Abstract
In estrogen-primed female rats, vaginal cervical stimulation (VCS) provided by male intromissions or by an experimenter enhances estrous behaviors exhibited by females during subsequent mating with a male. We tested the hypothesis that α1-adrenergic receptors, acting via the nitric oxide-cGMPprotein kinase G pathway, mediate VCS- induced facilitation of female reproductive behaviors. Ovariectomized, estradiol-primed rats received intracerebroventricular (icv) infusions of vehicle or pharmacological antagonists 15 or 60 min before VCS. Estrous behaviors (lordosis and proceptivity) in the presence of a male were recorded immediately (0 min), and 120 min following VCS. First we verified that VCS, but not manual flank stimulation alone, enhanced estrous behaviors when females received icv infusion of the vehicles used to administer drugs. Increased estrous behavior was apparent immediately following VCS and persisted for 120 min. We then infused prazosin, phenoxybenzamine (α1-adrenergic receptor antagonists), yohimbine, idaxozan (α2-adrenergic receptor antagonists), or propranolol (β–adrenergic receptor antagonist) 15 min prior to the application of VCS in females primed with 5 μg estradiol benzoate. Only α1-adrenergic antagonists inhibited VCS facilitation of estrous behavior, apparent 120 min after VCS. Finally, we administered specific inhibitors of soluble guanylyl cyclase, nitric oxide synthase or protein kinase G icv 15 or 60 min before VCS. All three agents significantly attenuated VCS facilitation of estrous behavior. These data support the hypothesis that endogenously released norepinephrine, acting via α1-adrenergic receptors, mediates the facilitation of lordosis by VCS, and are consistent with a mechanism involving α1-adrenergic activation of the nitric oxide/cGMP/protein kinase G pathway.
Keywords: Estrous behavior, vaginocervical stimulation, adrenergic receptors, nitric oxide, cGMP
Introduction
Estradiol (E2) primes the neural substrates that mediate estrous behaviors (lordosis and proceptive behaviors), and subsequently progesterone (P) acts on the E2-primed brain to trigger the onset and increase the intensity of these behaviors [2, 14, 15, 22, 45]. During a mating sequence in rats, females receive several mounts without vaginal intromission, mounts with intromission, and several ejaculations from a male, resulting in flank-perineal and vaginocervical stimulation (VCS). Normal VCS induces neuroendocrine and behavioral changes in female mammals that are critical to establish pregnancy [17, 20, 33] and accelerates the termination of sexual behavior in hamsters and rats [6, 35]. Artificial VCS and extravaginal stimuli, such as flank-perineal stimulation, facilitate lordosis in ovariectomized (ovx) female rats primed with E2 [38, 54].
During the estrous cycle and in ovx, hormone-treated rats, VCS induces the release of several neurotransmitters [43] and signaling molecules, including norepinephrine (NE) and nitric oxide (NO), in CNS areas that regulate various reproductive processes [24, 31, 37]. Sensory information from the uterus and cervix is transmitted in the anterolateral columns of the spinal cord via afferent fibers in the hypogastric, pelvic, and vagus nerves [5, 11, 13, 18, 39]. Many of these fibers terminate in the brainstem regions that give rise to the ventral NE bundle (e.g., nucleus of the solitary tract, a major site of convergence of visceral sensory information). NE neurons in this region then project to the hypothalamus. Bilateral transection of the pelvic nerve [5] or lesions of the ventral NE bundle [34] decrease the lordosis response and completely prevent the occurrence of VCS-induced pseudopregnancy [19, 21, 41].
Although the cellular mechanisms underlying the stimulation of sexual behavior by VCS are not clear, it is known that both NE and NO participate in the expression of hormone-dependent female sexual behaviors [7–9, 24, 27, 30, 40, 42, 46]. Thus, microdialysis studies demonstrate that NE release increases dramatically in the ventromedial hypothalamus when copulation begins in E2- and progesterone-treated female rats. Moreover, VCS rather than chemosensory or flank-perineal stimuli evoke NE release in sexually receptive rats [24, 58]. Studies in which NE, NE agonists, or NE antagonists were administered systemically or intracerebrally point to a critical role for hypothalamic α1-adrenergic receptors in the regulation of lordosis behavior in E2-primed rodents. For example, both NE- and P-facilitated lordosis is blocked by systemic or intrahypothalalmic administration of the α1-adrenergic antagonist prazosin [8, 12, 23, 25, 27, 40, 46, 58]. Erskine and colleagues (47) recently implicated α1-adrenoceptors in other responses to VCS by showing that infusion of an α1-adrenergic agonist as well as an α2-autoreceptor antagonist into the ventrolateral ventromedial hypothalamus facilitated pseudopregnancy induced by VCS while an opposite effect was found with administration of an α1 antagonist.
The NO pathway also plays a crucial role in the facilitation of lordosis behavior by P and its ring A-reduced metabolites [7–9, 30, 42]. A major mediator of cellular responses to NO is cGMP, which is synthesized when soluble guanylyl cyclase is stimulated by NO [3, 52, 53]. Intracerebroventricular (icv) infusions of a selective inhibitor of NO-stimulated soluble guanylyl cyclase decrease lordosis behavior in ovx rats treated with E2 plus P. Icv infusion of 8-bromo-cGMP also enhances reproductive behaviors in rats primed only with E2 and reverses the inhibitory effects of the α1-adrenoreceptor antagonist prazosin on lordosis behavior [8]. NE and the α1-adrenoreceptor agonist phenylephrine stimulate cGMP accumulation in hypothalamic and preoptic area slices, but only if tissue is derived from ovx females pretreated with both E2 and P. Thus, the NO/cGMP pathway most likely mediates the facilitatory effects of α1-adrenoreceptors on lordosis behavior in female rats, and prior exposure of the hypothalamus and preoptic area to both E2 and P is required to link α1-adrenoceptors to this pathway. The present study tested the hypothesis that the α1-adrenoceptor-NO-cGMP pathway, via activation of protein kinase G (PKG), is involved in the stimulation of female sexual behavior by VCS. This idea was tested by infusing selective inhibitors of adrenoreceptor subtypes and of the NO-cGMP-PKG pathway into the lateral ventricle of ovx, E2-primed female rats before application of VCS. VCS was administered using a vaginal probe that allowed us to deliver a quantifiable and reproducible stimulus to the vagina and cervix.
