Abstract
Adult age differences in the neural systems mediating semantic (context-independent) memory were investigated using positron emission tomography (PET). Younger (20–29 years) and older (62–70 years) participants performed lexical decision (word/nonword discrimination) and nonsemantic (simple visual search) baseline tasks during PET scanning. Within the lexical decision task, display duration and presentation rate were varied across scans. The behavioral data suggested that although an age-related slowing was evident in visual feature and response processing, the retrieval of semantic/lexical information was similar for younger and older adults. For both age groups, lexical-related activation occurred in inferior prefrontal and occipitotemporal regions of the left hemisphere. Differential activation, as a function of age group, was observed in the left occipitotemporal pathway as a result of older adults’ maintaining higher levels of neural activity in striate cortex (during visual search) and in inferior temporal cortex (during lexical decision). The prefrontal activation was similar for the two age groups. Thus, although this form of semantic memory retrieval does not undergo significant age-related decline, an age-related change in the associated pattern of neural activation is evident. These findings differ from previous neuroimaging studies of episodic (context-dependent) memory retrieval, which have suggested that age-related compensatory mechanisms are expressed primarily by greater activation of prefrontal regions for older adults than for younger adults.
Keywords: aging, brain function, semantic memory, PET, vision, cognition, reaction time, lexical decision, neuroimaging, information processing
Neuroimaging investigations using PET and functional magnetic resonance imaging (fMRI) have demonstrated that different types of memory are mediated by distinct but overlapping neural systems (Desgranges, Baron, & Eustache, 1998; Nyberg & Cabeza, 2000). Episodic and working memory tasks assess memory for events associated with specific sources or contexts (e.g., a recall or recognition test for previously presented items). Episodic encoding and retrieval processes are associated with activations in a widely distributed network, including the prefrontal, medial temporal, posterior midline, parietal, anterior cingulate, occipital, and cerebellar regions (Cabeza & Nyberg, 2000; Gabrieli, 2001). Different components of the network mediate different types of stimulus processing and task demands, with medial temporal and left prefrontal regions being prominent during encoding and right prefrontal regions being prominent during retrieval. Working memory is a related system that enables the temporary storage and active maintenance of information and relies on prefrontal, premotor, and posterior parietal regions (D’Esposito, 2001; Hartley & Speer, 2000).
In contrast to the episodic and working memory systems, semantic memory refers to memory for properties of items that are logically independent of specific contexts, such as the determination that a particular sequence of letters forms a word. Investigations of semantic memory processing have frequently used word naming and word/nonword discrimination (lexical decision) tasks to identify component processes of visual word identification (Taft, 1991). In the case of lexical decision (the task used in the present experiment), the pattern of statistical interaction among variables such as stimulus quality, word frequency, and context (e.g., priming) in reaction time (RT) suggests that word identification occurs as a multistage activation process (Besner & Smith, 1992; Borowsky & Besner, 1993). This process begins with the activation of information in an orthographic input lexicon (sensitive to stimulus quality). The output from this stage leads to activation in a separate semantic system (sensitive to word frequency). Participants monitor the level of activation in this latter system as the basis for the word/nonword decision, particularly when nonwords are pronounceable and cannot be distinguished from words at the orthographic level.
Neuroimaging investigations of word identification have most frequently reported activation of left hemisphere networks, particularly in inferior prefrontal and occipitotemporal regions (Binder & Price, 2001). Petersen, Fox, Snyder, and Raichle (1990), using PET, found that silent viewing of either words or pronounceable nonwords, relative to viewing a fixation point, led to increased regional cerebral blood flow (rCBF) in left medial extrastriate cortex (near the posterior lingual gyrus or lingual-fusiform border). Viewing consonant letter strings or false fonts (letterlike symbols) did not lead to activation in this region. These results suggest a central role for left medial extrastriate cortex in lexical access or the analysis of orthographic regularity (but cf. Price et al, 1994). In addition, Petersen et al. reported that when the false font condition was used as the baseline, left inferior prefrontal activation was evident for words but not for nonwords, which implicates this prefrontal region in semantic processing. Activation of the left inferior frontal gyrus has also been observed for more complex semantic decisions such as abstract/concrete judgments regarding words (Gabrieli et al., 1996). This type of semantic retrieval may be useful as a mechanism of encoding during episodic memory tasks (Demb et al., 1995; Wagner et al., 1998).
The activation of occipitotemporal and prefrontal regions of the left hemisphere during visual word identification has been generally confirmed in subsequent PET and fMRI experiments, although the functional role of specific cortical regions has not been established definitively. Herbster, Mintun, Nebes, and Becker (1997), for example, measured neural activation with PET while participants made vocal responses to visually presented words and nonwords. These authors found that reading aloud words, with either regular or irregular spelling, was associated with activation in left fusiform cortex (relative to a sensory-motor baseline), whereas reading aloud either nonwords or irregular words led to activation in the left inferior frontal gyrus. In contrast to Petersen et al. (1990), Herbster et al. proposed that a posterior cortical region, the left fusiform gyrus, mediated lexical retrieval in this task and that the left inferior prefrontal activation represented participants’ reliance on phonological processing. Other neuroimaging studies (Fiez, Raichle, Balota, Tallal, & Petersen, 1996; Howard et al., 1992; Vandenberghe, Price, Wise, Josephs, & Frackowiak, 1996), as well as direct recording of neural activity from indwelling electrodes (Nobre, Allison, & McCarthy, 1994), also suggest that specific regions within the occipitotemporal pathway are involved in the analysis of word meaning. The prefrontal activation during word identification tasks may represent self-directed retrieval and concurrent processing of semantic information, whereas the occipitotemporal regions may be more involved in long-term semantic storage (Fiez, 1997; Wagner, Desmond, Demb, Glover, & Gabrieli, 1997). Within the left inferior prefrontal cortex, it appears that the analysis of word meaning typically activates more anterior regions, Brodman areas (BAs) 47 and 10, whereas phonological processing activates more posterior regions, BAs 44 and 45 (Fiez, 1997).
Behavioral research on adult age differences in memory abilities suggest that different memory systems vary with regard to their trajectories of adult development as well as with regard to their patterns of neural activation. Older adults’ performance is typically worse than that of younger adults in tasks that involve self-directed encoding and retrieval of context-dependent information (Craik & Jennings, 1992). Consequently, a general theme from the behavioral findings is that working memory and episodic memory exhibit age-related decline, whereas semantic memory performance is less vulnerable to decline with aging (Balota, Dolan, & Duchek, 2000; Light, 1996; Mayr & Kliegl, 2000). A major contributing factor to age-related decline in episodic and working memory appears to be a slowing of information processing that applies across a wide range of component abilities (Madden, 2001; Salthouse, 1996). Age-related slowing affects episodic and working memory performance by limiting the rate at which items can be effectively encoded, rehearsed, and later retrieved. Access to semantic memory information, in contrast, does not appear to undergo significant age-related decline.
Studies of age-related effects in lexical decision performance suggest that the activation of semantic information is comparable for younger and older adults. The effects of word frequency and semantic priming, for example, remain relatively constant during later adulthood (Allen, Madden, & Slane, 1995; Madden, Pierce, & Allen, 1993; but cf. Balota & Ferraro, 1993). Other variables such as stimulus quality interact more reliably with age (Madden, 1988, 1992), consistent with age-related slowing at the orthographic input stage.
Several neuroimaging investigations have reported age-related changes in neural activation during episodic and working memory performance (Cabeza, 2001; Grady & Craik, 2000; Langley & Madden, 2000). A finding that has occurred in several experiments is that, during episodic retrieval conditions, activation of prefrontal cortex is relatively lateralized to the right hemisphere for younger adults but exhibits a bilateral pattern for older adults (Cabeza et al., 1997; Cabeza, Anderson, Mangels, Nyberg, & Houle, 2000; Madden et al., 1999). The age-related increase in prefrontal activation may represent the recruitment of neural systems, outside of the task-relevant cortical regions, to support the attentional demands of the memory task. This recruitment may be compensatory in the sense that aging is associated with a decline in efficiency of the neural systems mediating episodic encoding and retrieval (Grady et al., 1995; Grady, McIntosh, Rajah, Beig, & Craik, 1999).
The relation between behavioral performance and neural activation in memory tasks may also differ as a function of age. Rypma and D’Esposito (2000) found that, within dorsolateral prefrontal cortex (BAs 9 and 46, bilaterally), the relation between reaction time (RT) for retrieval from working memory and neural activation was positive for younger adults, whereas the correlation was negative for older adults. Rympa and D’Esposito accounted for their results in terms of a model of the relation between neural activation and response discriminability. In their model, the relation exhibits a sigmoidal shape, and increasing activation, within a certain range, would be beneficial for older adults (leading to faster responses) but detrimental for younger adults (leading to slower responses). Madden et al. (1999), taking a different approach, used stepwise multiple regression to determine the correlation between RT and rCBF activation, for a set of activated cortical regions, in an episodic recognition task. For both age groups, activation of right prefrontal cortex (BA 10) was related positively to recognition RT. The best fitting regression model for older adults, however, unlike that for younger adults, also included several cortical exhibiting negative correlations between RT and activation, which may represent the age-related changes in response discriminability proposed by Rypma and D’Esposito.
