens, not only does exogenous ethylene increase the severity of a fungal infection but also inhibitors of ethylene synthesis can significantly decrease the severity of infection. Since the enzyme ACC deaminase, when present in plant growth-promoting bacteria, can act to modulate the level of ethylene in a plant, lower the stress placed on plants by the presence of heavy metals and therefore ameliorate some of the apparent toxicity of heavy metals to plants.
High surface area-to-volume ratio
Soil rhizobacteria, with activity and a high surface area-to-volume ratio because of their small size and therefore providing a large contact area, may have the potential to act as microbial chelates associated with phytoremediation (Anderson et al., 1993; Kärenlampi et al., 2000; Sitaula et al., 1999). Indian mustard plants germinated on Se-containing media from axenic seeds coated with bacteria produced more root hairs and accumulated more Se than plants grown from axenic seeds (de Souza et al., 1999a). However, increased root surface area caused by bacteria cannot solely account for the increased heavy metals accumulation, because bacteria were not involved in the accumulation of other heavy metals. Antibiotic experiments performed with bulrush and rabbitfoot grass supplied with chromate and arsenate showed no differences between Cr and As accumulation in antibiotic-supplied and untreated plants (de Souza et al., 1999b).
Transform toxic heavy metals
The efficiency of phytoremediation is also influenced by the bioavailability of metals to plants in soil. Bacteria may transform toxic heavy metals to forms that are more readily taken up into roots. For example, bacteria could enhance Se accumulation in plants by reducing selenate to organic Se, and organoselenium forms like SeMet are known to be taken up at faster rates into roots than inorganic forms (Zayed et al., 1998). Huang et al.(2005) further depicts the relative changes as the percentages of the speciation concentration difference between bulked soil and rhizosphere to the concentration of bulked soil. Results showed that the relative changes of organic bound Cu, Zn and Pb were, respectively, +5%, +23%, +3% in the infected rhizosphere, and 0.8%, −3%, −2% in the noninfected rhizosphere. Thus, significant amounts of Cu, Zn and Pb were bounded by organic matter in the infected rhizosphere.
Soil rhizobateria can also directly influence metal bioavailability by altering their chemical properties, such as pH, organic matter content, redox state, etc. This can aid in the leaching of these contaminants from soils. The bioavailability of heavy metals in soils is a function of its solubility (Ernst, 1996) with pH and organic matter content being the main controlling factors (Gray et al., 1998). For example, a strain of Pseudomonas maltophilia was shown to reduce the mobile and toxic Cr6+ to nontoxic and immobile Cr3+, and also to minimize environmental mobility of other toxic ions such as Hg2+, Pb2+, and Cd2+ (Blake et al., 1993; Park et al., 1999).
Inhibition of plant pathogens
PGPR provides different mechanisms for suppressing plant pathogens. They include competition for nutrients and space (Elad and Baker, 1985; Elad and Chet, 1987), antibiosis by producing antibiotics viz., pyrrolnitrin, pyocyanine, 2,4-diacetyl phloroglucinol (Pierson and Thomashow, 1992) and production of siderophores (fluorescent yellow green pigment), viz., pseudobactin which limits the availability of iron necessary for the growth of pathogens (Kloepper et al., 1980; Lemanceau et al., 1992). Other important mechanisms include production of lytic enzymes such as chitinases and β-1,3-glucanases which degrade chitin and glucan present in the cell wall of fungi (Fridlender et al., 1993; Lim et al., 1991; Potgieter and Alexander, 1996; Velazhahan et al., 1999), HCN production (Défago et al., 1990) and degradation of toxin produced by pathogen (Borowitz et al., 1992; Duffy and Défago, 1997).
Stimulation of transport protein
Bacterial survival and proliferation in the environment as well as within various hosts are critically dependent on the uptake and sequestration of transition metals such as manganese, zinc, and iron. For example, cells may stringently regulate intracellular zinc levels, since high concentrations of zinc are toxic to cellular functions and have evolved several types of proteins involved in binding and transport of zinc (Claverys, 2001). Bacteria may also stimulate the sulfate transport protein, located in the root plasma membrane, which also transports selenate (Leggett and Epstein, 1956). Inorganic Hg uptake in higher plants has not been well investigated, but has been linked to the passive uptake of lipophilic chloride complexes in phytoplankton (Mason et al., 1996).
CONCLUSION
When evaluating the effect of rhizobacteria on phytoremediation in contaminated soil, regardless of the precise effects used by the bacterium to protect plants, the results from literature suggest that certain bacteria may eventually find a use in the development of phytoremediation strategies. In this regard, heavy metals may be removed from polluted soil either by increasing the metal-accumulating ability of plants or by increasing the amount of plant biomass. In heavily contaminated soil where the metal content exceeds the limit of plant tolerance, it may be possible to treat plants with plant growth-promoting rhizobacteria, increasing plant biomass and thereby stabilizing, revegetating, and remediating metal-polluted soils.
However, there are many areas of poor understanding or lack of information where more research is needed. They include:
1. Little has been done to investigate the microorganism induced changes in the rhizosphere of hyperaccumulator plants in relation to metal accumulation. Similarly, it is difficult to clarify specific features of microbial-plant and microorganism-soil interactions in the rhizosphere;
2. Further research is also needed to quantify the effect of rhizospheric processes induced by rhizobacteria on the phytoavailability of heavy metals;
3. Minimal work has been done to examine heavy metal speciation changes in the rhizosphere and to determine whether such changes could have altered the accumulation and distribution of heavy metals;
4. Rhizobacteria encounter soil solution before it enters the root and the sequestration of heavy metals by rhizobacteria from soil solution may play an important part in plant metals uptake. The role played by bacteria from soil solution in plant Cd uptake is still poorly understood;
5. Finally, we need to further understand the mechanisms involved in mobilization and transfer of metals in order to develop future strategies and optimize the phytoextraction process. Such knowledge may enable us to understand the role and mechanism of soil rhizobacteria on phytoremediation.