Material and methods
Animals and Surgery
Eighty seven adult Sprague–Dawley rats (200–260 g) bred in our colony in Tlaxcala were used and maintained on a 14-h light/10-h dark cycle with lights off at 1000 h. The females were bilaterally ovx under ether anesthesia and housed in groups of 4 per cage. Two weeks later, they were anesthetized with xylazine (4 mg/kg) and ketamine (80 mg/kg) and placed in a Kopf stereotaxic instrument (Tujunga, CA) for implantation of a stainless steel cannula (22 gauge, 17 mm long) into the right lateral ventricle following coordinates from the atlas of Paxinos and Watson [48] (A/P +0.80 mm, M/L -1.5 mm, D/V -3.5 mm with respect to bregma). A stainless steel screw was fixed to the skull and both cannula and screw were attached to the bone with dental cement. A dummy cannula (30 gauge) provided with a cap was introduced into the guide cannula to prevent clogging and contamination. Immediately after the cannula implantation the females were injected with penicillin (22,000 IU/kg). One week after surgery, all females received a s.c. injection of 5 μg of E2 benzoate (E2B; Sigma-Aldrich, St. Louis, MO) dissolved in 0.1 ml corn oil 40 h before drug infusions and manual flank stimulation (MFS)/VCS or MFS only. All drugs were purchased from Sigma-Aldrich and were administered icv in a volume of 2 μl through the guide cannula in the right lateral ventricle over 1 min, and another 1 min was allowed for drug diffusion before the removal of the infusion needle. All procedures used in these experiments followed the Mexican Law for the Protection of Animals and were approved by the Institutional Animal Care and Use Committee at CINVESTAV-Universidad Autónoma de Tlaxcala.
Behavioral Testing
Between 8 and 12 rats were assigned to each treatment group. Most females were tested twice with a two week interval between tests. These animals were incorporated into groups at random, but no rat received the same treatment twice. Control animals that received only MFS or that received MFS/VCS plus one of the vehicles used for drug infusion were tested only once. Forty hr after E2B injection, females received either 150 g of pressure into the vagina-cervix through a calibrated vaginal probe [38] together with MFS or MFS only without VCS for approximately 5 sec. MFS consisted of palpations applied with the finger thumb to both flanks and with the palm of the hand to the perineal area of the rat. Immediately (0 min) and 120 min following these procedures (MFS with or without VCS), females were placed in a circular plexiglas arena (53 cm diameter) until they received 10 mounts with pelvic thrusting from an experienced stimulus male. The lordosis quotient [LQ = (number of lordosis/10 mounts) X 100] was used to assess lordosis behavior. The intensity of lordosis was quantified according to the lordosis score (scale of 0–3) proposed by Hardy and DeBold [35]. Proceptivity was analyzed by determining the incidence of hopping, darting and ear-wiggling across the whole receptivity test. Females were considered proceptive when showing any of these behaviors.
Experiment 1. Effect of icv infusions of adrenergic antagonists on MFS/VCS-induced sexual behavior
This experiment tested the effects of α and β adrenergic antagonists on the response to MFS/VCS. E2B-primed animals were randomly assigned to receive an infusion of one adrenergic antagonist (α1, α2 or β) 15 min before receiving MFS/VCS. Two weeks later females were tested again with a different α or β adrenergic antagonist. The α1-adrenergic antagonists were prazosin and phenoxybenzamine; α2-adrenergic antagonists were yohimbine and idaxozan; and the β-adrenergic antagonist was propanolol. Because different drugs were dissolved in different vehicles (see below), separate control animals were primed with E2B and infused icv with one of these vehicles just before application of MFS/VCS. Additional controls received VCS alone. The vehicles infused and the number of animals in these control groups were: MFS without VCS (n=12); MFS/VCS + saline (n=12); MFS/VCS plus propylene glycol (n=10); MFS/VCS plus sterile distilled water (n=10); and MFS/VCS plus 10% dimethylsulfoxide (DMSO; vehicle for drugs in Experiment 2) (n=9). The doses, vehicles and number of animals infused with adrenergic antagonists were: 160 μg of prazosin (n= 9) prepared in saline containing 25% propylene glycol [8]; 5 μg of phenoxybenzamine (n=9) dissolved in propylene glycol [28]; 5 μg of idaxozan (n=8) dissolved in sterile distilled water; 5 μg of yohimbine (n=9) dissolved in sterile distilled water [4, 59]; and 5 μg of propanolol (n=8) dissolved in saline.
Experiment 2. Effect of icv infusion of NO pathway inhibitors prior to MFS/VCS
This experiment tested the effects of different inhibitors of the NO-cGMP-PKG pathway on estrous behavior induced by MFS/VCS in E2B-primed animals. E2B-primed animals were infused with an NO pathway inhibitor at various times before receiving MFS/VCS; two weeks later females were tested again with a different NO pathway inhibitor. Fifteen min before MFS/VCS, E2B-primed female rats were infused icv with the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME), a specific inhibitor of soluble guanylyl cyclase, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), or the specific PKG inhibitior KT5823. Drug-infused groups were compared with their corresponding vehicle-treated control groups (see description of Experiment 1). The doses, vehicles and number of animals utilized were: 500 μg of L-NAME (n=8) dissolved in saline, 22 μg of ODQ (n=8) and 0.12 μg of KT5823 (n=8), both dissolved in 10% DMSO. The doses of L-NAME, ODQ and KT5823 were selected on the basis of our previous work [7–9, 30]. L-NAME was purchased from Sigma-Aldrich. ODQ was obtained from Tocris Cookson (St. Louis, MO), and KT5823 was purchased from CalBiochem (La Jolla, CA). Additional animals received icv infusion of the same doses of L-NAME or ODQ (n=8/drug) 1 hr before application of MFS/VCS.
Verification of cannula placements
One day after the final test for lordosis, the animals were anesthetized with ether, and 1% methylene blue was administered through the cannula. The brain was removed and sectioned in the transverse plane to check the cannula position in the lateral ventricle. The animals whose cannulae were not in the ventricle were discarded from the experiment (6 animals).