Age differences in neural activation during semantic memory tasks have not been investigated extensively. It is thus unclear whether the similarity between younger and older adults’ semantic memory performance also occurs at the level of neural activation or is instead the result of the recruitment of additional neural systems on the part of older adults. Madden et al. (1996) obtained relevant evidence in a PET study of younger and older adults’ performance during a lexical decision (word/nonword discrimination) task. The behavioral data indicated that the age differences were consistent with a generalized slowing of processing speed rather than with specific changes in semantic retrieval. Analyses of differences in rCBF between task conditions suggested that the retrieval of semantic information sufficient to distinguish words from nonwords was mediated, for both age groups, by ventral occipitotemporal cortex. This activation was bilateral for both age groups, but a region in the left extrastriate cortex (BA 18) exhibited greater activation for younger adults than for older adults. The lexical decision conditions did not lead to prefrontal activation for either age group. Thus, the age-related slowing in performance and the age-related decrease in occipitotemporal activation suggested a corresponding decline in the efficiency of processing in this cortical region, and no compensatory recruitment of other neural systems was evident.
The absence of left inferior prefrontal activation in the Madden et al. (1996) data is surprising in view of previous reports of activation in this region for lexical and semantic processing (Demb et al., 1995; Gabrieli et al., 1996; Petersen et al., 1990; Wagner et al., 1998). The Madden et al. experiment, however, used passive viewing of words and nonwords as a baseline for the lexical decision condition, and thus the orthographic, phonological, and semantic processing activated automatically by viewing the words may have limited the ability to detect activation associated with lexical decision. In the present experiment we used a simple letter search task (search for a “c” in a letter string of alternating Ts and Zs) as a baseline condition. The stimuli in this search task were similar in visual structure to the lexical decision stimuli and required a yes/no response. The baseline search task was essentially a version of feature search in which the presence of a circular (and smaller) item among angular (and larger) items was sufficient for a yes response. This type of search is highly efficient and requires minimal attentional demands (Treisman, 1988; Wolfe, 1998). Thus, although the baseline task may recruit some processes (e.g., orienting to a specific location in the letter string) not required by lexical decision, it should not activate semantic information and consequently provide an appropriate control for visual display structure and response selection.
Our expectation was that comparisons among these conditions would be more sensitive to semantically driven activation of left inferior prefrontal cortex than those of the Madden et al. (1996) experiment. If there is a correspondence between age-related changes in neural activation for semantic (context-independent) and episodic (context dependent) memory retrieval, then the prefrontal activation should be either greater in magnitude or involve additional prefrontal regions, for older adults, as a result of compensation for age-related decline in the efficiency of information processing (Cabeza, 2001; Grady & Craik, 2000; Langley & Madden, 2000).
The word displays in the Madden et al. (1996) lexical decision conditions remained on the screen until the participant responded. The RTs varied significantly as a function of age group and task condition, and the resulting variation in the duration of the word displays, and in the total number of displays viewed during a scan, may have affected the observed pattern of activation. In the present experiment we examined age differences in rCBF activation under conditions that equated display duration and presentation rate for the two age groups. We varied duration and presentation rate across the lexical decision task conditions. Price et al. (1994) and Price, Moore, and Frackowiak (1996) demonstrated that sensory variables such as presentation rate and display duration are important determinants of the pattern of neural activation and visual word identification tasks. Price et al. (1994) found that more cortical regions were active at a shorter (150 ms) than at longer (981 ms or 1000 ms) display durations. Comparing lexical decision and nonsemantic (feature search) conditions, however, Price et al. (1994) noted that increasing display duration was associated with increasing activation in extrastriate cortex bilaterally. Price et al. (1996) reported that increasing presentation rate during word reading (both silently and aloud) led to increased activation in several regions, including occipitotemporal cortex bilaterally, as well as in motor regions, thalamus, and left dorsolateral prefrontal cortex (near Broca’s area).
In view of the age-related decline in the activation of occipitotemporal cortex reported by Madden et al. (1996), we expected that the increases in occipitotemporal activation associated with changes in duration and presentation rate would be more clearly evident for younger adults than for older adults. Prefrontal activation associated with the duration and rate variables, however, should be relatively greater for older adults, reflecting compensatory recruitment. Finally, within regions exhibiting an age-related change in rCBF activation, we examined the correlation between RT and the level of activation to determine whether the age difference in the direction of this correlation, which has been observed in the context of a working memory task (Rympa & D’Esposito, 2000), would also hold for a task involving the retrieval of semantic information.
METHOD
Participants
The participants were 12 younger adults between 20 and 29 years of age and 12 older adults between 62 and 70 years of age (Table 1). There were six women in each age group. The research procedures were approved by the Institutional Review Board of the Duke University Medical Center, and all participants gave written informed consent.
TABLE 1.
Participant Characteristics
| Younger adults
|
Older adults
|
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age (years) | 23.58 | 3.45 | 65.00 | 2.30 |
| Education (years) | 15.67 | 2.02 | 16.75 | 2.60 |
| Vocabulary | 63.25 | 5.41 | 63.92 | 3.68 |
| Digit symbol (accuracy) | 96.67 | 3.11 | 97.25 | 3.28 |
| Digit symbol (time) | 1,245.42 | 188.24 | 1,719.75 | 206.12 |
| Acuity | 15.42 | 1.44 | 20.42 | 8.38 |
| MMSE | 29.17 | 1.27 | 28.67 | 0.89 |
| Cortical volume (cm3) | 692.31 | 75.35 | 637.37 | 84.94 |
Note. Each group contained six women and six men. Vocabulary = raw score (of 70) on the Vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised (WAIS-R; Wechsler, 1981). Digit Symbol = percentage correct and median time per item (in milliseconds) on a computer version of the WAIS-R Digit Symbol subtest (Salthouse, 1992). Acuity = denominator of the Snellen fraction for corrected near vision (binocular). MMSE = score (of 30) on the Mini-Mental State Exam (Folstein et al., 1975). Cortical volume = height-adjusted volume of cortical gray matter. Cortical volume for one younger participant was lost due to movement artifact. Digit symbol (time) was significantly higher for older adults than for younger adults, p < .05, and cortical volume was marginally lower for older adults than for younger adults, p = .054. For all other variables, except age, the two groups differed nonsignificantly.
The younger adults were recruited from the Duke University campus and were primarily university staff and students. The older adults were recruited from the Duke Aging Center Subject Registry and from advertisements in the local media. Participants were right-handed, possessed at least a high school education, and were free from current health problems (e.g., diabetes, hypertension, and arteriosclerosis) as well as from previous significant medical events (e.g., head injury with loss of consciousness more than 5 min, stroke, transient ischemic attack, heart attack, and heart surgery), as determined by a screening questionnaire (Christensen, Moye, Armson, & Kern, 1992). All participants scored at least 27 (of 30) on the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975).
Participants completed the Vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised (WAIS-R; Wechsler, 1981) and a computer version of the WAIS-R Digit Symbol Substitution subtest (Salthouse, 1992). The latter test measures both accuracy and median time per item to code digit-symbol pairs. Corrected binocular acuity for near vision was measured with a Keystone Telebinocular vision tester (Mast/Keystone, Davenport, IA), using split-ring slides. Each participant possessed a corrected acuity of at least 20/40. Prior to PET testing, participants underwent MR imaging, and a neuroradiologist reviewed the images and excluded participants based on evidence of significant cerebral atrophy or structural abnormality.1 Two younger adults and four older adults were excluded and replaced on the basis of either the health screening or MR imaging.
Apparatus and Stimuli
On each trial, participants made a yes/no decision regarding a single string of visually presented letters (the target). Presentation of the targets and measurement of participants’ responses were controlled by a 486-processor (66-MHz) microcomputer (Gateway 2000, N. Sioux City, SD). The targets were presented on a Gateway Vivitron 15-inch video monitor, with a 285 × 213 mm display area. The monitor was positioned above the gantry opening of the PET scanner, facing downward at an approximately 45° angle at a viewing distance of approximately 81 cm. The character line on which the targets were presented was located in the upper third of the viewing area so that older adults who wore glasses would be viewing it through the distance portion of their lenses. The targets were presented as white letters against a dark gray background. Participants responded on each trial by means of a two-button response box connected to the game port of the microcomputer.
There were four task conditions: Baseline, Lexical A, Lexical B, and Lexical C. The three Lexical task conditions differed only in the duration and presentation rate of the targets (see “Procedure”). The target in the Baseline condition was a series of alternating Ts and Zs (e.g., TZTZT, and ZTZTZTZTZ); participants responded yes when a lowercase “c” was present within the letter string (e.g., TZTZTcT) and no otherwise. The other three conditions involved word/nonword discrimination (lexical decision), in which participants responded yes when the target was a word and no when it was a nonword. The characters in the letter strings (with the exception of yes-response trials in the Baseline condition) were all uppercase letters, 0.90° in height. The character space for each letter was approximately 0.67° wide, and the length of the targets ranged from 2.69° (4 letters) to 6.71° (10 letters).
