Abstract
Purpose
Human lactoferrin is a naturally occurring glycoprotein that inhibits cancer growth. Our purpose was to evaluate recombinant human lactoferrin as a chemotherapeutic agent against head and neck squamous cell carcinoma.
Experimental design
Controlled experiments both in vitro and in the murine model evaluating both the effect and mechanism of lactoferrin on cancer growth.
Results
In both human and murine cell lines, lactoferrin induced dose-dependent growth inhibition. Using flow cytometric analysis, lactoferrin was shown to induce G1/G0 growth arrest. This arrest appeared to be modulated by downregulation of cyclin D1. In the in vitro model, luminex data revealed that lactoferrin inhibited cellular release of proinflammatory and prometastatic cytokines including Interleukin 8, Interleukin 6, Granulocyte Macrophage Colony Stimulating Factor, and Tumor Necrosis Factor-alpha. Lactoferrin upregulated the cellular activation of Nuclear Factor Kappa B within 4 hours of cellular exposure. In C3h/HeJ mice implanted with SCCVII tumors, orally delivered lactoferrin inhibited tumor growth by 75% compared to control mice. Immunohistochemical analysis of harvested tumors revealed up to 20 fold increases of lymphocytes within treated animals. When mice were depleted of CD3+ cells, all lactoferrin-induced tumor inhibition was abrogated.
Conclusion
We conclude that human recombinant lactoferrin can inhibit the growth of HNSCCA via direct cellular inhibition as well as systemically via immunomodulation. Our data supports the study of human lactoferrin as an immunomodulatory compound with therapeutic potential.
Keywords: Lactoferrin, Head and neck squamous cell carcinoma, T lymphocyte, cytokines, immunomodulation
Introduction
Lactoferrin is an 80 kDa member of the transferrin family of iron-binding glycoproteins(1, 2). The protein is naturally occurring and is present in mammalian exocrine secretions, including breast milk, tears, nasal and bronchial mucus, cervical mucus and seminal fluid(3, 4). Lactoferrin has multiple known biologic activities including iron regulation, cellular growth and differentiation, antimicrobial defense, anti-inflammatory activity, and cancer protection(3, 5, 6). Many of the functions of lactoferrin are related to immune activation and modulation (7). It is hypothesized that immunomodulation by lactoferrin contributes to the lower rate of respiratory infections and malignancies in breastfed infants, but the exact mechanism of action of lactoferrin on the immune system is not currently understood(8–10). Specifically, the relative impact of lactoferrin on intrinsic cellular proliferation as well as immunomodulatory response is unknown.
Direct inhibition of cellular growth is one mechanism by which lactoferrin may inhibit cancer growth. Recent data has shown that lactoferrin induces direct cell cycle arrest in both breast and head and neck cancer cell lines(11, 12). Additionally lactoferrin can decrease cellular release of the proinflammatory cytokines including Interleukin-1 (IL-1), IL-6, IL-4, and TNF-α, recognized for their import in maintaining cellular viability and a malignant phenotype (13–17). Finally, lactoferrin decreases the activity of the transcription factor NF-κB(13). NF-κB is constitutively activated in HNSCCA and inhibition of this activation decreases cellular viability. Despite such well defined mechanisms for direct lactoferrin inhibition of tumor growth in vitro, the specific mechanism of growth inhibition in vivo is not understood.
Aside from the direct tumor cell growth inhibition, cellular immunity may also be enhanced by lactoferrin. For example, lactoferrin contributes to an increased Th1 response, which may explain the lower rate of allergies in breastfed children(18). Similarly, oral lactoferrin induces IL-18 release from murine intestinal epithelium, where it increases CD4+, CD8+, and NK activity(19, 20). Finally, oral lactoferrin has recently been shown to reconstitute the circulating and splenic CD4+ and CD8+ cell population in mice treated with cyclophosphamide(21). Most significantly, this increase in lymphocytes proved functional via reconstitution of the type IV hypersensitivity reaction in these mice(21).