Statistical analysis
To assess the effect of MFS vs MFS/VCS + vehicle in the first experiment, we used first the Kruskall Wallis test (significance level P< 0.05) for each of two the times tested. Subsequently, the results obtained at 0 and 120 min were compared using the Mann Whitney test (significance level p< 0.05). Proportion of females that displayed proceptivity was compared with the Fisher test. For the experiment involving adrenergic antagonists and vehicles, a Kruskall-Wallis test was performed to analyze lordosis behavior; when relevant the Mann Whitney test was used to compare lordosis behavior in specific groups receiving the experimental drug with control animals receiving the corresponding vehicle for each of the times tested (0 and 120 min). A similar procedure was used for analysis in experiments involving the NO-cGMP pathway inhibitors.
Results
Effect of MFS/VCS on the expression of female sexual behaviors
Table 1 shows that lordosis and proceptive behavior in the presence of a male were stimulated in ovx, E2B-primed female rats that received MFS/VCS plus vehicle, but not in females that received MFS alone. The Mann Whitney test showed a significant increase in LQ immediately after MFS/VCS (0 min), but not after MFS alone, in each one of the vehicle-treated groups (P<0.05 to P<0.001; see Table 1). This effect persisted for at least 120 min after MFS/VCS. Between 30 and 40% of females that were infused with vehicle and received MFS/VCS also began to exhibit proceptive behavior immediately (0 min) after MFS/VCS (Table 1). A significantly greater proportion of females displayed proceptive behavior at 120 min post-MFS/VCS plus vehicle (P < 0.01) than at 0 min. Females that received MFS without VCS did not display proceptive behaviors at any time. Because females receiving only MFS did not exhibit reproductive behavior, we attribute the effects of combined MFS/VCS to the VCS componenet and refer to VCS alone in discussing the results.
Table 1.
Infusion of different vehicles does not influence sexual behavior (lordosis and proceptivity) induced by VCS in ovx rats primed with E2B
| 0 min | 120 min | ||||
|---|---|---|---|---|---|
| N | LQ Mean ± SE | % Proceptve Females | LQ Mean ± SE | % Proceptive Females | |
| Saline | 12 | 49±10* | 33 | 74 ± 8* | 66* |
| Water | 10 | 52±9** | 40 | 74±10* | 70* |
| Propylene Glycol | 10 | 41±9+ | 30 | 70±7* | 60* |
| DMSO | 9 | 40±3+ | 33 | 80±5** | 66* |
| MFS only | 8 | 2.5±1.6 | 0 | 12±4 | 0 |
Estrous behavior in ovx female rats primed with 5 μg of E2B was tested 0 and 120 min after application of VCS. Some animals received manual flank stimulation (MFS) without VCS. Different vehicles were administered into the right lateral ventricle 15 min before VCS.
P< 0.05;
P< 0.01;
P< 0.001 VS MFS only.
Effect of icv infusions of α and β adrenergic antagonists on MFS/VCS-induced sexual behavior
Administration of prazosin (α1 antagonist) into the lateral ventricle significantly attenuated the MFS/VCS-induced increase in LQ at 120 min (P<0.01) post-MFS/VCS when compared to vehicle controls, but this difference was not significant at 0 min (Fig 1A). This inhibition of lordosis by prazosin persisted at 240 min post-MFS/VCS (P<0.05; data not shown). A different α1 antagonist, phenoxybenzamine, also attenuated MFS/VCS-induced increases in LQ at 120 min post-MFS/VCS (P < 0.01; Fig. 1A). In addition, MFS/VCS-induced proceptive behavior was significantly suppressed by both antagonists at 120 min post-MFS/VCS (P < 0.05; Fig 1B). We did not include control groups treated with prazosin or phenoxybenzamine without MFS/VCS, because previous studies showed that these compounds did not increase lordosis behavior [27, 46].
Fig. 1.
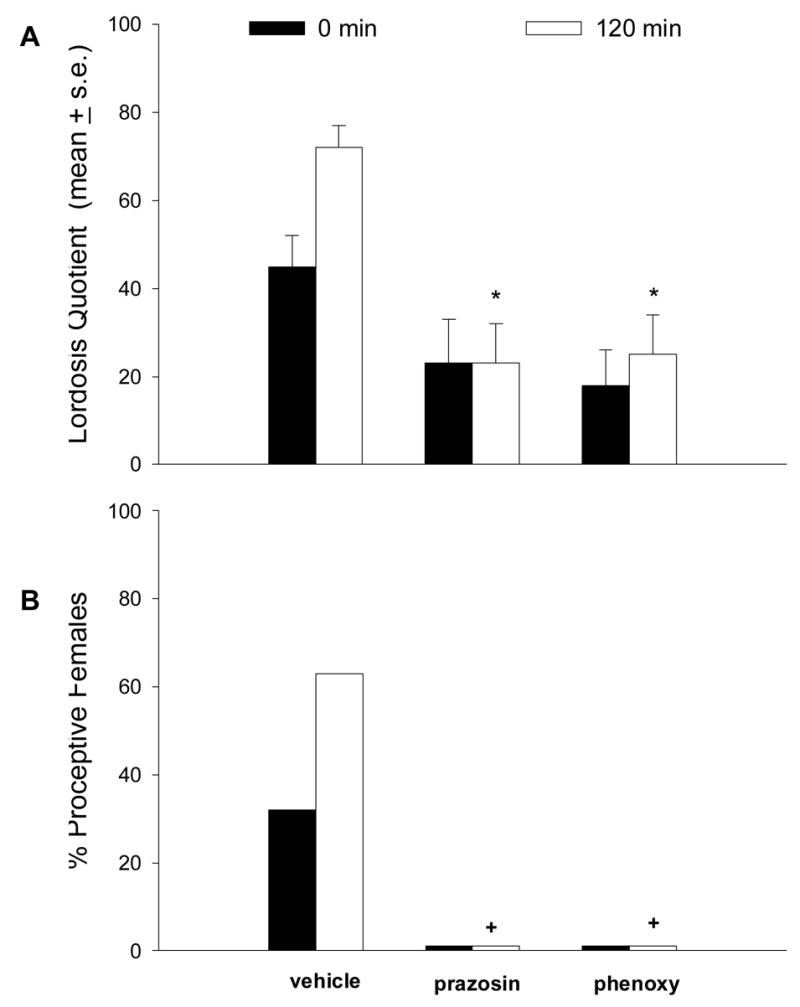
The facilitation of lordosis (A) and proceptive behavior (B) in ovx, E2B-primed rats produced by MFS/VCS is antagonized by icv infusion of the α1-adrenergic receptor antagonist prasozin (160 μg) or phenoxybenzamine (phenoxy, 5 μg). Drugs and vehicles were infused into the right lateral ventricle 15 min before application of MFS/VCS. Vehicle data are combined from the rows labeled saline and propylene glycol in Table 1. N = 8–12 rats per group. *P < 0.01; +P < 0.05 vs. corresponding group receiving MFS/VCS + vehicle.