To sum up, although the use of rhizobateria in combination with plants could provide high efficiency for phytoremediation, the microbial ecology in the rhizosphere is not yet fully understood.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 20577044), the National Basic Research Program (973) of China (No. 2002CB410804), and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT0536), China
References
- 1.Abou-Shanab RA, Angle JS, Delorme TA, Chaney RL, van Berkum P, Moawad H, Ghanem K, Ghozlan HA. Rhizobacterial effects on nickel extraction from soil and uptake by Alyssum murale . N Phytol. 2003;158(1):219–224. doi: 10.1046/j.1469-8137.2003.00721.x. [DOI] [Google Scholar]
- 2.Abou-Shanab RA, Delorme TA, Angle JS, Chaney RL, Ghanem K, Moawad H, Ghozlan HA. Phenotypic characterization of microbes in the rhizosphere of Alyssum murale . Int J Phytoremediation. 2003;5(4):367–379. doi: 10.1080/16226510390268766. [DOI] [PubMed] [Google Scholar]
- 3.Abou-Shanab RA, Ghozlan H, Ghanem K, Moawad H. Behaviour of bacterial populations isolated from rhizosphere of Diplachne fusca dominant in industrial sites. World J Microbiol Biotechnol. 2005;21(6-7):1095–1101. doi: 10.1007/s11274-004-0005-6. [DOI] [Google Scholar]
- 4.Aleem A, Isar J, Malik A. Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizospheric soil. Bioresour Technol. 2003;86(1):7–13. doi: 10.1016/S0960-8524(02)00134-7. [DOI] [PubMed] [Google Scholar]
- 5.Alkorta I, Garbisu C. Phytoremediation of organic contaminants. Bioresour Technol. 2001;79(3):273–276. doi: 10.1016/S0960-8524(01)00016-5. [DOI] [PubMed] [Google Scholar]
- 6.Alstrom S. Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere pseudomonads. J Gen Appl Microbiol. 1991;37(6):495–501. [Google Scholar]
- 7.Anderson TA, Guthrie EA, Walton BT. Bioremediation in the rhizosphere: plant roots and associated microbes clean contaminated soil. Environ Sci Technol. 1993;27(13):2630–2636. doi: 10.1021/es00049a001. [DOI] [Google Scholar]
- 8.Barber SA, Lee RB. The effect of microorganisms on the absorption of manganese by plants. N Phytol. 1974;73(1):97–106. doi: 10.1111/j.1469-8137.1974.tb04610.x. [DOI] [Google Scholar]
- 9.Bar-Ness E, Chen Y, Hadar Y, Marchner H, Romheld V. Siderophores of Pseudomonas putida as an iron source for dicot and monocot plants. Plant Soil. 1991;130(1-2):231–241. doi: 10.1007/BF00011878. [DOI] [Google Scholar]
- 10.Bar-Ness E, Hadar Y, Chen Y, Shanzer A, Libman J. Iron uptake by plant from microbial siderophores. Plant Physiol. 1992;99(4):1329–1335. doi: 10.1104/pp.99.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belimov AA, Kunakova AM, Gruzdeva EV. Influence of soil pH on the interaction of associative bacteria with barley. Microbiology (Moscow) 1998;67(4):463–469. [Google Scholar]
- 12.Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE, Borisov AY, Tikhonovich IA, Kluge C, Preisfeld A, et al. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol. 2001;47(7):642–652. doi: 10.1139/cjm-47-7-642. [DOI] [PubMed] [Google Scholar]
- 13.Belimov AA, Safronova VI, Mimura T. Response of spring rape to inoculation with plant growth-promoting rhizobacteria containing 1-aminocyclopropane-1-car-boxylate deaminase depends on nutrient status of the plant. Can J Microbiol. 2002;48(3):189–199. doi: 10.1139/w02-007. [DOI] [PubMed] [Google Scholar]
- 14.Belimov AA, Kunakova AM, Safronova VI, Stepanok VV, Yudkin LY, Alekseev YV, Kozhemyakov AP. Employment of rhizobacteria for the inoculation of barley plants cultivated in soil contaminated with lead and cadmium. Microbiology (Moscow) 2004;73(1):99–106. [PubMed] [Google Scholar]
- 15.Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol Biochem. 2005;37(2):241–250. doi: 10.1016/j.soilbio.2004.07.033. [DOI] [Google Scholar]
- 16.Bewley JD, Black M. Dormancy and the Control of Germination, Seeds: Physiology of Development and Germination. New York: Plenum Press; 1985. pp. 175–235. [Google Scholar]
- 17.Bingham FT, Pereyea FJ, Jarrell WM. Metal toxicity to agricultural crops. Metal Ions Biol Syst. 1986;20:119–156. [Google Scholar]
- 18.Blake RC, Choate DM, Bardhan S, Revis N, Barton LL, Zocco TG. Chemical transformation of toxic metals by a Pseudomonas strain from a toxic waste site. Environ Toxicol Chem. 1993;12(5):1365–1376. [Google Scholar]
- 19.Bogardt AH, Hemmingsen BB. Enumeration of phenanthracene-degrading bacteria by an overlayer technique and its use in the evaluation of petroleum-contaminated sites. Appl Environ Microbiol. 1992;58(7):2579–2582. doi: 10.1128/aem.58.8.2579-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollard EG. Involvement of Unusual Elements in Plant Growth and Nutrition. In: Lauchli A, Bielsky RL, editors. Inorganic Plant Nutrition. Encyclopedia of Plant Physiology, Vol. 15B. Berlin, Germany: Springer-Verlag KG; 1983. pp. 695–744. [Google Scholar]