Recent studies have suggested that lactoferrin holds promise as a cancer therapeutic agent. Lactoferrin treatment reduces colonic carcinogenesis in rats, and decreases solid tumor growth and metastases in mice(22–24) We have previously published data on the effect of lactoferrin on head and neck squamous cell carcinoma demonstrating tumor inhibition by intravenous, oral, and intratumoral dosing(25, 26). We have shown that in the murine model, lactoferrin induced growth inhibition of floor of mouth tumors is equal to that of the most common chemotherapeutic agent used for the treatment of HNSCCA, cisplatinum. The mechanism of this tumor inhibition remains to be discerned, but oral lactoferrin dosing was associated with increases in gut release of IL-18, NK activation and quantity of serum CD8+ cells(26).
The lactoferrin receptor (LFR) is expressed in many mammalian tissues including intestine, heart, spleen, liver, monocytes, lymphocytes, platelets, and salivary glands(27–30). The lactoferrin receptors found in the intestinal epithelium overlying peyers patches have the highest specific binding capability when compared to LFR at other sites (30). The murine lactoferrin receptor has 87% homology with the human receptor and demonstrates binding specific for human, mouse, and bovine lactoferrin(28, 31, 32). Recent data has shown the presence of the lactoferrin receptor on carcinoma cell lines(29).
Despite the data that lactoferrin inhibits the growth of squamous cell carcinomas of the head and neck, the mechanism of growth inhibition remains uncertain. Elucidating the mechanism of action of lactoferrin will allow us to evaluate its potential as a chemotherapeutic agent and to plan future studies to determine its role in clinical medicine. In this study we aimed to identify the key mechanism(s) of lactoferrin-induced tumor growth inhibition. We focused our attention on the effect of lactoferrin on HNSCCA in vitro and in vivo. Our data demonstrates iron-independent lactoferrin-induced dose-dependent cellular inhibition of squamous cell carcinoma cell lines that is associated with decreases in Cyclin D1 and increases in P19. We found that lactoferrin also reduces the cellular production of key proinflammatory and prometastatic cytokines. To further study the mechanism of lactoferrin growth inhibition, we used orthotopic and flank tumor models to show that lactoferrin inhibits HNSCCA in vivo and that this inhibition is associated with marked increases of lymphocytic infiltration into tumors. By depleting mice of lymphocytes, all tumor inhibition was abrogated leading suggesting that lactoferrin-induced tumor inhibition is the result of immunomodulation. These important findings as well as its known safety profile point to lactoferrin as a possible chemotherapeutic agent in the treatment of head and neck cancer.
METHODS AND MATERIALS
Cell Lines
SCCVII cell line is a spontaneously arising murine squamous cell carcinoma(33). O12 cell lines are well-characterized and derived from human SCC of the head and neck. UMSCC-9, -38 and -11B lines were derived from human SCC of the upper aerodigestive tract, with informed consent, at the University of Michigan (Ann Arbor, MI) and obtained from Dr. Thomas Carey. The cell lines were cultured in Eagle’s minimal essential media supplemented with 10% fetal bovine serum and penicillin/streptomycin, and maintained in 5% CO2 incubators at 37°C.
Animals
C3H/HeJ mice and Balb/C were purchased from Charles River laboratories (Wilmington, MA). The animal protocols were approved by the University of Maryland IACUC. The animal care facilities at the University of Maryland meet requirements of federal law (89–544 and 91–579) and National Institutes of health regulations and are accredited by the American Association for Accreditation of C3h/Hej or Balb/C mice 6–10 weeks old were anesthetized using intraperitoneal ketamine and xylazine and a 0.1 cc suspension of 5x105 SCCVII cells in Hanks Buffered Saline Solution are injected subcutaneously in the floor of the mouth or flank. Tumors were allowed to grow for a period of 5 days. On day 5, mice with orthotopic tumors were anesthetized with intraperitoneal avertin to adequate depth of anesthesia as determined by toe pinch. After betadine skin cleaning, a submental skin incision was performed with sharp scissors. A dissection into the floor of the mouth musculature with fine scissors revealed the tumors. Tumors were measured in three dimensions with calipers. The wounds were closed with vicryl sutures. After 10 days of oral lactoferrin dosing, mice were anesthetized, tumors were exposed, measured, and harvested. After serum was collected, mice were then sacrificed.