We also tested the effect of α2-adrenergic antagonists (idaxozan and yohimbine) and a β-adrenergic antagonist (propanolol) on MFS/VCS-induced increases in LQ and proceptivity (Table 2). None of these agents significantly affected either lordosis or proceptive behavior induced by MFS/VCS. The non-significant tendency for yohimbine to decrease estrous behaviors may reflect its partial α1 antagonist activity.
Table 2.
Icv administration of α2 (yohimbine and idaxozan) and β (propranolol) adrenergic antagonists did not affect VCS-facilitated lordosis and proceptive behavior of ovx rats primed with E2B
| 0 min | 120 min | ||||
|---|---|---|---|---|---|
| N | LQ Mean ± SE | % Proceptive Females | LQ Mean ± SE | % Proceptive Females | |
| Vehicle | 22 | 50 ± 6 | 36 | 74 ± 6 | 63 |
| Yohimbine | 9 | 40 ± 8 | 33 | 48 ±14 | 33 |
| Idaxozan | 8 | 50 ±11 | 62 | 68 ±12 | 37 |
| Propranolol | 8 | 75 ±6 | 57 | 84 ± 5 | 57 |
Estrous behavior in ovx female rats primed with 5 μg of E2B was tested 0 and 120 min after application of MFS/VCS. Vehicle data are combined from the rows labeled “saline” and “water” in Table 1. Adrenergic receptor antagonists (5 μg) were administered into the right lateral ventricle 15 min before VCS.
Effect of icv infusion of NO pathway inhibitors prior to MFS/VCS
In ovx, E2B-primed females that received icv injections of L-NAME or ODQ 15 min prior to MFS/VCS (Fig. 2A), LQ scores were significantly reduced at 120 min (P < 0.01 and 0.001 respectively vs corresponding vehicle). However, neither L-NAME nor ODQ prevented the immediate (0 min) increase in lordosis behavior shown in control rats following MFS/VCS (P > 0.05; Fig. 2A). LQ scores in rats receiving MFS/VCS plus L-NAME or ODQ did not differ significantly from the group that received MFS without VCS (Table 1) at any time point. Administration of L-NAME and ODQ significantly reduced proceptivity at 120 min post-MFS/VCS (Table 3). Thus, 12% and 0% of females treated with L-NAME or ODQ, respectively, showed proceptive behavior, compared to 67% of the MFS/VCS plus vehicle controls. We did not include a control group treated with L-NAME or ODQ without MFS/VCS, because previous studies showed that these compounds did not increase lordosis behavior [30].
Fig. 2.
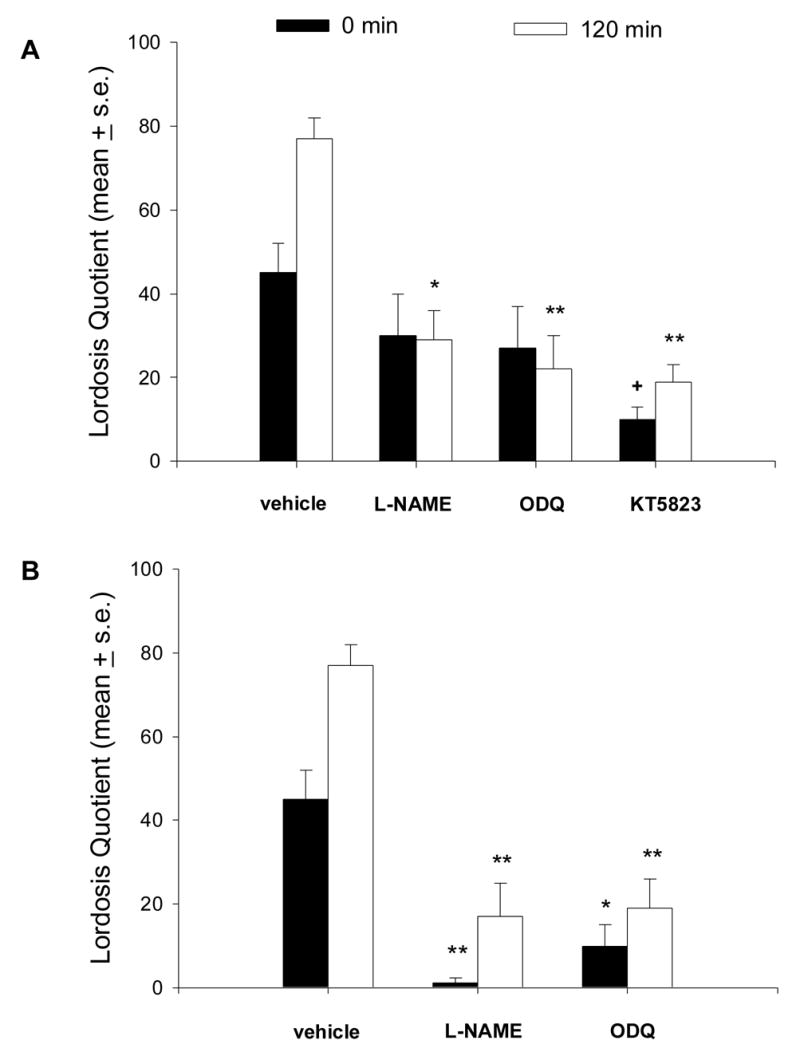
The facilitation of lordosis in ovx, E2B-primed rats produced by MFS/VCS is antagonized by icv infusion of the NO synthase inhibitor LNAME (500 μg), the soluble guanylyl cyclase inhibitor ODQ (22 μg) or the PKG inhibitor KT5823 (0.12 μg). Drugs and vehicle were infused into the right lateral ventricle 15 (A) or 60 min (B) before application of MFS/VCS. 1. N = 8–12 rats per group. **P< 0.001; *P < 0.01; +P < 0.05 vs. corresponding group receiving MFS/VCS + vehicle.
Table 3.