The lexical items were drawn from a set of 720 words and 720 nonwords. These items were grouped into 12 sets of 60 words and 12 sets of 60 nonwords such that within each set, the length of the targets ranged from 4 to 10 letters. Across the 24 sets of 60 items, mean target length per set ranged from 6.75 to 6.95 letters. All of the nonwords were pronounceable but nonhomophonic with real words (e.g., roatle). The words were selected to have their primary usage as a noun (e.g., pretzel), with a standard frequency index (SFI; Carroll, Davies, & Richman, 1971) between 20 and 65. (Increasing SFI represents increasing frequency of occurrence in printed text.) Across the 12 sets of words, the mean SFI ranged from 41.69 to 42.20.
In the task condition with the highest rate of target presentation (Lexical C), each of the trial blocks contained 240 trials, comprising 4 of the 60-item sets (2 sets of words and 2 sets of nonwords). In the Lexical A and Lexical B conditions, each trial block contained 120 trials, comprising 1 set of words and 1 set of nonwords. Each of the Baseline trial blocks also contained 120 trials, comprising 60 trials on which the “c” was present and 60 trials on which it was absent. The Baseline letter strings were five, seven, or nine letters in length. The Baseline trial blocks each contained 20 trials for each combination of target length and yes/no response. The position of the “c” was varied on the Baseline trials requiring a yes response.
Procedure
In a separate testing session prior to PET testing, participants performed the health screening, psychometric, and visual acuity tests, as well as a practice version of the Baseline and Lexical task conditions. This practice test included 80 trials for the Lexical C condition and 40 trials for each of the other task conditions. None of the word and nonword targets used during the practice testing were used in the subsequent PET session.
The conditions differed in the duration and presentation rate of the targets (Table 2). Changes in the duration of the target and interstimulus interval between the offset of the target and the onset of the next warning signal led to changes in target presentation rate. Thus, comparing the Lexical A and Baseline conditions provided a test of semantic versus nonsemantic tasks, holding target duration and presentation rate constant. Comparing Lexical B and Lexical A provided a test of increasing target duration holding presentation rate constant. Comparing Lexical B and Lexical C was a test of increasing presentation rate holding target duration constant. The duration and presentation rate values were selected on the basis of pilot testing, to lead to changes in RT for both age groups without differentially affected older adults’ accuracy.
TABLE 2.
Task Conditions
| Condition | Trials per block | Target duration (ms) | ISI (ms) | Target rate |
|---|---|---|---|---|
| Baseline | 120 | 750 | 750 | 1/2000 ms |
| Lexical A | 120 | 750 | 750 | 1/2000 ms |
| Lexical B | 120 | 375 | 1125 | 1/2000 ms |
| Lexical C | 240 | 375 | 750 | 1/1625 ms |
Note. ISI = interstimulus interval, measured from the offset of the target stimulus to the onset of the subsequent trial’s warning signal. Each trial began with a 500-ms warning signal (an asterisk in the middle of the screen). The screen was blank during the ISI. The Baseline condition required a yes/no decision regarding whether a string of alternating Ts and Zs contained a lowercase “c.” The Lexical conditions (A–C) required a yes/no decision regarding whether the letter string was a word.
At the beginning of the PET testing session, participants performed a block of practice trials for each of the task conditions. There were 40 practice trials for the Lexical C condition and 20 trials for each of the other task conditions (divided equally between yes and no responses). For both the practice and the test trials, participants were reminded of the task instructions and response assignments before each block. The experimenter instructed participants to respond as quickly as possible while maintaining accuracy.
During PET testing, each participant performed a run of 12 blocks of trials corresponding to the 12 PET scans. Each trial block contained an equal number of yes and no response trials, ordered randomly. Within the run of 12 blocks of trials, a particular sequence of the 4 task conditions was repeated 3 times. Sixteen block orders were constructed such that across the block orders, each task condition occurred 4 times within each 4-block section of the run. Each word and nonword target occurred only once in the run for an individual participant. Across the 16 block orders, each word occurred 8 times in the Lexical C condition and 4 times in each of the other two lexical conditions. Eight of the block orders were assigned to each age group, and each of the orders was usd at least once within each age group. Participants rested the index and middle fingers of the right hand on the two response buttons. Assignment of the yes and no responses to the two response buttons was counterbalanced across participants but remained constant for each participant.
Positron Emission Tomography
The measurement of rCBF was conducted with a General Electric Advance whole-body PET scanner containing 18 detector rings. Data were acquired simultaneously from 35 imaging planes (18 direct planes and 17 cross planes) separated by 4.25 mm. The axial field of view was 15 cm, and the intrinsic in-plane and axial spatial resolutions were approximately 5 mm FWHM. Data acquisition was performed in the septa-out, three-dimensional mode (DeGrado et al., 1994).
Radiotracer injection was performed through an intravenous catheter placed in the participant’s left arm at the beginning of the testing session. The participant was positioned in the tomograph with his or her head aligned in a plane approximately parallel to the glabella-inion line. Alignment was conducted with the assistance of a low-power laser. Prior to the emission scans, a 5-min transmisison scan was performed using a pair of 3- to 10-mCi 68Ge rotating pin sources. Participants performed the practice trials following the transmission scan.
During the 12 emission scans, the radiotracer was administered as an intravenous bolus injection of approximately 10 mCi of H215O. Presentation of the visual displays was initiated 30–60 s prior to radio-tracer injection and continued for approximately 4–6.5 min, depending on the task condition. The PET data acquisition began automatically when the radioactivity count rate exceeded a preset threshold of 75,000 counts/s (random-corrected) and continued for 1 min. Radiotracer injections were separated by 10 min. Reconstruction of the PET image data was performed with filtered back-projection using a Hann filter transaxially and a ramp filter axially (Kinahan & Rogers, 1990). Images comprised 128 × 128 pixels (2 × 2 mm2) for each of 35 slices. The data were corrected for random coincidences, attenuation, scattered radiation, and dead time.
Changes in rCBF between task conditions were analyzed with the 1999 version of the Statistic Parametric Mapping software (SPM99; Wellcome Department of Cognitive Neurology, London, UK; Friston et al., 1995). For each participant, a mean PET image was derived from the three scans associated with each task condition. The 12 scans for each subject were reordered to the same sequence of task conditions and then realigned using the scan for the first Baseline condition as a reference. The scans were normalized and transformed, after realignment, into a standard stereotaxic space (Talairach & Touroux, 1988). This procedure began with a 12 parameter affine transformation, followed by piecewise (contiguous transverse slices) nonlinear matching, constrained by a set of smooth basis functions (Friston et al., 1996). An additional 15-mm FWHM isotropic Gaussian kernel was used to smooth the spatially normalized images.
The SPM analyses of the task condition contrasts used a fixed effects model of the mean PET images. An analysis of covariance was applied to the PET counts on a voxel-by-voxel basis, including global activity as a confounding covariate. The increase in rCBF between task conditions was represented by a linear contrast (i.e., image subtraction), which was reversed to yield the corresponding decrease in rCBF. The resulting set of voxel t values for each subtraction formed the statistical parametric map SPM{t}. The t values were transformed to the unit normal distribution SPM{Z} and then characterized in terms of spatial extent (k) of clusters of contiguously activated voxels and the peak height (u) and of the local maxima within the clusters. Voxels were thresholded for height at Z = 2.33 (p = .01, unconnected). The local maxima within each cluster were defined as voxels with Z values greater than all voxels within 8 mm.
Significance of the peak height was estimated using distributional approximations from the theory of Gaussian fields. The significance level referred to the probability that the observed peak height of a local maximum of activation is greater than would be expected by chance (p[Zmax > u]). Corrected p values were obtained for estimates applied to the entire volume analyzed. Voxel-level rCBF change was considered to be significant statistically if the associated cluster size was at least 100 voxels and the SPM corrected probability level for the peak height of the local maximum was less than .05.
To assess age differences associated with the task condition contrasts, the rCBF data for the two age groups were combined, and a masked analysis was conducted that included two simultaneous linear contrasts: one representing the task condition subtraction of interest (for one age group) and the other representing the difference between the two age groups for that subtraction. The task condition contrast for the selected age group was used as a mask for the age difference contrast. For each task condition contrast, the younger adults’ activation map was used as a mask for the contrast defining the change in the younger adults’ activation relative to that of the older adults, and the older adults’ activation map was used as a mask to identify the change in the older adults’ activation relative to that of the younger adults. Thus, the masked analysis identified, from those voxels that were significantly activated for a particular age group (at p < .05, corrected), the subset of voxels that exhibited a greater degree of change in activation than in the other age group. This approach was used for identifying age differences in both rCBF increases and decreases between task conditions. The p values for the interaction contrasts were corrected on the basis of the number of regions identified in the contrast used as the mask. Additional analyses were conducted on the PET radioactivity counts of the voxels identified as local maxima in the SPM maps.
RESULTS
Lexical Decision Performance
The performance data of primary interest were the correct RTs for the yes/no response (Fig. 1). Error rates were relatively low (less than 6.0% for each combination of age group and task condition), but were also analyzed as an adjunct to the RT analyses. For each age group, failures to respond occurred on less than 1.50% of the trials.
FIG. 1.