- 21.Borowitz JJ, Stankie-Dicz M, Lewicka T, Zukowska Z. Inhibition of fungal cellulase, pectinase and xylanase activity of plant growth promoting fluorescent pseudomonads. Bull OILB/SROP. 1992;15(4):103–106. [Google Scholar]
- 22.Bradley R, Burt AJ, Read DJ. The biology of mycorrhyza in the Ericaceae. VIII. The role of mycorrhyzal infection in heavy metal resistance. N Phytol. 1982;91(2):197–202. doi: 10.1111/j.1469-8137.1982.tb03306.x. [DOI] [Google Scholar]
- 23.Brookes PC, McGrath SP. Effects of metal toxicity on the size of the soil microbial biomass. Eur J Soil Sci. 1984;35(2):341–346. doi: 10.1111/j.1365-2389.1984.tb00288.x. [DOI] [Google Scholar]
- 24.Brown MT, Wilkins DA. Zinc tolerance of mycorrhyial Betula. N Phytol. 1985;99(1):101–106. doi: 10.1111/j.1469-8137.1985.tb03640.x. [DOI] [Google Scholar]
- 25.Budzikiewicz H. Siderophores of fluorescent Pseudomonas L. Nat Foresche. 1997;52C:413–420. [PubMed] [Google Scholar]
- 26.Burd GI, Dixon DG, Glick BR. A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol. 1998;64(3):3663–3668. doi: 10.1128/aem.64.10.3663-3668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burd GI, Dixon DG, Glick BR. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol. 2000;46(3):237–245. doi: 10.1139/cjm-46-3-237. [DOI] [PubMed] [Google Scholar]
- 28.Chander K, Brookes PC. Effects of heavy metals from past applications of sewage sluge on microbial biomass and organic matter accumulaiton in a sandy loam soil and silty loam UK soil. Soil Biol Biochem. 1991;23(10):927–932. doi: 10.1016/0038-0717(91)90172-G. [DOI] [Google Scholar]
- 29.Chaney RL, Brown SL, Li YM, et al. US-EPA “Phytoremediation: State of Science”, 2000 May 1-2. Boston, MA: 2000. Progress in Risk Assessment for Soil Metals, and In-situ Remediation and Phytoextraction of Metals from Hazardous Contaminated Soils. [Google Scholar]
- 30.Chaudri AM, McGrath SP, Giller KE. Survival of the indigenous population of Rhizobium leguminosarum biovar trifolii in soil spiked with Cd, Zn, Cu and Ni salts. Soil Biol Biochem. 1992;24(7):625–632. doi: 10.1016/0038-0717(92)90040-5. [DOI] [Google Scholar]
- 31.Cheng S, Grosse W, Karrenbrock F, Thoennessen M. Efficiency of constructed wetlands in decontamination of water polluted by heavy metals. Ecol Eng. 2002;18(3):317–325. doi: 10.1016/S0925-8574(01)00091-X. [DOI] [Google Scholar]
- 32.Claverys JP. A new family of high-affinity ABC manganese and zinc permeases. Res Microbiol. 2001;152(3-4):231–243. doi: 10.1016/S0923-2508(01)01195-0. [DOI] [PubMed] [Google Scholar]
- 33.Crowley DE, Wang YC, Reid CPP, Szansiszlo PJ. Mechanism of iron acquisition from siderophores by microorganisms and plants. Plant Soil. 1991;130(1-2):179–198. doi: 10.1007/BF00011873. [DOI] [Google Scholar]
- 34.Cunningham SD, Berti WR. Remediation of contaminated soils with green plants: an overview. In Vitro Cell Dev Biol. 1993;29(4):207–212. [Google Scholar]
- 35.Cunningham SD, Ow DW. Promises and prospects of phytoremediation. Plant Physiol. 1996;110(5):715–719. doi: 10.1104/pp.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham SD, Berti WR, Huang JW. Phytoremediation of contaminated soils. Trends Biotechnol. 1995;13(9):393–397. doi: 10.1016/S0167-7799(00)88987-8. [DOI] [Google Scholar]
- 37.Davies FTJr, Puryear JD, Newton RJ. Mycorrhizal fungi enhance accumulation and tolerance of chromium in sunflower (Helianthus annuus) J Plant Physiol. 2001;158(6):777–786. doi: 10.1078/0176-1617-00311. [DOI] [Google Scholar]
- 38.de Souza MP, Chu D, Zhao M, Zayed AM, Ruzin SE, Schichnes D, Terry N. Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian mustard. Plant Physiol. 1999;119(2):565–573. doi: 10.1104/pp.119.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Souza MP, Huang CP, Chee N, Terry N. Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta. 1999;209(2):259–263. doi: 10.1007/s004250050630. [DOI] [PubMed] [Google Scholar]
- 40.Défago G, Berling CH, Burger U, et al. Suppression of Black Root Rot of Tobacco and Other Root Diseases by Strains of Pseudomonas fluorescens: Potential Applications and Mechanisms. In: Hornby D, editor. Biological Control of Soilborne Plant Pathogens. Oxon, UK: CAB International, Wellingford; 1990. pp. 93–108. [Google Scholar]
- 41.Dell’Amico E, Cavalca L, Andreoni V. Analysis of rhizobacterial communities in perennial Graminaceae from polluted water meadow soil, and screening of metal-resistant, potentially plant growth-promoting bacteria. FEMS Microbiol Ecol. 2005;52(2):153–162. doi: 10.1016/j.femsec.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Delorme TA, Gagliardi JV, Angle JS, Chaney RL. Influence of the zinc hyperaccumulator Thalaspi caerulescens J. and C. Presl and the nonmetal accumulator Trifolium pratense L. on soil microbial populations. Can J Microbiol. 2001;47(8):773–776. doi: 10.1139/cjm-47-8-773. [DOI] [PubMed] [Google Scholar]