For flank tumors, mice tumor volumes were measured daily using the formula: (width2 X Length)/2. Statistical Analysis was performed using Students T-test.
Using a 20 gauge ball tip feeding needle, human recombinant lactoferrin (Agennix, Houston, TX) was delivered enterally from days 5 through 15 in varying doses. The lactoferrin was delivered into the mices’ stomach. No anesthesia was used for oral gavage feeding.
Measurement of Cell Proliferation by MTT Assay
Murine cell line SCCVII and Human cell lines 012 were plated in a 96-well microtiter plate and incubated overnight. Cells were washed twice with PBS and exposed to varying doses of human recombinant lactoferrin (Agennix, Houston, TX) ranging from 0 to 250 μM in serum-free KGM. Cell density was determined using an MTT cell proliferation assay (Boehringer Mannheim, Indianapolis, IN) per the manufacturers instructions. MTT-labeling reagent was added at daily from days 1 through 6 after lactoferrin treatment, and colorimetric ODs were measured at 570 nm by a microplate reader (Bio-Tek Systems, Winooski, Vermont). For iron saturation experiments, lactoferrin was saturated with elemental iron at a 2:1 molar ratio (iron:LF) for 10 minutes prior to adding it to the medium. Cell density was again determined using an MTT cell proliferation assay (Boehringer Mannheim, Indianapolis, IN). MTT-labeling reagent was added at daily from days 1 through 6 after lactoferrin treatment, and colorimetric ODs were measured at 570 nm by a microplate reader (Bio-Tek Systems). Statistical Analysis was performed using Students T-test.
Measurement of NF-κB Activity
The determination of the NF-κB DNA binding activity was performed using both electrophoretic mobility shift assay (EMSA) and NF-κB-specific ELISA. UMSCC9 and SCCVII cells were cultured with Lactoferrin (250 μM) and nuclear extracts were harvested at 30 minutes and 60 minutes using the method of Beg with minor modifications(34). Briefly, cells were rinsed with PBS and harvested from tissue culture flasks by gentle scraping. After spinning down the cells and removing the PBS, an equivalent volume of lysis buffer was added to resuspend the cell pellet. A protease inhibitor cocktail tablet (Complete, Mini; Boehringer, Mannheim, Germany) was added per 10 ml of lysis buffer before use. Then samples were spun at 18,000 3 g for 20 min at 4°C. Supernatants were aliquoted, snap frozen, and stored at −80°C.
The protein concentration was determined by the method of Lowry using bovine serum albumin (BSA) as a standard. EMSA was performed using EMSA chemiluminescence kit (Pierce, Rockford IL) according to the manufacturer’s protocols. A biotin-labeled oligonucleotide containing an NF-κB DNA-binding consensus sequence, 5’-AGT TGA GGG GAC TTT CCC AGG C-3’ (Panomics, Inc.), and a unlabeled oligonucleotide, 5’-AGT TGA GGC GAC TTT CCC AGG C-3’, was used to study NF-kB DNA binding activity. Oct-1 was used as control oligonucleotide. Briefly, nuclear extracts were preincubated in a reaction mixture for 20 min, and biotin end-labeled oligonucleotide containing the NF-κB consensus sequence was added. Five microliters of loading buffer was added to each sample. A 20-μl aliquot of the samples was electrophoresed through a 6% nondenaturing polyacrylamide gel. Finally, the gel was dried and exposed to x-ray film.