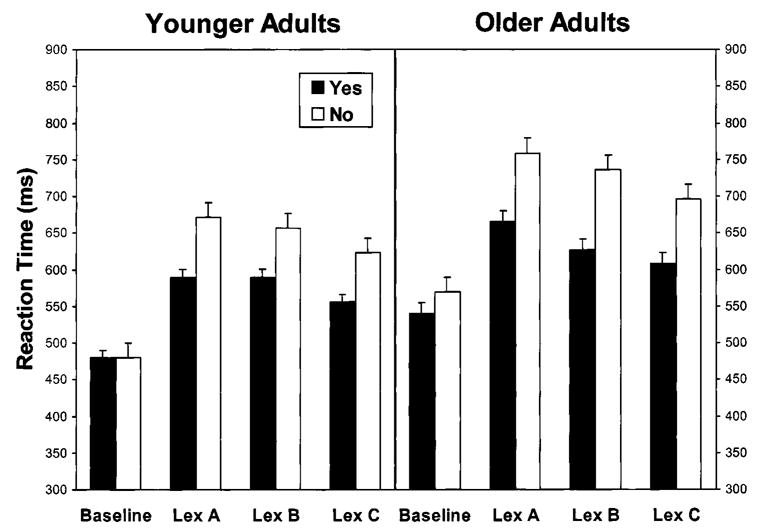
Means of median reaction time for correct responses as a function of age group, task condition, and response time. Means are accompanied by standard error bars. Lex = Lexical. Task conditions are described in Table 2. In the Baseline condition, yes responses = target present and no responses = target absent. In the lexical decision conditions, yes responses = word target and no responses = nonword target.
In a split-plot analysis of variance (ANOVA) of the median RTs for all four task conditions, age group was a between-subjects variable and task condition and response type were within-subjects variables. All of the main effects were significant: age group, F(1, 22) = 13.84, MSE = 16,596, p < .001; task condition, F(3, 66) = 109.79, MSE = 2,061, p < .001; and response type, F(1, 22) = 141.95, MSE = 1,506, p < .001. The interactions of Age Group × Response Type, F(1, 22) = 5.75, MSE = 1,506, p < .05, and Task Condition × Response Type, F(3, 66) = 50.75, MSE = 18,637, p < .001, were also significant. Further analysis focused on the three comparisons of pairs of task conditions that corresponded to the central components of the rCBF analyses: Lexical A versus Baseline, Lexical A versus Lexical B, and Lexical C versus Lexical B.
Lexical A versus Baseline
This comparison represents the retrieval of semantic information involved in distinguishing words from nonwords relative to the visual feature discrimination processes involved in the search task. In the ANOVA of the RT data for the Lexical A and Baseline conditions, all of the main effects were significant: Older adults’ responses were 78 ms slower than those of younger adults, F(1, 22) = 14.75, MSE = 9,920, p < .001; RT was 154 ms higher for lexical decision than for visual search, F(1, 22) = 211.35, MSE = 2,696, p < .0001; and no responses were 51 ms slower than yes responses, F(1, 22) = 86.36, MSE = 731, p < .001. The Task Condition × Response Type interaction was also significant, F(1, 22) = 110.24, MSE = 281, p < .001, because the difference between yes and no responses was more pronounced in the lexical decision task (88 ms) than in the search task (15 ms).
An ANOVA of the error rates for these two conditions yielded a significant main effect for task condition, F(1, 22) = 20.89, MSE = 0.000654, p < .001, representing a higher error rate for the Lexical A condition (3.80%) than for the Baseline condition (1.41%). The only other significant effect for error rates was the Task Condition × Response Type interaction, F(1, 22) = 5.07, MSE = 0.000247, p < .05. This latter effect occurred because errors on no-response trials were slightly higher than on yes-response trials (by 1.17%) in the lexical decision task, whereas the yes-response errors were relatively higher (by 0.28%) in the search task.
Lexical A versus Lexical B
This comparison represents the effect of varying display duration during the lexical decision task. All three main effects were significant in the ANOVA of the RT data: Older adults’ responses were 70 ms slower than those of younger adults, F(1, 22) = 9.21, MSE = 12,661, p < .01; RT was 19 ms higher for the longer duration targets (Lexical A) than for the shorter duration targets (Lexical B), F(1, 22) = 12.13, MSE = 717, p < .01; and no responses (nonword targets) were 87 ms slower than yes responses (word targets), F(1, 22) = 154.71, MSE = 1,177, p < .001. The interaction terms for Age Group × Task Condition, F(1,22) = 4.56, MSE = 717, p < .05, and Age Group × Task Condition × Response Type, F(1, 22) = 4.64, MSE = 301, p < .05, were significant. The three-way interaction represented the fact that although both age groups increased their RT for the longer duration nonwords relative to the shorter duration nonwords (15 ms for younger adults and 23 ms for older adults), the older adults also increased their RT for longer duration words relative to shorter duration words (by 38 ms), whereas the younger adults did not.
Analyses of the error rate data yielded only a main effect of task condition, F(1, 22) = 8.28, MSE = 0.000206, p < .01, reflecting a higher error rate for shorter duration targets (4.64%) than for longer duration targets (3.80%).
Lexical C versus Lexical B
This comparison represents the effect of varying presentation rate in the lexical decision task. All three main effects were significant in the ANOVA: Older adults’ responses were 61 ms slower than those of younger adults, F(1, 22) = 9.60, MSE = 9,094, p < .01; RT was 31 ms higher with the slower presentation rate (Lexical B) than with the faster presentation rate (Lexical C), F(1, 22) = 22.42, MSE = 1,067, p < .001; and responses to nonword targets were 82 ms slower than responses to word targets, F(1, 22) = 158.48, MSE = 1,023, p < .0001. The Age Group × Response Type interaction was also significant, F(1, 22) = 6.19, MSE = 1,023, p < .05. Although the RT difference between word and nonword targets was in the same direction for both age groups, the difference was greater for older adults (98 ms) than for younger adults (66 ms).
The only significant effect in the error rate data was a main effect of task condition, F(1, 22) = 10.10, MSE = 0.000235, p < .01, reflecting an increase in error rate with the faster presentation rate (5.64%) relative to the slower presentation rate (4.64%).
Regional Cerebral Blood Flow
The changes in rCBF associated with the task condition contrasts are presented in Table 3. Table 3 also lists the coordinates of the local maxima in a standard stereo-taxic space (Talairach & Tournoux, 1988), the Brodmann’s area (BA) designation, and the gyral location. The rCBF activations for the younger adults are presented in Fig. 2 and the older adults’ activations are presented in Fig. 3.
TABLE 3.
Regions of rCBF Change as a Function of Age Group and Task Condition Subtraction
| Region size (k) | p(Zmax> u) | Z | x | y | z | BA | Location | Direction | Interaction |
|---|---|---|---|---|---|---|---|---|---|
| Lexical A minus Baseline (semantic effect) for younger adults | |||||||||
| 7245 | .0001 | 6.22 | −27 | −89 | −15 | 18 | Inferior occipital gyrus | Inc | |
| .0001 | 6.12 | −20 | −93 | −16 | 18 | Lingual gyrus | Inc | ||
| .0001 | 6.09 | −19 | −96 | −9 | 17 | Striate | Inc | ||
| .001 | 5.48 | −36 | −44 | −25 | Cerebellum | Inc | |||
| .031 | 4.59 | −29 | −15 | −34 | 36 | Parahippocampal gyrus | Inc | ||
| .035 | 4.56 | −40 | −63 | −18 | Cerebellum | Inc | |||
| 4257 | .010 | 4.85 | −33 | 18 | 17 | 45 | Inferior frontal gyrus | Inc | |
| 374 | .025 | 4.65 | 17 | −69 | 49 | 7 | Superior parietal lobe | Dec | |
| Lexical A minus Baseline (semantic effect) for older adults | |||||||||
| 8055 | .0001 | 7.71 | −43 | −60 | −19 | Cerebellum | Inc | ||
| .0001 | 6.01 | −36 | −40 | −23 | Cerebellum | Inc | |||
| .0001 | 5.68 | −27 | −89 | −17 | 18 | Inferior occipital gyrus | Inc | ||
| 6636 | .0001 | 6.66 | −33 | 26 | 1 | 47 | Inferior frontal gyrus | Inc | |
| .0001 | 6.05 | −40 | 1 | 21 | 44 | Inferior frontal gyrus | Inc | ||
| 1693 | .023 | 4.67 | 55 | −46 | 20 | 22 | Superior temporal gyrus | Dec | |
| Lexical A minus Baseline (semantic effect) age group interactions | |||||||||
| 342 | .0313 | 3.07 | −22 | −102 | −2 | 17 | Striate | Inc | Y> O |
| 719 | .0003 | 4.43 | −54 | −52 | −15 | 37 | Inferior temporal gyms | Inc | O > Y |
| 183 | .0019 | 3.68 | 17 | −71 | 49 | 7 | Superior parietal lobe | Dec | Y > O |
| Lexical A minus B (increasing duration effect) for younger adults | |||||||||
| 1998 | .0001 | 6.03 | −20 | −100 | −5 | 17 | Striate | Inc | |
| .017 | 4.74 | −24 | −93 | −19 | Cerebellum | Inc | |||
| .044 | 4.50 | 22 | −96 | −7 | 17 | Striate | Inc | ||
| Lexical A minus Lexical B (increasing duration effect) for older adults | |||||||||
| 5019 | .0001 | 6.15 | 20 | −91 | −8 | 17 | Striate | Inc | |
| .0001 | 6.01 | −26 | −89 | −15 | 18 | Fusiform gyms | Inc | ||
| Lexical C minus Lexical B (increasing rate effect) for younger adults | |||||||||
| 1894 | .047 | 4.49 | −20 | −100 | −4 | 17 | Striate | Inc | |
Note. See Method and Table 2 for a description of task conditions. Region size (k) = number of voxels in cluster; Z = t value of local maxima of activation within clusters scaled to a unit normal distribution; p(Zmax > u) = probability that the observed peak height of local maximum of activation is greater than would be expected by chance; x, y, z = coordinates (in millimeters) in the standard stereotaxic space of Talairach and Tournoux (1988); x = right/left hemisphere, negative indicates left hemisphere; y = anterior/posterior coordinate, negative indicates posterior to the zero point (anterior commissure); z = superior/inferior coordinate, negative indicates inferior to the AC–PC line; BA = Brodmann’s area; Direction = rCBF increase (Inc) or decrease (Dec). Interaction = change in rCBF greater for younger adults than for older adults (Y > O) or greater for older adults than for younger adults (O > Y). Task condition contrasts that did not yield significant results are not reported. Probability levels of less than .0001 have been rounded to .0001.