- 43.Dueck TA, Visser P, Ernest WHO, Schat H. Vesiculararbuscular mycorrhyzae decrease zinc toxicity to grasses in zinc polluted soil. Soil Biol Biochem. 1986;18(3):331–333. doi: 10.1016/0038-0717(86)90070-2. [DOI] [Google Scholar]
- 44.Duffy BK, Défago G. Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phyotpathology. 1997;87(12):1250–1257. doi: 10.1094/PHYTO.1997.87.12.1250. [DOI] [PubMed] [Google Scholar]
- 45.Duffy BK, Défago G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol. 1999;65(6):2429–2438. doi: 10.1128/aem.65.6.2429-2438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egamberdiyeva D, Höflich G. Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J Arid Environ. 2004;56(2):293–301. doi: 10.1016/S0140-1963(03)00050-8. [DOI] [Google Scholar]
- 47.Elad Y, Baker R. The role of competition for iron and carbon in suppression of chlamydospore germination of Fusarium oxysporum . Phytopathology. 1985;75:190–195. [Google Scholar]
- 48.Elad Y, Chet I. Possible role of competition for nutrition in biocontrol of Pythium damping-off by bacteria. Phytopathology. 1987;77:190–195. [Google Scholar]
- 49.Ellis RJ, Timms-Wilson TM, Bailey MJ. Identification of conserved traits in fluorescent pseudomonads with antifungal activity. Environ Microbiol. 2000;2(3):274–284. doi: 10.1046/j.1462-2920.2000.00102.x. [DOI] [PubMed] [Google Scholar]
- 50.Elsgaard L, Petersen SO, Debosz K. Effects and risk assessment of linear alkylbenzene sulfonates in agricultural soil. 1. Short-term effects on soil microbiology. Environ Toxicol Chem. 2001;20(8):1656–1663. doi: 10.1897/1551-5028(2001)020<1656:EARAOL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 51.Ernst WHO. Bioavailability of heavy metals and decontamination of soils by plants. Appl Geochem. 1996;11(1-2):163–167. doi: 10.1016/0883-2927(95)00040-2. [DOI] [Google Scholar]
- 52.Filip Z. International approach to assessing soil quality by ecologically-related biological parameters. Agric Ecosyst Environ. 2002;88(2):689–712. [Google Scholar]
- 53.Foy CD, Chaney RL, White MC. The physiology of metal toxicity in plants. Annu Rev Plant Physiol. 1978;29(1):511–566. doi: 10.1146/annurev.pp.29.060178.002455. [DOI] [Google Scholar]
- 54.Fridlender M, Inbar J, Chet I. Biological control of soilborne plant pathogens by a β-1,3-glucanase-producing Pseudomonas cepacia . Soil Biol Biochem. 1993;25(9):1211–1221. doi: 10.1016/0038-0717(93)90217-Y. [DOI] [Google Scholar]
- 55.Gadd GM. Heavy metal accumulation by bacteria and other microorganisms. Experientia. 1990;46(8):834–840. doi: 10.1007/BF01935534. [DOI] [Google Scholar]
- 56.Garbisu C, Alkorta I. Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol. 2001;77(3):229–236. doi: 10.1016/S0960-8524(00)00108-5. [DOI] [PubMed] [Google Scholar]
- 57.Garbisu C, Hernandez-Allica J, Barrutia O, Alkorta I, Becerril JM. Phytoremediation: a technology using green plants to remove contaminants from polluted areas. Rev Environ Health. 2002;17(3):173–188. doi: 10.1515/reveh.2002.17.3.173. [DOI] [PubMed] [Google Scholar]
- 58.Giller KE, Witter E, McGrath SP. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils. Soil Biol Biochem. 1998;30(10-11):1389–1414. doi: 10.1016/S0038-0717(97)00270-8. [DOI] [Google Scholar]
- 59.Glick BR. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 60.Glick BR. Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv. 2003;21(5):383–393. doi: 10.1016/S0734-9750(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 61.Glick BR, Jacobson CB, Schwarze MMK, Pasternak JJ. 1-aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhyzobacterium Pseudomonas putida GR 12-2 do not stimulate canola root elongation. Can J Microbiol. 1994;40(2):911–915. [Google Scholar]
- 62.Glick BR, Karaturovic DM, Newell PC. A novel procedure for rapid isolation of plant growth promoting pseudomonads. Can J Microbiol. 1995;41(6):533–536. [Google Scholar]
- 63.Glick BR, Penrose DM, Li JP. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol. 1998;190(1):63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 64.Glick BR, Patten CL, Holguin G, et al. Biochemical and Genetic Mechanisms Used by Plant Growth-Promoting Bacteria. London: Imperial College Press; 1999. [Google Scholar]
- 65.Gray CW, McLaren RG, Roberts AHC, Condron LM. Sorption and desorption of cadmium from some New Zealand soils: effect of pH and contact time. Aust J Soil Res. 1998;36(2):199–216. doi: 10.1071/S97085. [DOI] [Google Scholar]