NF-κB DNA binding activity was assessed with trans-active motif (trans-AM) NF-B transcription factor assay kits (Active Motif, Rixensart, Belgium) according to the manufacturer’s instructions. This ELISA-like test measures the level of the active form of NF-κB contained in cell extracts specifically able to bind to an oligonucleotide containing the NF-κB consensus site (5′-GGGACTTTCC-3′) attached to a 96-well plate(35). UMSCC9, SCCVII, and UMSCC11B cell lines were incubated with lactoferrin (250 μM) and/or 500 pg/ml of IL-1α (positive control). Nuclear extracts were harvested at specific time points. 20-lg extracts were added to the 96-well plates. The binding of NF-κB to the DNA was visualized by anti–p65/Rel-A antibodies that specifically recognize activated NF-κB. Antibody binding was determined as absorbance values at 450 nm.
Flow Cytometric Analysis
For cell cycle analysis, 1x105 SCCVII or O12 cells were incubated in 25 cm2 flasks. Human recombinant lactoferrin (250 μM) was added to the medium at 24 hours and the cells were incubated for up to 48 hours. Adherent cells were trypsinized, and fixed in 70% ethanol at 4°C.
Before analysis, cells were washed once in PBS and resuspended in 1ml propidium iodide working solution (PBS, 0.5mg/ml RNAse, and 0.1mg/ml propidium iodide). Cell cycle distribution was determined by flow cytometry using a FACScan cytofluorimeter and the FACSDIVA software (Becton Dickinson, San Jose, CA).
For lymphocyte analyses, mouse spleens were placed in 3 ml of media (i.e. RPMI 1640 with 10% FBS) in a 6-well plate. Using sterile forceps, the spleen was placed on a sterile wire mesh screen, and pushed through with the plunger of a 10ml syringe into a 50-ml tube. The cells were pelleted by centrifugation (300–400xg) for 5 min at 4°C and the supernatant was aspirated. After standard lysis of erythrocytes and washing with PBS, the cell number in the spleen cell suspension was counted. 5 × 105 cells were double-labeled with anti-CD4 (PE)/anti-CD3 (FITC), anti-CD8 (PE)/anti-CD3 (FITC) (Becton Dickinson, Franklin Lakes, NJ) and processed on FACSDiva (Becton Dickinson, Franklin Lakes, NJ).
For apoptosis analysis, SCCVII and O12 tumor cells were incubated for 24 hours at which time 250 μM lactoferrin was added to the media. The assay was performed at 10 minutes, 20 minutes, 1 hour and 4 hours after exposure to the lactoferrin. The cells were washed by resuspending in 500 lL cold (2–8 °C) 1X PBS, pelleted by centrifugation and resuspended in Annexin V incubation reagent (which contains propidium iodide) at a concentration of 1 x 105−1 x 106 cells per 100 lL. Cells were incubated in the dark for 15 minutes before being collected by centrifugation. Cells were resuspended in 100 lL 1X Binding buffer containing fluorescent streptavidin conjugate and incubated in the dark for 15 minutes at 18–24 °C. After calibration of the flow cytometer, samples will be analyzed within one hour for maximal signal. Treated cells were also stained separately with Annexin V and propidium iodide to define the boundaries of each population.
Quantitative real-time RT-PCR
Cells were plated into 100 mm dishes at 5 × 106/dish. After being incubated at 37°C for 24 hours, the cells were exposed to lactoferrin at 250 uM. After another 24 hours, the cells were harvested. Total RNA was extracted from 1 × 107 cells using Qiagen’s RNeasy Mini kit (Qiagen, CA) according to the manufacturer’s directions. The RNA quality was exclusively evaluated by denaturing agarose gel electrophoresis. Cell cycle micoarrays were performed using according to the manufacturers instructions(Superarray, Frederick, MD). To verify the microarray data, real-time quantitative RT-PCR was performed with a LightCycler 2.0 Instrument, LightCycler FastStart DNA Masterplus SYBR Green I kit and LightCycler control Kit DNA (Roche, Indianapolis, IN) according to the manufacturer’s protocol. The following LightCycler conditions were used: precycling hold at 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 10 s, and extension at 72 °C for 12 s. The fluorescence was measured at the end of each cycle to construct amplification curves. To confirm amplification specificity, the PCR products were subjected to a melting curve analysis. Quantitation of transcripts was calculated based on a titrated standard curve co-run in the same experiment and calibrated with the expression level of a housekeeping gene (β-actin). All samples were done in triplicate. The primer sequences appear in Table 1.