Icv administration of L-NAME, ODQ and KT5823 reduced proceptivity induced by VCS in ovx rats primed with 5 μg of E2B
| 0 min | 120 min | ||||
|---|---|---|---|---|---|
| % Proceptve Females | % Proceptve Females | ||||
| N | 15 minutes | 60 minutes | 15 minutes | 60 minutes | |
| Vehicle | 21 | 33 | 67 | ||
| L-NAME 500 μg | 8 | 12 | 0 | 12+ | 0+ |
| ODQ 22 μg | 8 | 12 | 0 | 0** | 0+ |
| KT5823 0.12 μg | 8 | 0 | 0** | ||
Proceptive behavior in ovx female rats primed with 5 μg of E2B was tested 0 and 120 min after application of VCS. Drugs or vehicle were infused into the right lateral ventricle 15 min before VCS as in Fig. 2A. Vehicle data are combined from the rows labeled “saline” and “DMSO” in Table 1. Other females were injected with L-NAME or ODQ 60 min before application of VCS (see Fig. 2B for lordosis data).
P<0.05;
P<0.001 vs. corresponding vehicle.
We also infused L-NAME or ODQ 60 min prior to MFS/VCS in some animals to determine whether a longer pre-exposure to these drugs would block the immediate increase in estrous behavior observed after MFS/VCS. Under these conditions, both L-NAME and ODQ attenuated the immediate increase (0 min) in LQ in addition to attenuating lordosis observed at 120 min post-MFS/VCS (Fig 2B). At 240 min post-MFS/VCS, the LQ scores of the MFS/VCS plus L-NAME group were still significantly decreased (P < 0.0 vs. vehicle; data not shown). Both inhibitors completely blocked the MFS/VCS-induced increase in proceptive behavior observed at 120 min post-MFS/VCS (P < 0.05; Table 3).
Because PKG is the major target of NO-dependent cGMP synthesis, we next tested the effect of a PKG inhibitor, KT5823, on MFS/VCS-induced lordosis and proceptive behaviors (Fig. 2A). When KT5823 was infused icv 15 min prior to MFS/VCS, the MFS/VCS-induced increase in lordosis was significantly attenuated at 0 and 120 min post-MFS/VCS (0 min, P < 0.05; 120 min, P < 0.001 vs. vehicle). As shown in Table 3, KT5823 also prevented the MFS/VCS-induced increase in proceptive behavior observed at 120 min (P < 0.01).
Discussion
The present results support the hypothesis that VCS facilitates female sexual behaviors (lordosis and proceptivity) through release of NE, which then activates α1-adrenergic receptors. The data are also consistent with the hypothesis that α1-adrenoceptors mediate VCS facilitation of estrous behaviors via the NO-cGMP-PKG pathway. Administration of two different α1-adrenergic receptor antagonists (prazosin, phenoxybenzamine) as well as inhibitors of NO synthase (L-NAME), NO-stimulated guanylyl cyclase (ODQ), and PKG (KT5823) all attenuated the VCS-induced increase in lordosis and proceptive behaviors observed in E2-primed female rats during mating with a male. Sexually receptive female rats exhibit elevated release of NE in the ventromedial hypothalamus during mating tests with males [24]. When a vaginal mask was used to permit receptive females to receive flank-perineal stimulation but to prevent them from receiving VCS, elevated NE was not detected in the hypothalamus [24]. Therefore, VCS resulting from male intromissions appears to be necessary for the mating-induced release of NE in the ventromedial hypothalamus. Present findings are consistent with our previous conclusion that the released NE facilitates reproductive behaviors by a pathway involving α1-adrenergic receptor activation of the NO-cGMP-PKG pathway [8].
Our results are in agreement with previous studies showing that α1, but not α2- or β-adrenergic receptors, mediate NE stimulation of female sexual behavior. In the present study, the facilitatory effect of VCS on lordosis was blocked by icv infusions of the α1 antagonists, prazosin and phenoxybenzamine, but not by β or α2 antagonists. Consistent with a role for α1-adrenoceptors in the stimulation of lordosis, Nock and Feder [46] found that phenoxybenzamine (α1 antagonist) reduced the facilitation of lordosis by the NE agonist clonidine in E2-primed females. Similarly, systemic or intrahypothalamic administration of the α1 antagonist prazosin blocked the stimulation of lordosis by hormone treatment or by NE infusion into the ventromedial hypothalamus of female rats [23, 27]. Likewise, administration of the α1 agonists phenylephrine or methoxamine, but not the α2 agonist clonidine or the β agonist isoproterenol, facilitated lordosis in E2-primed rats [4, 40]. Intrahypothalamic application of antagonists specific for α2- and β-adrenergic receptors directly into the ventromedial hypothalamus also fails to attenuate E2 and P-induced lordosis behavior [23]. Combined administration of the α2 agonist clonidine and β agonist isoproterenol is reported to enhance lordosis behavior 15 min after intrahypothalamic infusion in rats [40]. Nonetheless, most evidence suggests that the predominant effect of β-adrenergic receptor activation in the hypothalamus is to inhibit lordosis in rats [28]. Interestingly, E2 modifies activity of both β- and α1-adrenergic receptors in the hypothalamus and preoptic area, attenuating β-adrenergic while augmenting α1-adrenergic responses [25, 49, 57]. It is tempting to speculate that the attenuation of NE action at hypothalamic β receptors along with the potentiation of NE action at α1 receptors are functionally related to E2 priming of lordosis behavior.
There is little direct evidence that VCS induces the production specific second messengers. However, Meredith et al. [44] tested the hypothesis that VCS induces the phosphorylation of DARPP-32, a phosphoprotein activated by the cAMP-protein kinase A and cGMP-PKG systems [36, 56]. They found that VCS increased the number of cells expressing phosphorylated DARPP-32 immunoreactivity in several brain areas related to lordosis expression (preoptic area, ventromedial hypothalamus, etc.). In agreement with these findings, we have preliminary evidence that several kinase systems participate in VCS-induced stimulation of estrous behavior (unpublished observations). The present results support the participation of cGMP-PKG pathway, which can be activated through α1-adrenoreceptors. Specifically, we demonstrated that ODQ and KT5823, selective inhibitors of soluble guanylyl cyclase and PKG, respectively, attenuated VCS-facilitated lordosis and proceptive behaviors. Taken together, these results suggest that some of the neuronal effects of VCS, and perhaps other social and environmental stimuli, are mediated by neurotransmitter-induced phosphorylation of different proteins, perhaps DARPP-32, progestin receptors, or different coactivator proteins.