- 66.Gremion F, Chatzinotas A, Kaufmann K, von Sigler W, Harms H. Impacts of heavy metal contamination and phytoremediation on a microbial community during a twelve-month microcosm experiment. FEMS Microbiol Ecol. 2004;48(2):273–283. doi: 10.1016/j.femsec.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Grichko VP, Glick BR. Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem. 2001;39(1):11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- 68.Hall JA, Peirson D, Ghosh S, Glick BR. Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Isr J Plant Sci. 1996;44(2):37–42. [Google Scholar]
- 69.Han JG, Sun L, Dong XZ, Cai ZQ, Sun XL, Yang HL, Wang YS, Song W. Characterization of a novel plant growth-promoting bacteria strain Delftia tsuruhatensis HR4 both as a diazotroph and a potential biocontrol agent against various plant pathogens. Syst Appl Microbiol. 2005;28(1):66–76. doi: 10.1016/j.syapm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Hasnain S, Sabri AN. Growth Stimulation of Triticum Aestivum Seedlings under Cr-Stresses by Non Rhizospheric Pseudomonad Strains. Abstracts of the 7th International Symposium on Biological Nitrogen Fixation with Non-Legumes. the Netherlands: Kluwer Academic Publishers; 1996. p. 36. [Google Scholar]
- 71.Heggo A, Angle JS, Chaney RL. Effect of vesicular-arbuscular mycorrhyzae fungi on heavy metal uptake by soybeans. Soil Biol Biochem. 1990;22(6):865–869. doi: 10.1016/0038-0717(90)90169-Z. [DOI] [Google Scholar]
- 72.Hinsinger P. Bioavailability of soil inorganic P in the rhizospere as affected by root-induced chemical changes: a review. Plant Soil. 2001;237(2):173–195. doi: 10.1023/A:1013351617532. [DOI] [Google Scholar]
- 73.Huang Y, Tao S, Chen YJ. The role of arbuscular mycorrhiza on change of heavy metal speciation in rhizosphere of maize in wastewater irrigated agriculture soil. J Environ Sci. 2005;17(2):276–280. (in Chinese) [PubMed] [Google Scholar]
- 74.Imsande J. Iron, sulfur, and chlorophy II deficiencies: a need for an integrative approach in plant physiology. Physiologia Plantarum. 1998;103(1):139–144. doi: 10.1034/j.1399-3054.1998.1030117.x. [DOI] [Google Scholar]
- 75.Jacobson CB, Pasternak JJ, Glick BR. Partial purification and characterization of the enzyme ACC deaminase from the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol. 1994;40(2):1019–1025. [Google Scholar]
- 76.Jordan MJ, LeChevalier MP. Effects of zinc-smelter emissions on forest soil microflora. Can J Microbiol. 1975;21:1855–1865. doi: 10.1139/m75-269. [DOI] [PubMed] [Google Scholar]
- 77.Kabata-Pendias A, Pendias H. Trace Elements in the Soil and Plants. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- 78.Kamnev AA. Phytoremediation of Heavy Metals: An Overview. In: Fingerman M, Nagabhushanam R, editors. Recent Advances in Marine Biotechnology, Vol. 8: Bioremediation. USA: Science Publishers Inc., Enfield (NH); 2003. pp. 269–317. [Google Scholar]
- 79.Kamnev AA, Tugarova AV, Antonyuk LP, Tarantilis PA, Polissiou MG, Gardiner PH. Effects of heavy metals on plant-associated rhizobacteria: comparison of endophytic and non-endophytic strains of Azospirillum brasilense. J Trace Elem Med Biol. 2005;19(1):91–95. doi: 10.1016/j.jtemb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Kanazawa K, Higuchi K, Nishizawa NK, Fushiya S, Chino M, Mori S. Nicotianamine aminotransferase activities are correlated to the phytosiderophore secretion under Fe-deficient conditions, in Gramineae. J Exp Bot. 1994;45(12):1903–1906. doi: 10.1093/jxb/45.12.1903. [DOI] [Google Scholar]
- 81.Kärenlampi S, Schat H, Vangronsveld J, Verkleij JAC, van der Lelie D, Mergeay M, Tervahauta AI. Genetic engineering in the improvement of plants for phytoremediation of metal polluted soils. Environ Pollut. 2000;107(2):225–231. doi: 10.1016/S0269-7491(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 82.Killham K, Firestone MK. Vesicular arbuscular mycorrhyzal mediation of grass response to acidic and heavy metal deposition. Plant Soil. 1983;72(1):39–48. doi: 10.1007/BF02185092. [DOI] [Google Scholar]
- 83.Kloepper JW, Schroth MN, Miller TD. Effects of rhizosphere colonization by plant growth promoting rhizobacteria on potato plant development and yield. Phytopathology. 1980;70:1078–1082. [Google Scholar]
- 84.Kloepper JW, Lifshitz R, Zablotowicz RM. Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol. 1989;7(2):39–44. doi: 10.1016/0167-7799(89)90057-7. [DOI] [Google Scholar]
- 85.Konopka A, Zakharova T, Bischoff M, Oliver L, Nakatsu C, Turco RF. Microbial biomass and activity in lead-contaminated soil. Appl Environ Microbiol. 1999;65(5):2256–2259. doi: 10.1128/aem.65.5.2256-2259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kumar PBA, Dushenkov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995;29(5):1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- 87.Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J. Indole-3-acetic acid: a reciprocal signalling molecule in bacteria-plant interactions. Trends Microbiol. 2000;8(7):298–300. doi: 10.1016/S0966-842X(00)01732-7. [DOI] [PubMed] [Google Scholar]