Table 1.
RT-PCR primer sequences.
Luminex assay for Cellular Cytokines
1x105 SCCVII cells were incubated in 25 cm2 flasks overnight. The following morning, the media was changed and 200 μM of lactoferrin was added. After 24 hours, the media was removed, centrifuged and the supernatant was collected for Luminex analysis per the manufacturers instructions (Luminex, Austin, TX).
The plates were read utilizing Luminex technology (Austin, TX) and IS software and the final concentrations are calculated using Upstate multiplexing software (Upstate, San Francisco, CA). Statistical Analysis were performed using Students T-test.
In vivo CD3 depletion
4–6 week old C3H/HeJ females were injected with 200 μg of purified anti-CD3 (145-2C11) MoAb (hamster IgG, anti murine CD3, produced in our lab) on day −1, 0, and day 7 by IP. The mice were divided into 2 groups (Nolactoferrin treated control andlactoferrin treated) with 5 mice in each group. SCCVII cells (1x105) were injected into the flanks of each mouse with hypodermic method on day 0. On day 5, the designed treatment was then performed. For thelactoferrin treated groups, lactoferrin was delivered by gavage feeding at doses of 65mg/kg/d from day 5 to day 14. Control mice were treated with PBS. No anesthesia was used for oral gavage feeding. The tumor growth was evaluated every other day from day 5, and the tumors were measured in two dimensions with calipers. The tumor volume was calculated by formula (width2 X length)/2. The mice were sacrificed on day 15. The tumors were harvested and were embedded in OCT for immunohistochemistry studies. Blood and spleen were collected for flow cytometry analysis.
Immunohistochemistry
Tumors were harvested on day15 and then immediately embedded in OCT. 5μm thick cryostat sections were cut, mounted on superfrost plus slides (Fisher), and then fixed in ice-cold acetone for 5 minutes. After the slides were air-dried for 30 minutes, standard staining procedure was performed for the presence of infiltration of CD4 and CD8. The slides were rinsed with PBS to remove the OCT. After that, the endogenous peroxidase activity was blocked by incubating the slides in 0.3% H2O2 solution in PBS for 10 minutes. The slides were rinsed in PBS, and incubated with 3% FBS in PBS for 30min at room temperature to block the non-specific binding. The sections were then incubated for 1h with monoclonal antibodies (Purified rat anti-mouse CD4 and purified rat anti-mouse CD8(BD Pharmingen, Indianapolis, IN). After washing with PBS, the sections were incubated for 30 min with biotinylated anti-Rat IgG (H+L) secondary antibody (Vector Laboratories, Burlingame, CA). The slides were washed with PBS again, and incubated for 30 min with ABC reagent (Vector Laboratories, Burlingame, CA). After washing with PBS once more, the slides were incubated in the DAB (BD Pharmingen) substrate solution for about 10 min. The slides were finally washed in water, and counterstained with Hematoxylin (Vector Laboratories, Burlingame, CA) to facilitate routine light microscopic. Spleen sections served as positive control. Each section was counted three times and the mean was calculated. Statistical Analysis were performed using Students T-test.