There is evidence that mating activates NOergic neurons in several brain areas important for regulation of sexual behavior [32, 60]. Given the importance of NO in the regulation of female reproductive function, activated NOergic neurons in these brain areas may directly or indirectly be involved in integrating mating signals to trigger neuroendocrine changes. In the present study, we show that a NOS inhibitor (L-NAME), a specific inhibitor of NO-stimulated guanylyl cyclase (ODQ) and a PKG inhibitor (KT5823) all significantly attenuated lordosis behavior induced by VCS. cGMP is a key mediator of cellular responses to NO. Fernández-Guasti et al. [26] were the first to demonstrate the stimulatory effects of guanine nucleotides on lordosis in E2-primed rats, indicating a potential role of cGMP in the regulation of this behavior. The Etgen laboratory subsequently showed that PKG mediates the facilitation of lordosis by cGMP, and that the ability of cGMP to facilitate lordosis in E2-primed rats is blocked by the progestin receptor antagonist RU 486 [9], suggesting that cGMP activates brain progestin receptors. We have also shown that inhibitors of NO synthesis, of NO-stimulated guanylyl cyclase, and of PKG significantly attenuate lordosis behavior induced by P and two of its ring A-reduced metabolites [30]. These results, together with those obtained in the present study, support the hypothesis that the NO-cGMP-PKG pathway is involved in lordosis induced by membrane mechanisms.
It seems likely that the NO-cGMP-PKG signaling pathway is activated by NE (via α1 adrenoreceptors) in response to VCS, and that this signaling pathway mediates the facilitatory effect of VCS on estrous behavior. Biochemically, α1-adrenoreceptors are well positioned to activate NO synthesis, a calcium-calmodulin activated enzyme, because they elevate intracellular calcium both by opening calcium channels and by mobilizing intracellular calcium stores [16]. Behavioral experiments showed that icv infusion of a cell-permeable cGMP analog reverses the inhibitory effect of the α1-adrenoreceptor antagonist prazosin on hormone-dependent lordosis behavior in female rats [8]. Moreover, E2 and P together greatly enhanced NO-stimulated cGMP synthesis in female rat hypothalamic slices and in GT1-1 cells [10]. Interestingly, NE and phenylephrine (α1 agonist) do not activate cGMP production in hypothalamic slices unless the tissue is prepared from animals exposed to both E2 and progesterone [8]. These results suggest that NO-stimulated cGMP production acts downstream of α1-adrenoreceptor activation to enhance lordosis responsiveness and that several steps in this pathway are hormone-regulated.
We cannot rule out the possibility that estrous behavior induced by VCS in ovx, E2-primed female rats requires the release of P from the adrenal cortex. The adrenal cortex secretes P in response to mating stimuli [29, 51, 55], as well as to a variety of other stimuli [50]. Removal of the adrenals increases progestin receptor levels in the brain [22]. VCS decreases progestin receptor immunoreactivity within 1 h in ovx, E2-primed female rats; however, VCS does not decrease progestin receptor immunoreactivity in ovx, adrenalectomized E2-primed female rats [1]. Because we find that stimulation of α1-adrenergic receptors in the hypothalamus only promotes cGMP synthesis if female rats are treated with both E2 and P [8], adrenal P may be necessary for NE to facilitate VCS-induced estrous behaviors.
In conclusion, the present data support the hypothesis that NE released in the hypothalamus in response to VCS activates α1-adrenoreceptors. These receptors in turn couple to the NO-cGMP-PKG pathway to mediate the VCS-dependent facilitation of lordosis and proceptive behaviors observed in E2-primed rats.
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Guadalupe Domínguez López. This work was supported by PROMEP program and by DHHS grant MH41414.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auger AP, LaRiccia LM, Moffatt CA, Blaustein JD. Progesterone, but not progesterone-independent activation of progestin receptors by a mating stimulus, rapidly decreases progestin receptor immunoreactivity in female rat brain. Horm Behav. 2000;37:135–144. doi: 10.1006/hbeh.1999.1565. [DOI] [PubMed] [Google Scholar]
- 2.Boling JM, Blandau R. The estrogen-progesterone induction of mating response in the spayed female rat. Endocrinology. 1939;25:359–364. [Google Scholar]
- 3.Brann D, Bhat GK, Lamar CA, Mahesh VB. Gaseous transmitters and neuroendocrine regulation. Neuroendocrinology. 1997;65:385–395. doi: 10.1159/000127201. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell JD, Clemens LG. Norepinephrine infusions into the medial preoptic area inhibit lordosis behavior. Pharmacol Biochem Behav. 1986;24:1015–1023. doi: 10.1016/0091-3057(86)90450-8. [DOI] [PubMed] [Google Scholar]
- 5.Carlson RR, DeFeo VJ. Role of the pelvic nerve vs the abdominal sympathetic nerves in the reproductive function of the female rat. Endocrinology. 1965;77:1014–1022. doi: 10.1210/endo-77-6-1014. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS. Stimuli contributing to the decrement in sexual receptivity of female golden hamsters (Mesocricetus auratus) Anim Behav. 1973;21:827–834. doi: 10.1016/s0003-3472(73)80108-3. [DOI] [PubMed] [Google Scholar]
- 7.Chu HP, Etgen AM. A potential role of cyclic GMP in the regulation of lordosis behavior of female rats. Horm Behav. 1997;3:125–132. doi: 10.1006/hbeh.1997.1413. [DOI] [PubMed] [Google Scholar]