- 88.Lasat HA. Phytoextraction of toxic metals: a review of biological mechanisms. J Environ Qual. 2002;31(1):109–120. [PubMed] [Google Scholar]
- 89.Leggett JE, Epstein E. Kinetics of sulfate adsorption by barley roots. Plant Physiol. 1956;31:222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemanceau P, Bakker PAHM, Dekogel WJ, Alabouvette C, Schippers B. Effect of pseudobactin 358 produced by Pseudomonas putida WSC358 on suppression of Fusarium wilt of carnations by non pathogenic Fusarium oxysporum . Appl Environ Microbiol. 1992;58(3):2978–2980. doi: 10.1128/aem.58.9.2978-2982.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leong J. Siderophores: their biochemistry and possible role in control of plant pathogens. Annu Rev Phytopathol. 1986;24(1):187–209. doi: 10.1146/annurev.py.24.090186.001155. [DOI] [Google Scholar]
- 92.Lim H, Kim Y, Kim S. Pseudomonas stutzeri YLP-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol. 1991;57(2):510–516. doi: 10.1128/aem.57.2.510-516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu A, Hamel C, Hamilton RI, Ma BL, Smith DL. Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza. 2000;9(6):331–336. doi: 10.1007/s005720050277. [DOI] [Google Scholar]
- 94.Lonergan DJ, Jenter H, Coates JD, Phillips EJP, Schmidt T, Lovley DR. Phylogenetic analysis of dissimilatory Fe(lll)-reducing bacteria. J Bacterial. 1996;176(3):2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loper JE, Henkels MD. Utilization of heterologous siderophore enhances levels of iron available to Pseudomonas putida in rhizosphere. Appl Environ Microbiol. 1999;65(12):5357–5363. doi: 10.1128/aem.65.12.5357-5363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lovley DR. Dissimilatory metal reduction. Annu Rev Microbial. 1993;47(1):263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 97.Lovley DR. Bioremediation of organic and metal contaminants with dissimilatory metal reduction. J Ind Microbiol. 1995;14(2):85–93. doi: 10.1007/BF01569889. [DOI] [PubMed] [Google Scholar]
- 98.Lovley DR, Coates JD, Saffarini DA, et al. Dissimilatory Iron Reduction. In: Winkelman G, Carrano CJ, editors. Iron and Related Transition Metals in Microbial Metabolism. New York: Harwood Academic Publishers; 1997. [Google Scholar]
- 99.Ma JF, Nomoto K. Inhibition of mugineic acid-ferric complex in barley by copper, zinc and cobalt. Physiologia Plantarum. 1993;89(2):331–334. doi: 10.1034/j.1399-3054.1993.890213.x. [DOI] [Google Scholar]
- 100.Marschner H. Mineral Nutrition of Higher Plants. London: Academic Press; 1995. p. 889. [Google Scholar]
- 101.Masalha J, Kosegarten H, Elmaci O, Mengal K. The central role of microbial activity for iron acquisition in maize and sunflower. Biol Fertil Soils. 2000;30(5-6):433–439. doi: 10.1007/s003740050021. [DOI] [Google Scholar]
- 102.Mason RP, Reinfelder JR, Morel FMM. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol. 1996;30(6):1835–1845. doi: 10.1021/es950373d. [DOI] [Google Scholar]
- 103.Maurhofer M, Reimmann C, Sacherer SP, Heeb S, Haas D, Defago G. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus. Phytopathology. 1998;88(2):678–684. doi: 10.1094/PHYTO.1998.88.7.678. [DOI] [PubMed] [Google Scholar]
- 104.Mayak S, Tirosh T, Glick BR. Effect of wild-type and mutant plant growth promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul. 1999;18(2):49–53. doi: 10.1007/PL00007047. [DOI] [PubMed] [Google Scholar]
- 105.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004;166(2):525–530. doi: 10.1016/j.plantsci.2003.10.025. [DOI] [Google Scholar]
- 106.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 2004;42(6):565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 107.Mayer AM, Poljakoff-Mayber A. The Germination of Seeds. Oxford: Pergamon Press; 1989. [Google Scholar]
- 108.McGrath SP. Effects of Heavy Metals from Sewage Sludge on Soil Microbes in Agricultural Ecosystems. In: Ross SM, editor. Toxic Metals in Soil-Plant Systems. New York: Wiley; 1994. pp. 247–273. [Google Scholar]
- 109.McGrath SP, Chaudri AM, Giller KE. Long-term effects of metals in sewage sluge on soils, microorganisms and plants. J Ind Microbiol. 1995;14(2):94–104. doi: 10.1007/BF01569890. [DOI] [PubMed] [Google Scholar]
- 110.McGrath SP, Zhao FJ, Lombi E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil. 2001;232(1-2):207–214. doi: 10.1023/A:1010358708525. [DOI] [Google Scholar]
- 111.Mengoni A, Barzanti R, Gonnelli C, Gabbrielli R, Bazzicalupo M. Characterization of nickel-resistant bacteria isolated from serpentine soil. Environ Microbiol. 2001;3(11):691–698. doi: 10.1046/j.1462-2920.2001.00243.x. [DOI] [PubMed] [Google Scholar]