FIG. 2.
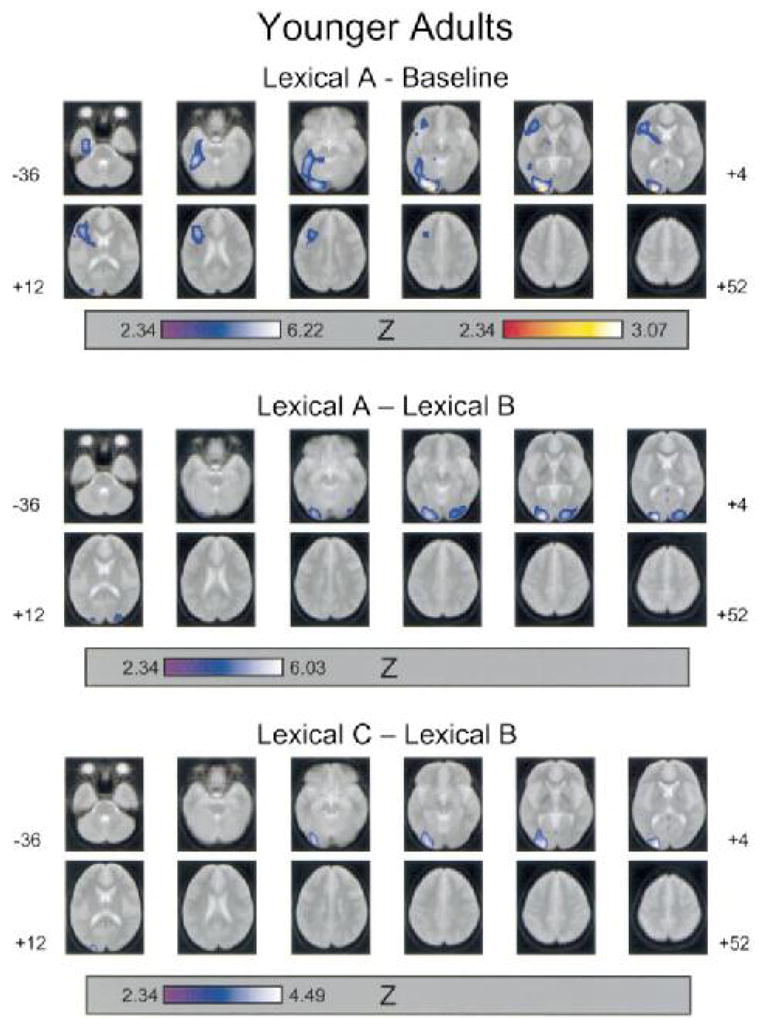
Areas of increased rCBF between task conditions for younger adults. Task conditions are described in Table 2. Regions of rCBF increase were identified from the SPM{Z}map, using a threshold for the peak height of voxel change (u) of Z = 2.33, p = .01 (unconnected). Regions of rCBF increase (p < .05, corrected) are superimposed (blue color scale) on a composite MR image of the 12 younger participants. Images are axial slices between −36 mm and +52 mm on the superior/inferior dimension (in 8 mm steps) with negative values referring to locations inferior to the AC-PC line. Images are presented in standard neurologic orientation (right = right; anterior = top). Regions with significant local maxima are listed, with their three-dimensional coordinates in standardized (Talairach) space and Brodmann’s area designations, in Table 4. The red color scale represents those voxel clusters for which the local maxima were significantly greater (p < .05, corrected) than in the corresponding task condition subtraction for the older adults’ data.
FIG. 3.
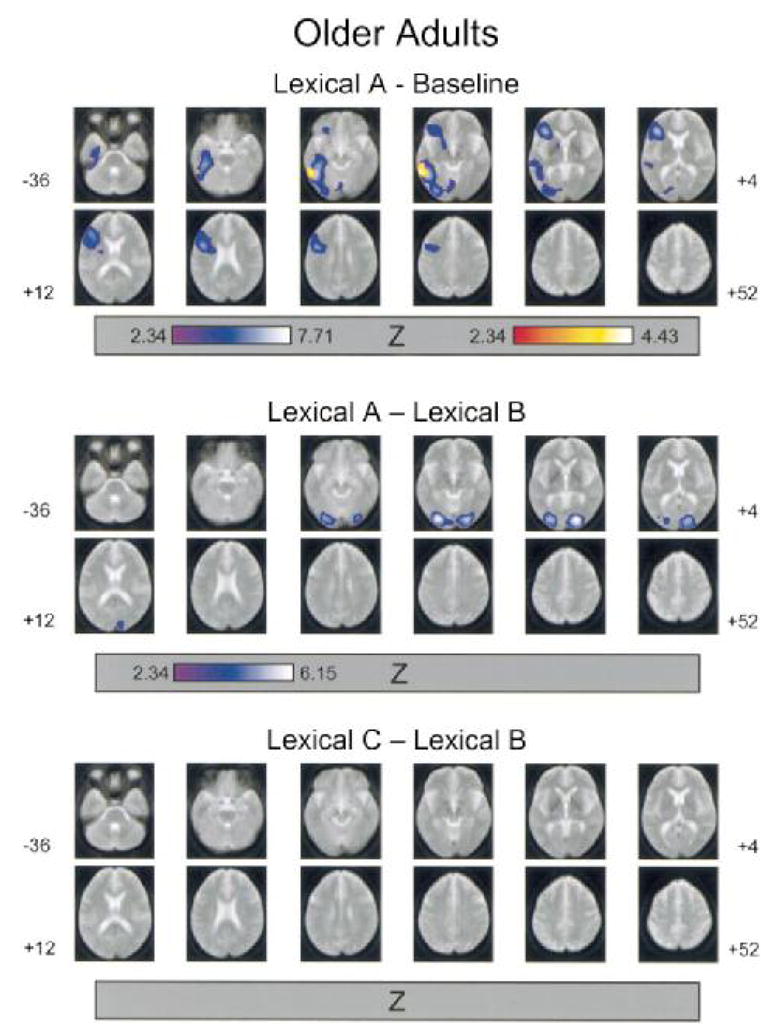
Areas of increased rCBF between task conditions for older adults. See Table 2 for a description of the task conditions and the legend to Fig. 2 for a description of image parameters. Regions of rCBF increase (p < .05, corrected) are superimposed (blue color scale) on a composite MR image of the 12 older participants. The red color scale represents those voxel clusters for which the local maxima were significantly greater (p < .05, corrected) than in the corresponding task condition subtraction for the younger adults’ data.
Lexical A minus Baseline
For both age groups, activation associated with semantic processing comprised two large clusters, one in the occipitotemporal pathway and one in the inferior prefrontal cortex. For the analyses conducted within each age group, all of the local maxima were located in the left hemisphere. The younger adults’ occipitotemporal activation included local maxima in striate cortex (BA 17), inferior occipital and lingual gyri (BA 18), and parahippocampal gyrus (BA 36). The older adults’ occipitotemporal activation included a local maximum in the inferior occipital gyrus (BA 18). Both age groups exhibited local maxima in the inferior frontal gyrus (BA 45 for younger adults and BA 44 for older adults) and in the cerebellum.
The interaction consists indicated that activation in the left striate cortex (BA 17) was greater in magnitude for younger adults than for older adults, whereas activation in the left inferior temporal gyrus (BA 37) was relatively greater for older adults.
The decreases in rCBF associated with this contrast comprised local maxima in the right superior parietal lobe (BA 7) for younger adults and in the right superior temporal gyrus (BA 22) for older adults. The age difference contrast indicated that the younger adults’ decrease in rCBF in parietal cortex was significantly greater than for the older adults.
Lexical A minus Lexical B
In the lexical decision task, increasing target duration was associated with increased rCBF in occipitotemporal cortex bilaterally. Local maxima for younger adults were in striate cortex (BA 17) bilaterally and the left cerebellum. The local maxima for older adults were in the right striate cortex (BA 17) and left fusiform gyrus (BA 18). The age difference contrast yielded no significant voxels for either age group.
The analyses of the decreases in rCBF within each age group yielded no local maxima.
Lexical C minus Lexical B
For younger adults, increasing the presentation rate of lexical decision targets led to rCBF activation with a local maximum in the left striate cortex (BA 17), whereas no activation was evident for older adults. The age difference contrast, however, yielded no significant voxels for either age group.