Gastrointestinal Co-Culture
4–6 week old C3H/HeJ female mice were euthanized and the intestine was harvested from stomach to cecum. Fat and surrounding tissues were removed and the intestine was irrigated and flushed with wash buffer containing Hanks Balanced Salt Solution supplemented with Fetal Bovine Serum, Glutamine, Pencillin, Gentamicin, Streptomycin, and Amphoteracin B. The tissue was longitudinally incised, rewashed and transferred to 100x15mm dish with 15ml of complete DMEM medium with/without 250μM of LF and cultured at 37°C with 5%CO2 for 1hr, 4hr, and 8hr. Supernatants were collected at each time point, centrifuged, and analyzed by IL-18 ELISA (R&D systems. Minneapolis, Minnesota)
Results
Lactoferrin inhibits growth of human and murine squamous cell carcinoma cell lines in vitro
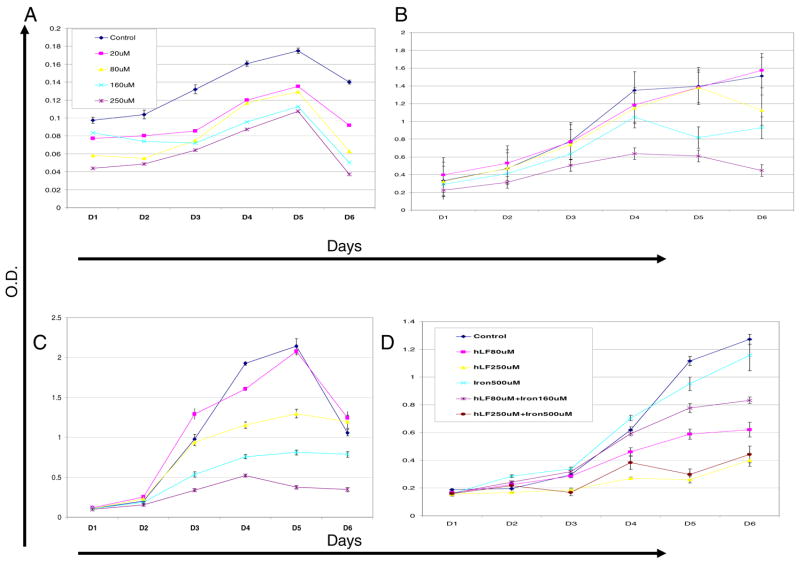
Although others have shown that lactoferrin inhibits tumor cell growth in vitro, we sought to evaluate the effect of lactoferrin on the growth of both murine and human squamous cell carcinoma cell lines. Recombinant human lactoferrin induces a dose-dependent growth inhibition of cancer cell growth via MTT assay (Figure 1). This was consistent in all cell lines tested. To demonstrate that this growth was not dependent on the iron binding ability of lactoferrin, lactoferrin was saturated with iron at 2:1 molar ration (iron:LF) for 10 min prior adding it to the medium. The growth inhibition was unaffected by iron saturation. These results demonstrate that lactoferrin inhibits cancer cell growth via an iron-independent, but dose-dependent, mechanism.
Figure 1.
Murine and human squamous cell carcinoma cell lines were cultured in medium containing increasing doses of lactoferrin in doses ranging from 0 μM to 250 μM. Daily MTT assays were performed to assess cell growth. A. Human cell line UMSCC9 cultured with varying doses of lactoferrin in the medium. There is a dose-dependent decrease in cell number from day 3 and beyond (P<0.05). B. Murine cell lines O12 cultured with varying doses of lactoferrin in the medium. There is a dose-dependent decrease in cell number from day 3 and beyond (P<0.05). C. Murine cell line SCCVII cultured with varying doses of lactoferrin in the medium. D. O12 cells were cultured with varying doses of lactoferrin as well as molar ratios of iron to saturate the lactoferrin. The iron binding state of lactoferrin had no effect on tumor.
Lactoferrin induces NF-κB Activation in squamous cell carcinoma cell lines
Based upon the previously published data that lactoferrin inhibits NF-κB activity, we hypothesized that lactoferrin would decrease constitutive activation of cellular NF-κB. UMSCC9, SCCVII, and UMSCC11B cell lines were incubated with Lactoferrin (250 μM) and nuclear extracts were harvested at specific time points. There was increased NF-κB activity at 4 hours of lactoferrin exposure that returned to baseline by 24 hours (Figure 2a). The experiment was then repeated using electromobility shift assay which confirmed the induction of active NF-κB (Figure 2b). Cells were then incubated for 4 hours in medium with lactoferrin (250μM) and in the presence and absence of Interleukin-1alpha (IL-1α) as a positive control. Cell extracts were collected and the NF-κB ELISA confirmed induction of NF-κB equal to that of the IL-1α. When lactoferrin and IL-1 were combined in the medium, the induction was greater than IL-1 alone for the initial 16 hours [p=0.02 (1 hour); 0.004 (4hours); 0.05 (8 hours); 0.01 (16 hours)], and equal to the IL-1 at 24 hours (p=0.8). These results were confirmed with EMSA shown in figure 2B and repeated with SCCVII cell lines (data not shown). These results do not support our initial hypothesis and demonstrate that lactoferrin upregulates NF-κB activity. Based upon these experiments, NF-κB likely does not contribute to lactoferrin-induced growth inhibition.