- 112.Meyer JM. Pyoverdines: pigments siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol. 2000;174(3):135–142. doi: 10.1007/s002030000188. [DOI] [PubMed] [Google Scholar]
- 113.Mishra D, Kar M. Nickel in plant growth and metabolism. Bot Rev. 1974;40:395–452. [Google Scholar]
- 114.Nie L, Shan S, Rashid A, Burd GI, George DD, Glich BR. Phytoremediation of arsenate contaminated soil by transgenic canola and the plant growth-promoting bacterium Enterobacter cloacae CAL2. Plant Physiol Biochem. 2002;40(4):355–361. doi: 10.1016/S0981-9428(02)01375-X. [DOI] [Google Scholar]
- 115.Oostendorp M, Sikora RA. Seed-treatment with antagonistic rhizobacteria for the suppression of Heterodera schachtii early root infection of sugar beet. Rev Nematol. 1989;12(1):77–83. [Google Scholar]
- 116.Oostendorp M, Sikora RA. In vitro interrelationship between rhizosphere bacteria and Heterodera schachtii . Rev Nematol. 1990;13(3):269–274. [Google Scholar]
- 117.Pan B, Bai YM, Leibovitch S, Smith DL. Plant-growth-promoting rhizobacteria and kinetin as ways to promote corn growth and yield in a short-growing-season area. Eur J Agron. 1999;11(3-4):179–186. doi: 10.1016/S1161-0301(99)00029-5. [DOI] [Google Scholar]
- 118.Park CH, Keyhan M, Matin A. Purification and characterization of chromate reductase in Pseudomonas putida . Abs Gen Meet American Soc Microbial. 1999;99(4):536–548. [Google Scholar]
- 119.Patten CI, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42(3):207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- 120.Patten CL, Glick BR. The role of bacterial indoleacetic acid in the development of the host plant root system. Appl Environ Microbiol. 2002;68(8):3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Penrose DM, Glick BR. Levels of 1-aminocyclopropane-1-carboxylic acid (ACC) in exudates and extracts of canola seeds treated with plant growth-promoting bacteria. Can J Microbiol. 2001;47(4):368–372. doi: 10.1139/cjm-47-4-368. [DOI] [PubMed] [Google Scholar]
- 122.Pierson LS, Thomashow LS. Cloning and heterologous expression of the phenazine biosynthetic locus from Pseudomonas aureofaciens . Mol Plant-Microb Interact. 1992;5(4):330–339. doi: 10.1094/mpmi-5-330. [DOI] [PubMed] [Google Scholar]
- 123.Potgieter H, Alexander M. Susceptibility and resistance of several fungi to microbial lysis. J Bacteriol. 1996;91(4):1526–1532. doi: 10.1128/jb.91.4.1526-1532.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Probanza A, Lucas García JA, Ruiz Palomino M, Ramos B, Gutiérrez Mañero FJ. Pinus pinea L. seedling growth and bacterial rhizosphere structure after inoculation with PGPR Bacillus (B. licheniformis CECT 5106 and B. pumilus CECT 5105) Appl Soil Ecol. 2002;20(2):75–84. [Google Scholar]
- 125.Ramos B, Lucas García JA, Probanza A, Barrientos ML, Gutiérrez Mañero FJ. Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis . Environ Exp Bot. 2003;49(1):61–68. doi: 10.1016/S0098-8472(02)00059-X. [DOI] [Google Scholar]
- 126.Reid CP, Szaniszlo PJ, Crowley DE. Siderophore Involvement in Plant Iron Nutrition. In: Swinburne TR, editor. Iron Siderophores and Plant Diseases. New York: Plenum Press; 1986. pp. 29–42. [Google Scholar]
- 127.Römheld V, Marschner H. Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr. 1986;2:155–204. [Google Scholar]
- 128.Sabry SA, Ghozlan HA, Abou-Zeid DM. Metal tolerance and antobiotic resistance patterns of a bacterial population isolated from sea water. J Appl Microbiol. 1997;82(2):245–252. doi: 10.1111/j.1365-2672.1997.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 129.Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biol Technol. 1995;13(5):468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- 130.Sandaa RA, Torsvik V, Enger O, Daae LF, Castberg T, Hahn D. Analysis of bacterial communities in heavy metal-contaminated soils at different levels of resolution. FEMS Microbiol Ecol. 1999;30(3):237–251. doi: 10.1111/j.1574-6941.1999.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 131.Sanità di Toppi L, Gabrielli R. Response to cadmium in higher plants. Environ Exp Bot. 1999;41(2):105–130. doi: 10.1016/S0098-8472(98)00058-6. [DOI] [Google Scholar]
- 132.Saxena PK, Krishnaraj S, Dan T, et al. Phytoremediation of Heavy Metal Contaminated and Polluted Soils. In: Prasad MNV, Hagemeyer J, editors. Heavy Metal Stress in Plants: from Molecules to Ecosystems. Berlin: Springer; 1999. pp. 305–329. [Google Scholar]
- 133.Sharma A, Johri BN, Sharma AK, Glick BR. Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck) Soil Biol Biochem. 2003;35(7):887–894. doi: 10.1016/S0038-0717(03)00119-6. [DOI] [Google Scholar]
- 134.Sikora RA. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annu Rev Phytopathol. 1992;30(1):245–270. doi: 10.1146/annurev.py.30.090192.001333. [DOI] [Google Scholar]