The analyses of the decreases in rCBF within each age group yielded no local maxima.
PET radioactivity counts and reaction time
Using an approach similar to the fMRI study of Rympa and D’Esposito (2000), we examined age-related changes in the correlation between regional activation and RT within task conditions. Unlike event-related fMRI, however, which measures signal change within each condition relative to a prestimulus baseline, the measures of rCBF activation implemented in the present SPM analyses refer to the difference between task conditions. As an estimate of within-condition activation we analyzed variation in the PET radioactivity counts. These counts, representing the tissue concentration of H215O, are a nearly linear function of quantitative changes in rCBF as determined from arterial blood sampling (Herscovitch, Markham, & Raichle, 1983).
As indicated previously, the SPM analyses of the increases in rCBF (Table 3) yielded two local maxima of interactive effects between age group and task condition. Both of these voxels were associated with the Lexical A minus Baseline subtraction. One voxel was located in left striate cortex (BA 17), and the other was located in left inferior temporal cortex (BA 37). We analyzed the PET count data for the voxel corresponding to each local maxima, normalized to each participant’s whole-brain gray matter mean value for the corresponding task condition. These latter values were obtained by averaging all of the voxels that were above 80% of the mean voxel value for the entire brain image (for each participant and task condition). Whereas the SPM contrast represent differences in the level of activation between task conditions, the voxel count values represent the level of activation within each task condition, relative to the whole-brain mean (Madden et al., in press).
To explore age differences in the relationship between voxel activations and RT performance, we analyzed Pearson correlations between standardized RTs and normalized count values for the left striate and left inferior temporal voxels. These correlations were conducted within each age group for the Baseline and Lexical A conditions separately. Following Rympa and D’Esposito (2000), task condition mean RTs were standardized by subtracting the group mean RT for the task condition from the individual participant’s mean RT and dividing the result by the group standard deviation. The only significant result was a positive correlation, r = .722, p < .01, between younger adults’ RT values and left striate count values in the Lexical A condition. The corresponding correlation for the older adults, r = .233, was not significant, but a test of the difference between the correlations for the two age groups, using Fisher’s r-to-z transform, indicated that younger and older adults’ correlations differed nonsignificantly, p > .10.
The PET radioactivity counts are also useful for more precisely characterizing the nature of the observed age differences in activation. Although the SPM contrasts provide a measure of age differences between task conditions (i.e., interaction effects), different patterns of activation could lead to the same type of age difference in the SPM data. When comparing an experiment task condition and a baseline condition, for example, a lower level of activation (i.e., the rCBF difference between conditions) for older adults than for younger adults could be due either to a relatively high level of rCBF within the baseline condition for the older adults or to a relatively high level of rCBF within the experimental condition for the younger adults.
We first determined whether the age differences in the voxel count data were consistent with the SPM results by performing an ANOVA of the Lexical A minus Baseline difference scores for the voxel counts. As in the SPM analysis, the activation associated with lexical processing, for the left striate voxel, was greater for younger adults than for older adults, F(1, 22) = 5.85, MSE = 0.003943, p < .05, whereas the activation for the left inferior temporal voxel was greater for older adults than for younger adults, F(1, 22) = 16.14, MSE = 0.008768, p < .001.
Analyses of the voxel count values within the task conditions demonstrated that, for the left striate voxel, the age-related decline in Lexical A activation was due to age differences within the Baseline condition. Within this latter condition, the count values were higher for older adults than for younger adults, F(1, 22) = 6.86, MSE = 0.001694, p < .05, whereas the age group effect was not significant in the Lexical A condition. In addition, for the left striate voxel, the younger adults exhibited a higher count value in the Lexical A condition than in the Baseline condition, F(1, 11) = 14.71, MSE = 0.000424, p < .01, whereas the older adults’ task condition effect was nonsignificant.
In contrast, the age-related increase in activation associated with the Lexical A minus Baseline contrast, with the local maximum in the left inferior temporal cortex, was due to age differences within the lexical decision condition. The count level for this voxel in the Lexical A condition was marginally higher for older adults than for younger adults, F(1, 11) = 3.65, MSE = 0.00175, p < .07, whereas the count values were comparable for the two age groups in the Baseline condition, F < 1.0. The lexical-related increase in the count values for the left inferior temporal voxel was significant for older adults, F(1, 11) = 24.08, MSE = 0.00019, p < .001, but not for younger adults.
DISCUSSION
The primary goal of this experiment was to investigate adult age differences in neural activation for a visual word identification task involving semantic (context-independent) memory retrieval (lexical decision). To extend previous findings (Madden et al., 1996), we used a nonsemantic baseline task (visual search), equated display duration and presentation rate for the two age groups, and examined the effects of varying display duration and presentation rate on the pattern of regional activation. In younger adults, visual word identification tasks typically lead to activation of neural networks in left inferior prefrontal cortex mediating phonological and self-directed semantic analyses, as well as in left occipitotemporal cortex mediating visual feature extraction and matching visual patterns to lexical representations (Binder & Price, 2001; Fiez, 1997). We predicted that age-related decline in the efficiency of sensory processing would be reflected in a corresponding decline in occipitotemporal activation. We expected that the pattern of prefrontal activation, in contrast, would be more pronounced for older adults than for younger adults, reflecting compensatory mechanisms to support either visual feature analysis or semantic retrieval. For voxels exhibiting an age difference in activation, we also examined the correlation between the magnitude of activation (from normalized PET radioactivity counts) and RT, to test the hypothesis that this correlation differs for younger and older adults (Rympa & D’Esposito, 2000).
Lexical Decision Performance
The comparison between the Lexical A and Baseline conditions demonstrated that for both age groups, performance was slower and less accurate for word/nonword discrimination than for letter search, indicating that the lexical decision task required additional or more complex information processing operations than those necessary to locate a “c” among Ts and Zs. In addition, the increase in RT for no responses, relative to yes responses, was greater for lexical decision than for letter search, and this effect was comparable for the two age groups. Although the older adults’ RTs were significantly higher than those of the younger adults, the difference between the Lexical A and Baseline conditions did not vary significantly as a function of age group. This pattern is consistent with previous findings indicating that the activation and retrieval of semantic memory information are less vulnerable to age-related decline than are other forms of memory performance (Light, 1996; Mayr & Kliegl, 2000).
Across the lexical decision conditions, the changes in display duration and presentation rate led to measurable changes in performance. Main effects for both of these variables were evident in the analyses of both RT and error rate, representing the fact that as additional time was available (i.e., increasing display duration or decreasing presentation rate), participants’ lexical decision responses were slowed. This pattern was modified by two interactions with age group. In the comparison of the two presentation rate conditions (Lexical C versus Lexical B), an Age Group X Response Type interaction was significant, which represented an age-related increase in the difference between yes (word) and no (nonword) responses. This age difference did not interact with the presentation rate variable, however, and thus the specific relation of this effect to presentation rate is not clear. In the comparison of the two display duration conditions, (Lexical A versus Lexical B), the three-way interaction of Age Group X Task Condition X Response Type was significant in the RT data because the increase in RT for longer duration displays was most clearly evident in the older adults’ responses to word targets. This type of age difference appears to represent a response-level effect in which older adults are more inclined than younger adults to respond more slowly as display duration increases.
rCBF Activation: Task Condition Effects
The most frequently activated cortical regions in visual word identification tasks include the inferior prefrontal and occipitotemporal regions of the left hemisphere (Binder & Price, 2001). The Madden et al. (1996) comparison of younger and older adults’ lexical decision performance yielded no left inferior prefrontal activation in the lexical decision conditions, but that experiment used passive viewing of words and nonwords as a baseline, which allowed phonological and semantic information to be activated in the baseline condition. In the present experiment we used a nonsemantic (visual search) baseline for assessing the activation associated with the lexical decision task. We predicted that this baseline would be more appropriate than passive viewing of words and nonwords for detecting left inferior prefrontal activation during lexical decision. This expectation was confirmed in that the Lexical A minus Baseline contrast yielded activation of the left inferior frontal gyrus for both age groups, with local maxima in BA 45 for younger adults and BA 44 for older adults (Figs. 2 and 3).
Although the components of lexical decision performance leading to this activation cannot be identified definitively, Fiez (1997) noted that within the inferior prefrontal cortex of the left hemisphere, different types of linguistic processing lead to consistently distinct regional activations. Tasks emphasizing phonological processing tend to activate posterior regions of prefrontal cortex (e.g., BAs 44 and 45), whereas tasks emphasizing semantic category information (e.g., abstract/concrete) lead to activation of more anterior regions (e.g., BAs 47 and 10). Both types of processing rely on the retrieval and use of context-independent memory information. If this interpretation is correct, then the activation obtained in this experiment would most likely represent the additional phonological processing associated with the word/nonword discrimination task relative to the search task (finding a “c” among Ts and Zs).