Figure 2.
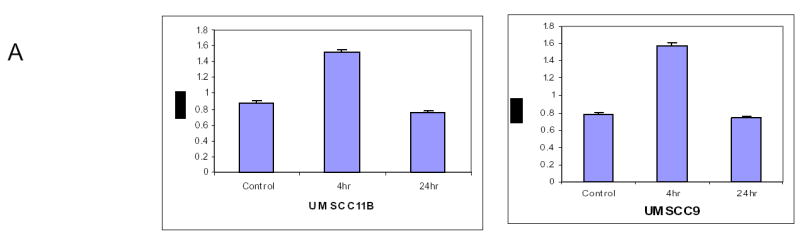
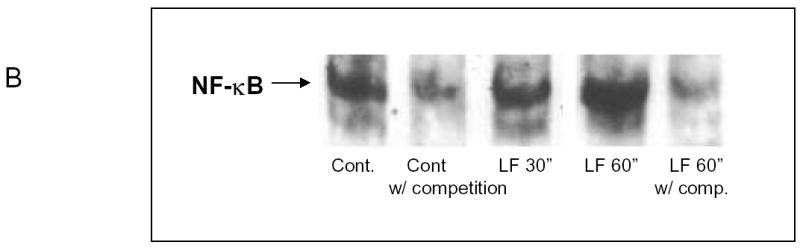

Lactoferrin Induces NF-κB activation in head and neck squamous cell carcinoma. A.UMSCC9 and UMSCC11B cell lines were cultured with Lactoferrin (250 μM) for 4 hours and 24 hours. Nuclear extracts were harvested and ELISA for the p50 subunit was performed and read on a plate reader at 450nm. Within 4 hours Lactoferrin induced activation and nuclear translocation of NF-κb compared to controls (p=0.04). At 24 hours of lactoferrin exposure, the amount of activated NF-κB returns to that of control tumors (p=0.27). B. To confirm the NF-κB activation, UMSCC9 cells were cultured with Lactoferrin (250 μM) and nuclear extracts were harvested at 30 minutes and 60 minutes. EMSA was performed for activated subunit of NFκB and read on photopaper. There is specific increase in NF-κB binding at both 30 and 60 minutes, which can be alleviated with unlabeled competitive sequence. C. UMSCC9 cell lines were cultured with Lactoferrin (250 μM), IL-1α (500 pg/ml as positive control), and Lactoferrin + IL-1α. Nuclear extracts were harvested and an ELISA for the p50 subunit was performed and read on a plate reader at 450nm. At all time points, there was increased activated NF-κB compared to controls (p<0.05). When compared to IL-1α alone, the amount of activated NF-κB in the Lactoferrin + IL-1α group was greater at 1 hour (p=0.017), 4 hours (p=0.003), and 16 hours (p=0.013).