- 135.Sikora RA, Hoffmann-Hergarten S. Importance of plant health-promoting rhizobacteria for the control of soil-borne fungal diseases and plant parasitic nematodes. Arab J Plant Prot. 1992;10(4):53–58. [Google Scholar]
- 136.Sitaula BK, Almas A, Bakken LR, Singh BR. Assessment of heavy metals associated with bacteria in soil. Soil Biol Biochem. 1999;31(2):315–316. doi: 10.1016/S0038-0717(98)00104-7. [DOI] [Google Scholar]
- 137.Smalle J, van der Straeten JD. Ethylene and vegetative development. Physiologia Plantarum. 1997;100(3):593–605. doi: 10.1034/j.1399-3054.1997.1000322.x. [DOI] [Google Scholar]
- 138.Smith SE, Read DJ. Mycorrhizal Symbiosis. San Diego: Academic Press Inc; 1997. [Google Scholar]
- 139.Steenhoudt O, Vanderleyden J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev. 2000;24(4):487–506. doi: 10.1111/j.1574-6976.2000.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 140.Tam PCF. Heavy metal tolerance by ectomycorrhyzal fungi and metal amelioration by Pisolithus tinctorium . Mycorrhiza. 1995;5(3):181–187. doi: 10.1007/s005720050057. [DOI] [Google Scholar]
- 141.van Loon LC. Regulation of Pathogenesis and Symptom Expression in Diseased Plants by Ethylene. In: Fuchs Y, Chalutz E, editors. Ethylene: Biochemical, Physiological and Applied Aspects. the Hague, the Netherlands: Martinus Nijhoff/Dr. W. Junk; 1984. pp. 171–180. [Google Scholar]
- 142.Velazhahan R, Samiyappan R, Vidhyasekaran P. Relationship between antagonistic activities of Pseudomonas fluorescens isolates against Rhizoctonia solani and their production of lytic enzyme. J Plant Dis Prot. 1999;106(3):244–250. [Google Scholar]
- 143.Vidhyasekaran P, Muthamilan M. Evaluation of powder formulation of Pseudomonas fluorescens Pf1 for control of rice sheath blight. Biocontrol Sci Technol. 1999;9(1):67–74. doi: 10.1080/09583159929910. [DOI] [Google Scholar]
- 144.Viswanathan R, Samiyappan R. Proceedings of the Sugar Technology Association of India, Vol. 61. New Delhi, India: Sugar Technology Association; 1999. Induction of Systemic Resistance by Plant Growth Promoting Rhizobacteria against Red Rot Disease Caused by Collectotrichum falcatum Went in Sugarcane; pp. 24–39. [Google Scholar]
- 145.Wallace A, Wallace GA, Cha JW. Some modifications in trace elements toxicities and deficiencies in plants resulting from interactions with other elements and chelating agents. The special case of iron. J Plant Nutr. 1992;15(2):1589–1598. [Google Scholar]
- 146.Wang YT, Shen H. Bacterial reduction of hexavalent chromium. J Ind Microbiol. 1995;14(2):159–163. doi: 10.1007/BF01569898. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Brown HN, Crowley DE, Szaniszlo PJ. Evidence for direct utilization of a siderophore, ferroxamine B, in axenically grown cucumber. Plant Cell Environ. 1993;16(5):579–585. doi: 10.1111/j.1365-3040.1993.tb00906.x. [DOI] [Google Scholar]
- 148.Weber O, Scholz RW, Bvhlmann R, Grasmuck D. Risk perception of heavy metal soil contamination and attitudes toward decontamination strategies. Risk Anal. 2001;21(5):967–977. doi: 10.1111/0272-4332.215165. [DOI] [PubMed] [Google Scholar]
- 149.Wei L, Kloepper JW, Tuzun S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth promoting rhizobacteria. Phytopathology. 1991;81(11):1508–1512. [Google Scholar]
- 150.Wei L, Kloepper JW, Tuzun S. Induced systemic resistance to cucumber diseases and increased plant growth by plant growth promoting rhizobacteria under field conditions. Phytopathology. 1996;86(2):221–224. doi: 10.1094/Phyto-86-221. [DOI] [Google Scholar]
- 151.Wenzel WW, Lombi E, Adriano DC. Biochemical Processes in the Rhizosphere: Role in Phytoremediation of Metal-Polluted Soils. In: Prasad MNV, Hagemeyer J, editors. Heavy Metal Stress in Plants: from Molecules to Ecosystems. Berlin: Springer; 1999. pp. 273–303. [Google Scholar]
- 152.Whiting SN, de Souza MP, Terry N. Rhizosphere bacteria mobilize Zn for hyperaccumulation by Thlaspi caerulescens . Environ Sci Technol. 2001;35(15):3144–3150. doi: 10.1021/es001938v. [DOI] [PubMed] [Google Scholar]
- 153.Xie H, Pasternak JJ, Glick BR. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr Microbiol. 1996;32(2):67–71. doi: 10.1007/s002849900012. [DOI] [Google Scholar]
- 154.Yang X, Baligar VC, Martens DC, Clark PB. Plant tolerance to nickel toxicity. II. Nickel effect on influx and transport of mineral nutrients in four plant species. J Plant Nutr. 1996;19(2):265–279. [Google Scholar]
- 155.Zayed AM, Lytle CM, Terry N. Accumulation and volatilization of different chemical species of selenium by plants. Planta. 1998;206(2):284–292. doi: 10.1007/s004250050402. [DOI] [Google Scholar]
- 156.Zehnder G, Kloepper J, Yao C, Wei G. Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth promoting rhizobacteria. J Econ Entomol. 1997;90(2):391–396. [Google Scholar]