The left occipitotemporal activation noted in previous studies of visual word identification was also replicated in the contrast between the Lexical A and Baseline conditions. Both age groups exhibited extensive occipitotemporal activation, with local maxima in both striate (BA 17) and extrastriate (BA 18) regions for younger adults and in the extrastriate (BA 18) region for older adults. This activation also extended into the cerebellum for both age groups. The displays in the lexical decision and visual search conditions contained, on average, the same number of letters, and thus the visual sensory impact of the two conditions was comparable in this sense. But the variety of letters was relatively greater in the Lexical A condition. It is also likely that the level of visual feature analysis was more fine-grained in the word/nonword discrimination task than in the visual search task. These differences between the conditions undoubtedly contributed to the occipitotemporal activation associated with the lexical decision task, but the strong lateralization of the activation to the left hemisphere suggests that linguistic analyses were also a determining factor. The present results are consistent with other neuroimaging evidence indicating that the left occipitotemporal cortex is critically involved with semantic processing during visual word identification, either by mediating a direct route between the visual word form and a lexical representation or by supporting the active retrieval of word meaning (Herbster et al., 1997; Nobre et al., 1994).
The changes in display duration and presentation rate led to changes in neural activation as well as in lexical decision performance. For both age groups, increasing display duration from 375 to 750 ms led to activation of occipital cortex bilaterally. The local maxima were in striate cortex (BA 17) bilaterally for younger adults and in the left fusiform gyrus (BA 18) and right striate cortex for older adults. This pattern of activation is generally consistent with the results of Price et al. (1994), who also found that increasing display duration in a lexical decision task led to bilateral occipital activation, although in their experiment the local maxima were located in extrastriate cortex (BA 18) rather than in the striate region. The fact that the duration-related activation in the present experiment was bilateral, whereas the occipitotemporal activation related to the lexical decision task (Lexical A minus Baseline) was localized to the left hemisphere, suggest that increasing duration led primarily to an increase in the amount of peripheral visual processing (i.e., feature-level analyses) without changing significantly the cognitive operations involved in the lexical decision task. Although the RT data indicated that older adults slowed their responses to word targets, as a function of increasing display duration, more than younger adults, this strategic difference led to no corresponding age difference in the patter of rCBF activation.
The activation associated with presentation rate was limited to an rCBF increase in younger adults’ left striate cortex. Price et al. (1996) reported significant effects of activation related to increasing presentation rate in several brain regions, including occipitotemporal cortex. Participants in the Price et al. (1996) experiment, however, performed word reading (silently and aloud) rather than lexical decision, and the presentation rate values were more widely separated (from 1 word/3000 ms to 2 words/1000 ms) than in the present study (from 1 target/2000 ms to 1 target/1625 ms). Thus, the present changes in the speed of display presentation affected participants’ performance, but not to a degree that led to substantial changes in neural activation as measured by PET.
The only decreases in rCBF that were significant in the SPM analyses were associated with the Lexical A minus Baseline contrast (Table 3). The younger adults exhibited a decrease in the superior parietal lobe of the right hemisphere (BA 7), and the older adults’ decrease was located in the superior temporal gyrus of the right hemisphere (BA 22). These decreases may represent an inhibition of spatial and auditory processing, respectively, during performance of the visual word identification task.
rCBF Activation: Age Group Effects
The pattern of neural activation across the various task condition contrasts was generally similar for the two age groups. Both younger and older adults exhibited left occipitotemporal and left inferior prefrontal activation for the lexical decision task relative to the visual search baseline, and the rCBF changes associated with display duration and presentation rate varied nonsignificantly as a function of age group. We did observe significant age-related change in rCBF activation, within the occipitotemporal pathway for the lexical decision task. The Lexical A minus Baseline subtraction yielded relatively greater activation for younger adults in left striate cortex (BA 17) and relatively greater activation for older adults in left inferior temporal cortex (BA 37). The age difference in left striate activation is consistent with Madden et al. (1996), who reported an age-related decline in activation, for lexical decision compared to passive viewing of words and nonwords, at the border of the lingual and fusiform gyri of the left hemisphere.
The age-related increase in left inferior temporal activation is a new finding for a visual word identification task, but Grady et al. (1994) obtained a similar result in a face-matching task. Grady et al. noted that an age-related decline in activation of occipital regions supporting early visual processes (e.g., visual feature extraction) was accompanied by an age-related increase in activation in the more ventral occipitotemporal regions supporting visual object identification, including BA 37. Grady et al. suggested that older adults rely more than younger adults on higher order visual processing to compensate for an age-related decline in the efficiency of visual sensory functioning. In the present instance, the strong lateralization of the age-related changes to the left hemisphere suggests that the stimulus identification processes are linguistic in nature.
In contrast to our initial prediction, the age difference in the pattern of regional activation did not include an age-related increase in prefrontal activation. The rCBF increase in the left inferior prefrontal cortex, associated with the Lexical A minus Baseline contrast, varied nonsignificantly as a function of age group. In this regard the age differences associated with a context-independent semantic memory task (word identification) diverge from those associated with context-dependent episodic memory tasks. Episodic memory retrieval typically leads to right prefrontal activation for younger adults but to a more bilateral pattern of prefrontal activation for older adults (Cabeza et al., 1997; Madden et al., 1999), perhaps as a compensatory mechanism for age-related decline in the efficiency of encoding and retrieval of contextual information (Cebeza, 2001; Grady & Craik, 2000; Langley & Madden, 2000).
It may be relevant that the display duration and presentation rate variables led to no significant change in the prefrontal activation. These variables did influence performance but were selected so that they would not affect accuracy substantially. Price et al. (1994, 1996), using briefer display durations and faster presentation rates than in the present experiment, observed prefrontal activation related to these variables. Using values of these variables that have a greater effect on lexical decision performance may elicit age-related compensatory activation of prefrontal cortex. Madden et al. (in press), however, reported that in a visual search task with a minimal memory component (search for an upright L among rotated Ls), prefrontal activation was comparable for younger and older adults even though search accuracy was substantially worse for the older adults. As in the present experiment, Madden et al. (in press) found that age differences were evident primarily in the form of an age-related decline in activation of the occipitotemporal pathway.
rCBF Activation: Changes in Normalized PET Radioactivity Counts
Rypma and D’Esposito (2000) proposed a model of age differences in the relation between RT and activation based on the assumption of a sigmoidal relation between activation and response discriminability. The model predicts that within a particular range of activation, the associated changes in signal strength lead to a positive RT-activation correlation for younger adults, but a negative correlation for older adults. Rympa and D’Esposito demonstrated that this model accounted for age differences in dorsolateral prefrontal activation and RT for working memory retrieval. This model does not appear to characterize the age differences obtained in the present lexical decision task, which involved semantic memory retrieval. For those regions exhibiting age-related change in rCBF activation (left striate and left inferior temporal cortex), the correlation between RT and within-condition activation (normalized PET radioactivity counts) differed nonsignificantly as a function of age group. However, the finding that, for the local maximum in the left striate cortex, the correlation between activation and lexical decision RT was significant and positive for younger adults, but was nonsignificant for older adults, may warrant additional exploration of the model in other semantic tasks. In addition, we conducted our correlational analyses within individual brain regions (at the local maxima of significant age difference contrasts). It would thus also be valuable to explore age differences in semantic memory tasks using other types of analyses, such as network analyses of functional and effective connectivity, which can reveal the functional relations among brain regions (Nyberg & McIntosh, 2001).
The analyses of the PET count values were also informative regarding the age differences in occipitotemporal activation for the Lexical A minus Baseline contrast. The SPM analyses demonstrated that the lexical decision task was associated with greater activity in left striate cortex for the younger adults than for the older adults, whereas lexical-related activation in left inferior temporal cortex was relatively greater for the older adults. From the normalized count values, however, we determined that the age difference in left striate activation occurred primarily as the result of the older adults’ relatively high level of activation in the Baseline condition, whereas the age difference in left occipitotemporal activation was a consequence of the older adults’ higher count level in the Lexical A condition. Consequently, the age difference in left striate activation appears to represent older adults’ maintaining a high level of neural activity in regions mediating relatively early visual processes (e.g., feature extraction) during the visual search task.
The age difference in left inferior temporal activation, in contrast, reflects the older adults’ higher level of neural activity in a region mediating more complex visual identification processes during the lexical decision task. Although the differences between the Lexical A and Baseline conditions in RT and error rate were comparable for younger and older adults, it appears that older adults maintain this comparability by means of a differential level of neural activity within the occipitotemporal pathway, in relation to task demands, rather than by means of compensatory recruitment of prefrontal regions. Madden et al. (in press), using a similar analysis of normalized PET count values for a visual search task, also found that an age-related decline in occipitotemporal activation was the result of older adults maintaining a relatively higher level of activity in the less complex (baseline) condition, without an age-related increase in prefrontal activation. This pattern of age-related change may be compensatory, but is expressed as differential activation within the task-relevant (occipitotemporal) neural pathway rather than as the recruitment of (prefrontal) regions outside this pathway. An intriguing possibility is that the recruitment of prefrontal regions is driven specifically by the requirement to retrieve or actively maintain context-dependent memory information.