- 8.Chu HP, Etgen AM. Ovarian hormone dependence of α1-adrenoceptor activation of the nitric oxide-cGMP pathway: relevance for hormonal facilitation of lordosis behavior. J Neurosci. 1999;19:7191–7197. doi: 10.1523/JNEUROSCI.19-16-07191.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu HP, Morales JC, Etgen AM. Cyclic GMP may potentiate lordosis behaviour by progesterone receptor activation. J Neuroendocrinol. 1999;11:107–113. doi: 10.1046/j.1365-2826.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu HP, Sarkar G, Etgen AM. Estradiol and progesterone modulate the nitric oxide/cyclic gmp pathway in the hypothalamus of female rats and in GT1-1 cells. Endocrine. 2004;24:177–84. doi: 10.1385/ENDO:24:2:177. [DOI] [PubMed] [Google Scholar]
- 11.Collins JJ, Lin CE, Berthoud HR, Papka RE. Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res. 1999;295:43–54. doi: 10.1007/s004410051211. [DOI] [PubMed] [Google Scholar]
- 12.Crowley WR, Nock B, Feder HH. Facilitation of lordosis behavior by clonidine in female guinea pigs. Pharmacol Biochem Behav. 1978;8:207–209. doi: 10.1016/0091-3057(78)90339-8. [DOI] [PubMed] [Google Scholar]
- 13.Cueva-Rolon R, Sansone G, Bianca R, Gomez LE, Beyer C, Whipple B, Komisaruk BR. Vagotomy blocks responses to vaginocervical stimulate after genitospinal neurectomy in rats. Physiol Behav. 1996;1:19–24. doi: 10.1016/0031-9384(95)02245-7. [DOI] [PubMed] [Google Scholar]
- 14.Delville Y. Progesterone-facilitated sexual receptivity: a review of arguments supporting a nongenomic mechanism. Neurosci Biobehav Rev. 1991;15:407–414. doi: 10.1016/s0149-7634(05)80033-8. [DOI] [PubMed] [Google Scholar]
- 15.Edwards DA, Whalen RE, Nadler RD. Induction of estrous: estrogen-progesterone interaction. Physiol Behav. 1968;3:29–33. [Google Scholar]
- 16.Esbenshade TA, Theroux RL, Minneman KP. Increased voltage-dependent calcium influx produced by α1B-adrenergic receptor activation in rat medullary thyroid carcinoma 6–23 cells. Mol Pharmacol. 1994;45:591–598. [PubMed] [Google Scholar]
- 17.Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- 18.Erskine MS, Weaver CE., Jr The role of ovarian sympathetic innervation in the control of estrous responsiveness in the rat. Horm Behav. 1988;22:1–11. doi: 10.1016/0018-506x(88)90026-8. [DOI] [PubMed] [Google Scholar]
- 19.Erskine MS. Role of pelvic and pudendal nerves in display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav Neurosci. 1992;106:690–697. doi: 10.1037//0735-7044.106.4.690. [DOI] [PubMed] [Google Scholar]
- 20.Erskine MS. Mating induced increases in fos protein in preoptic area and medial amygdala of cycling female rats. Brain Res Bull. 1993;32:447–451. doi: 10.1016/0361-9230(93)90289-n. [DOI] [PubMed] [Google Scholar]
- 21.Erskine MS. Prolactin release after mating and genitosensory stimulation in females. Endocr Rev. 1995;16:508–528. doi: 10.1210/edrv-16-4-508. [DOI] [PubMed] [Google Scholar]
- 22.Etgen AM. Progestin receptors and the activation of female reproductive behavior: a critical review. Horm Behav. 1984;18:411–430. doi: 10.1016/0018-506x(84)90027-8. [DOI] [PubMed] [Google Scholar]
- 23.Etgen AM. Intrahypothalamic implants of noradrenergic antagonists disrupt lordosis behavior in female rats. Physiol Behav. 1990;48:31–36. doi: 10.1016/0031-9384(90)90256-4. [DOI] [PubMed] [Google Scholar]
- 24.Etgen AM, Morales JC. Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexual receptive female rats. J Neuroendocrinol. 2002;14:213–218. doi: 10.1046/j.0007-1331.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- 25.Etgen AM, Ungar S, Petitti N. Estradiol and progesterone modulation of norepinephrine neurotransmission: implications for the regulation of female reproductive behavior. J Neuroendocrinology. 1992;4:255–271. doi: 10.1111/j.1365-2826.1992.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Guasti A, Rodríguez-Manzo G, Beyer C. Effect of guanine derivatives on lordosis behavior in estrogen primed rats. Physiol Behav. 1983;31:589–592. [PubMed] [Google Scholar]
- 27.Fernádez-Guasti A, Larsson K, Beyer C. Potentiative action of α and β-adrenergic receptor stimulation in inducing lordosis behavior. Pharmacol Biochem Behav. 1985;22:613–617. doi: 10.1016/0091-3057(85)90283-7. [DOI] [PubMed] [Google Scholar]
- 28.Foreman MM, Moss RL. Role of hypothalamic alpha and beta adrenergic receptors in the control of lordotic behavior in the ovariectomized estrogen-primed rats. Pharmacol Biochem Behav. 1978;9:235–241. doi: 10.1016/0091-3057(78)90170-3. [DOI] [PubMed] [Google Scholar]
- 29.Frye CA, McCormick CM, Coopersmith C, Erskine MS. Effects of paced and non-paced mating stimulation on plasma progesterone, 3alpha-diol and corticosterone. Psychoneuroendocrinology. 1996;21:431–439. doi: 10.1016/0306-4530(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 30.González-Flores O, Etgen AM. The nitric oxide pathway participates in estrous behavior induced by progesterone and some of its ring A-reduced metabolites. Horm Behav. 2004;45:50–57. doi: 10.1016/j.yhbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Guevara-Guzmán R, Barrera-Mera B, de la Riva C, Kendrick KM. Release of classical transmitters and nitric oxide in the rat olfactory bulb, evoked by vaginocervical stimulation and potassium, varies with the oestrous cycle. Eur J Neurosci. 2000;12:80–88. doi: 10.1046/j.1460-9568.2000.00882.x. [DOI] [PubMed] [Google Scholar]
- 32.Guevara-Guzmán R, Buzo E, Larrazolo A, de la Riva C, Da Costa AP, Kendrick KM. Vaginocervical stimulation-induced release of classical neurotransmitters and nitric oxide in the nucleus of the solitary tract varies as a function of the oestrous cycle. Brain Res. 2001;898:303–313. doi: 10.1016/s0006-8993(01)02207-7. [DOI] [PubMed] [Google Scholar]
- 33.Gunnet JW, Freeman ME. The mating-induced release of prolactin: A unique neuroendocrine response. Endocrinol Rev. 1983;4:44–61. doi: 10.1210/edrv-4-1-44. [DOI] [PubMed] [Google Scholar]
- 34.Hansen S, Stanfield EJ, Everitt BJ. The effects of lesions of lateral tegmental noradrenergic neurons on components of sexual behavior and pseudopregnancy in female rats. Neuroscience. 1981;6:1105–1117. doi: 10.1016/0306-4522(81)90075-0. [DOI] [PubMed] [Google Scholar]