Conclusions
Performance in these visual search and lexical decision tasks was consistent with other behavioral research in suggesting that age-related change in the activation and retrieval of semantic information is minimal. Although an age-related response slowing was evident, the increase in RT and error rate associated with lexical decision, relative to a simple visual search task, was similar in magnitude for younger and older adults. The rCBF activation confirmed the results of previous neuroimaging investigations, which have proposed that visual word identification is mediated by neural systems in the occipitotemporal and inferior prefrontal regions of the left hemisphere. The regional pattern of activation was generally similar for the two age groups. The similarity in prefrontal activation suggests that, unlike episodic memory tasks, semantic memory retrieval does not lead older adults to activate additional prefrontal regions as a compensatory mechanism. Activation associated with visual word identification exhibited an age-related decline in visual sensory (striate) cortex, and an age-related increase in an inferior temporal region related to object recognition. Analyses of normalized PET radioactivity counts indicated that older adults maintained a relatively higher level of activity in the left striate cortex in the baseline (visual search) task, as well as a higher level of activity in left inferior temporal cortex in the lexical decision task. Older adults’ mechanism for supporting performance of this semantic task appears to involve differential activation within the occipitotemporal region rather than the recruitment of additional prefrontal (or other) regions.
Footnotes
Transaxial MR images were acquired with a 1.5-T scanner (Signa; GE Medical Systems, Milwaukee, WI). The slice thickness was 3 mm with no interslice gap. The repetition time (TR) for the T1-weighted images was 600 ms, and the excitation time (TE) was 20 ms, with two excitations (NEX). The T2-weighted images had a TR of 2.50 s, TE values of 20 and 80 ms, and 1 NEX. For both age groups, participants were excluded from PET testing if the MR image contained any signal abnormality indicating a mass, ventricular enlargement, or atrophy atypical for age; flow signal abnormality within intracranial vessels; extraaxial fluid collection; or any focal signal abnormality within caudate, putamen, globus pallidus, thalamus, brain stem, or cerebellum. Because the frequency of white-matter hyperintensities on MR increases with age (Gunning-Dixon & Raz, 2000), different exclusion criteria were adopted for the two age groups on this variable. For younger adults, any focal area of hyperintensity on T2-weighted images was an exclusion criterion, whereas for older adults, the criterion was the presence of focal supratentorial hyperintense white matter signal abnormalities greater than 3 mm.
Cortical volumes (Table 1) were estimated for each participant’s MR image using the method described by Madden et al. (1996). There was an age-related decrease in cortical volume, which is consistent with previous MR investigations (Raz et al., 1993,1997). Madden et al. (in press) reported that the magnitude of the age-related decline in cortical gray matter volume was predicted accurately (to within 2 cm3) by a function noted by Raz (2000): a decrease of 2% per decade. The present participants, an independent sample of individuals who underwent the same health screening used by Madden et al. (in press), exhibited a similar magnitude of age-related decline in cortical volume. The mean cortical gray matter volume for the younger adults (primarily individuals in their 20s) was 692 cm3. An age-related decrease of 2% per decade would yield a predicted volume of 638 cm3 for individuals in their 60s, and the observed value for the present sample of older adults (mean age = 65.0 years) was 637 cm3.
This work was supported by grants R01 AG11622 and R37 AG02163 from the National Institute on Aging. We are grateful to Susanne M. Harris, Sharon Hamblen, and Mary Hawk for technical assistance.
References
- Allen PA, Madden DJ, Slane SD. Visual word encoding and the effect of adult age and word frequency. In: Allen PA, Bashore TR, editors. Age differences in word and language processing. Amsterdam; North-Holland: 1995. pp. 30–71. [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy young and older adults. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford, UK: Oxford Univ. Press; 2000. pp. 395–409. [Google Scholar]
- Balota DA, Ferraro FR. A dissociation of frequency and regularity effects in pronunciation performance across young adults, older adults, and individuals with senile dementia of the Alzheimer’s type. Journal of Memory and Language. 1993;32:573–592. [Google Scholar]
- Besner D, Smith MC. Models of visual word recognition: When obscuring the stimulus leads to a clearer view. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18:468–482. [Google Scholar]
- Binder J, Price CJ. Functional neuroimaging of language. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2001. pp. 187–251. [Google Scholar]
- Borowsky R, Besner D. Visual word recognition: A multistage activation model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:813–840. doi: 10.1037//0278-7393.19.4.813. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Functional neuroimaging of cognitive aging. In: Cabeza R, Kingstone A, editors. Handbook of functional of cognition. Cambridge, MA: MIT Press; 2001. pp. 331–377. [Google Scholar]
- Cabeza R, Anderson ND, Mangels JA, Nyberg L, Houle S. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. Journal of Cognitive Neuroscience. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B. The American heritage word frequency book. New York: American Heritage; 1971. [Google Scholar]
- Christensen KJ, Moye J, Armson RR, Kern TM. Health screening and random recruitment for cognitive aging research. Psychology and Aging. 1992;7:204–208. doi: 10.1037//0882-7974.7.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 51–110. [Google Scholar]
- D’Esposito M. Functional neuroimaging of working memory. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2001. pp. 293–327. [Google Scholar]
- DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner. Journal of Nuclear Medicine. 1994;35:1398–1406. [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Eustache F. The functional neuroanatomy of episodic memory: The role of the frontal lobes, the hippocampal formation, and other areas. NeuroImage. 1998;8:198–213. doi: 10.1006/nimg.1998.0359. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5:79–83. [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE. PET activation of posterior temporal regions during passive auditory word presentation and verb generation. Cerebral Cortex. 1996;6:1–10. doi: 10.1093/cercor/6.1.1. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ. Spatial realignment and normalization of images. Human Brain Mapping. 1996;3:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gabrieli JDE. Functional neuroimaging of episodic memory. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2001. pp. 253–291. [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Grady CL, Craik FIM. Changes in memory processing with age. Current Opinion in Neurobiology. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FIM. The effects of age on the neural correlates of episodic encoding. Cerebral Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neurospychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Speer NK. Locating and fractionating working memory using functional imaging: Storage, maintenance, and executive functions. Microscopy Research and Technique. 2000;51:45–53. doi: 10.1002/1097-0029(20001001)51:1<45::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintum MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Human Brain Mapping. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. Journal of Nuclear Medicine. 1983;24:782–789. [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R. The cortical localization of the lexicons. Brain. 1992;115:1769–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. Analytic 3D image reconstruction using all detected events. IEEE Transactions on Nuclear Science. 1990;37:773–777. [Google Scholar]
- Langley LK, Madden DJ. Neuroimaging of memory: Implications for cognitive aging. Microscopy Research and Technique. 2000;51:75–84. doi: 10.1002/1097-0029(20001001)51:1<75::AID-JEMT8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging. In: Bjork EL, Bjork RA, editors. Memory. San Diego: Academic Press; 1996. pp. 433–490. [Google Scholar]
- Madden DJ. Adult age differences in the effects of sentence context and stimulus degradation during visual word recognition. Psychology and Aging. 1988;3:167–172. doi: 10.1037//0882-7974.3.2.167. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Four to ten milliseconds per year: Age-related slowing of visual word identification. Journal of Gerontology: Psychological Sciences. 1992;47:P59–P68. doi: 10.1093/geronj/47.2.p59. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Speed and timing of behavioral processes. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 5. San Diego: Academic Press; 2001. pp. 288–312. [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Age-related slowing and the time course of semantic priming in visual word identification. Psychology and Aging. 1993;8:490–507. doi: 10.1037//0882-7974.8.4.490. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Coleman RE, Provenzale JM, DeGrado TR, Hoffman JM. Adult age differences in regional cerebral blood flow during visual word identification: Evidence from H215O PET. NeuroImage. 1996;3:127–142. doi: 10.1006/nimg.1996.0015. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Human Brain Mapping. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attentional guidance during visual search: Functional neuroanatomy by positron emission tomography. Psychology and Aging. doi: 10.1037//0882-7974.17.1.24. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Kliegl R. Complex semantic processing in old age: Does it stay or does it go? Psychology and Aging. 2000;15:29–43. doi: 10.1037//0882-7974.15.1.29. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Cabeza R. Brain imaging of memory. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. Oxford, UK: Oxford Univ. Press; 2000. pp. 501–517. [Google Scholar]
- Nyberg L, McIntosh AR. Functional neuroimaging: Network analyses. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. Cambridge, MA: MIT Press; 2001. pp. 49–72. [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Frackowiak RSJ. The effect of varying stimulus rate and duration on brain activity during reading. NeuroImage. 1996;3:40–52. doi: 10.1006/nimg.1996.0005. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Watson JDG, Patterson K, Howard D, Frackowiak RSJ. Brain activity during reading: The effects of exposure duration and task. Brain. 1994;117:1255–1269. doi: 10.1093/brain/117.6.1255. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Manwah, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD. Selective aging of human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Torres IJ, Spencer WD, Acker JD. Pathoclysis in aging human cerebral cortex: Evidence from in vivo MRI morphometry. Psychobiology. 1993;21:151–160. [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. What do adult age differences in the digit symbol substitution test reflect? Journal of Gerontology: Psychological Sciences. 1992;47:P121–P128. doi: 10.1093/geronj/47.3.p121. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Taft M. Reading and the mental lexicon. Hove, UK: Erlbaum; 1991. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Treisman A. Features and objects: The fourteenth Bartlett memorial lecture. The Quarterly Journal of Experimental Psychology. 1988;40A:201–237. doi: 10.1080/02724988843000104. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price CJ, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic repetition priming for verbal and pictorial knowledge: A functional MRI study of left inferior pre-frontal cortex. Journal of Cognitive Neuroscience. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale—revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. East Sussex, UK: Psychology Press; 1998. pp. 13–73. [Google Scholar]