- 35.Hardy DF, DeBold JF. Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol. 1972;78:400–408. doi: 10.1037/h0032536. [DOI] [PubMed] [Google Scholar]
- 36.Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- 37.Hubscher CH, Berkley KJ. Responses of neurons in caudal solitary nucleus of female rats to stimulation of vagina, cervix, uterine horn and colon. Brain Res. 1994;664:1–8. doi: 10.1016/0006-8993(94)91946-1. [DOI] [PubMed] [Google Scholar]
- 38.Komisaruk BR. Induction of lordosis in ovariectomized rats by stimulation of the vaginal cervix: Hormonal and neural interrelationships. In: Sawyer CH, Gorski RA, editors. Steroid Hormones and Brain Function. University of California Press; Berkley: 1971. pp. 127–136. [PubMed] [Google Scholar]
- 39.Komisaruk BR, Adler NT, Hutchison J. Genital sensory field: enlargement by estrogen treatment in female rats. Science. 1972;178:1295–1298. doi: 10.1126/science.178.4067.1295. [DOI] [PubMed] [Google Scholar]
- 40.Kow LM, Weesner GD, Pfaff DW. α1-adrenergic agonists act on the ventromedial hypothalamus to cause neuronal excitation and lordosis facilitation: electrophysiological and behavioral evidence. Brain Res. 1992;588:237–245. doi: 10.1016/0006-8993(92)91581-x. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann ML, Erskine MS. Induction of pseudopregnancy using artificial VCS: importance of lordosis intensity and prestimulus estrous cycle length. Horm Behav. 2004;45:75–83. doi: 10.1016/j.yhbeh.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Mani SK, Allen JM, Rettori V, McCann SM, O’Malley BW, Clark JH. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci USA. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masters DB, Jordan F, Beyer C, Komisaruk BR. Release of amino acids into regional superfusates of the spinal cord by mechano-stimulation of the reproductive tract. Brain Res. 1993;621:279–90. doi: 10.1016/0006-8993(93)90117-6. [DOI] [PubMed] [Google Scholar]
- 44.Meredith JM, Moffatt CA, Auger AP, Snyder GL, Greengard P, Blaustein JD. Mating-related stimulation induces phosphorylation of dopamine- and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female rat brain. J Neurosci. 1998;18:10189–10195. doi: 10.1523/JNEUROSCI.18-23-10189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moralí G, Beyer C. Neuroendocrine control of mammalian estrous behavior. In: Beyer C, editor. Endocrine Control of Sexual Behavior. New York: Raven Press; 1979. pp. 33–75. [Google Scholar]
- 46.Nock B, Feder HH. Noradrenergic transmission and female behavior of guinea pigs. Brain Res. 1979;166:369–380. doi: 10.1016/0006-8993(79)90222-1. [DOI] [PubMed] [Google Scholar]
- 47.Northrop LE, Shadrach JL, Erskine MS. Noradrenergic innervation of the ventromedial hypothalamus is involved in mating-induced pseudopregnancy in the female rat. J Neuroendocrinol. 2006;18:577–583. doi: 10.1111/j.1365-2826.2006.01453.x. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1997. [Google Scholar]
- 49.Petitti N, Karkanias GB, Etgen AM. Estradiol selectively regulates α1B-noradrenergic receptors in the hypothalamus and preoptic area. J Neurosci. 1992;10:3869–3876. doi: 10.1523/JNEUROSCI.12-10-03869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piva F, Gagliano P, Motta M, Martini L. Adrenal progesterone: Factors controlling its secretion. Endocrinology. 1973;93:1178–1184. doi: 10.1210/endo-93-5-1178. [DOI] [PubMed] [Google Scholar]
- 51.Plas-Roser S, Aron C. New data concerning the control by the adrenals of sexual receptivity in the rat. Physiol Behav. 1997;19:57–60. doi: 10.1016/0031-9384(77)90159-7. [DOI] [PubMed] [Google Scholar]
- 52.Pu S, Horvath TL, Diano S, Naftolin F, Kalra PS, Kalra SP. Evidence showing that β -endorphin regulates cyclic guanosine 3′,5′ monophosphate (cGMP) efflux: anatomical and functional support for an interaction between opiates and nitric oxide. Endocrinology. 1997;138:1537–1543. doi: 10.1210/endo.138.4.5086. [DOI] [PubMed] [Google Scholar]
- 53.Pu S, Kalra PS, Kalra SP. Ovarian steroid-independent diurnal rhythm in cyclic GMP/nitric oxide efflux in the medial preoptic area: possible role in preovulatory and ovarian steroid-induced LH surge. J Neuroendocrinology. 1998;10:617–625. doi: 10.1046/j.1365-2826.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- 54.Rodríguez Sierra JF, Crowley WR, Komisaruk BR. Vaginal stimulation in rats induces prolonged lordosis responsiveness and sexual receptivity. J Comp Physiol Psychol. 1975;89:79–85. doi: 10.1037/h0076442. [DOI] [PubMed] [Google Scholar]
- 55.Roser S, Roos J, Aron C. Role played by the adrenal gland progesterone activity on the early mating behavior of female rats during 4 days cycles. C R Seances Soc Biol Fil. 1973;167:927–929. [PubMed] [Google Scholar]
- 56.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 57.Ungar S, Makman MH, Morris SA, Etgen AM. Estrogen uncouples beta-adrenergic receptor from the stimulatory guanine nucleotide-binding protein in female rat hypothalamus. Endocrinology. 1993;133:2818–2826. doi: 10.1210/endo.133.6.8243309. [DOI] [PubMed] [Google Scholar]
- 58.Vathy I, Etgen AM. Hormonal activation of female sexual behavior is accompanied by hypothalamic norepinephrine release. J Neuroendocrinol. 1989;1:383–388. doi: 10.1111/j.1365-2826.1989.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 59.Vincent PA, Thornton J, Feder HH. Possible role of alpha-2-noradrenergic receptors in modulation of sexual behavior in female guinea pigs. Neuroendocrinology. 1987;46:10–13. doi: 10.1159/000124790. [DOI] [PubMed] [Google Scholar]
- 60.Yang SP, Voogt JL. Mating-activated nitric oxide-producing neurons in specific brain regions in the female rat. Brain Res. 2002;950:79–87. doi: 10.1016/s0006-8993(02)03004-4. [DOI] [PubMed] [Google Scholar]