Lactoferrin inhibits cell cycle progression with arrest at the G1/G0 checkpoint
It has previously been reported that lactoferrin induces G1/G0 growth arrest in head and neck and breast cancer cell lines. To determine the mechanism of growth inhibition in our cell lines, both SCCVII and O12 cell lines were plated and cultured overnight. After 24 hours, the media was changed and 250 μM of lactoferrin was added to the media. The cells were cultured for 24 and 48 hours and were harvested and analyzed by flow cytometry. In both cell lines and at both time points there were increases in cells in the G1/G0 phase compared to controls, with resultant decreases in the percentage of cells in the S phase (Figure 3a). We performed cell cycle microarrays (Superarray, Frederick, Maryland) which demonstrated decreases in Cyclin D1 in lactoferrin treated controls, with resultant increases in p19 (data not shown). This was confirmed via quantitative PCR (Figure 3b). To determine whether lactoferrin induces apoptosis, cells were cultured in 250 μM of lactoferrin. At 6 timepoints ranging from 2 hours to 48 hours, they were costained with Annexin and PI and were analyzed with flow cytometry. TNFα was used as a positive control. There was no induction of apoptosis at any time point in the lactoferrin group (data not shown). These results demonstrate that lactoferrin does not induce apoptosis in HNSCCA and inhibits growth via cell cycle arrest.
Figure 3.
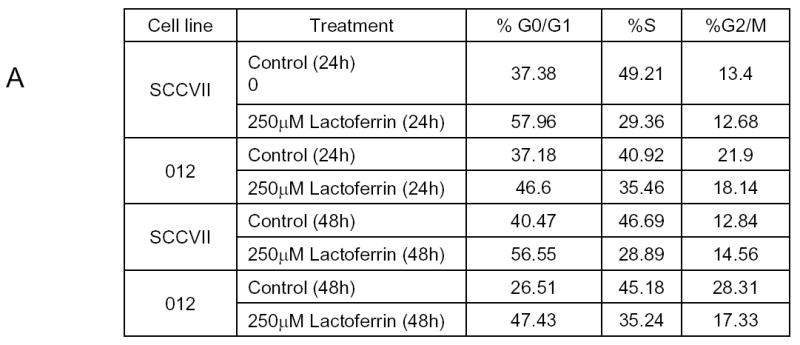
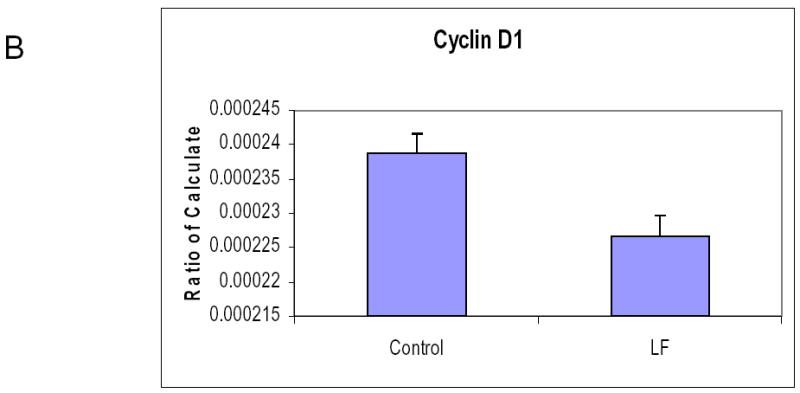
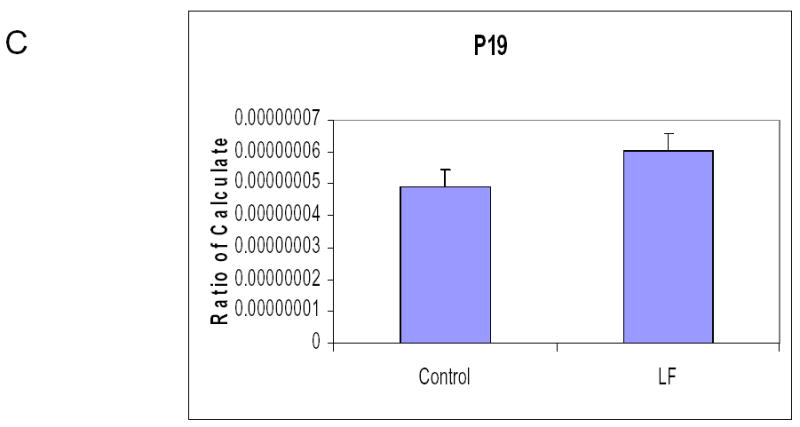
Lactoferrin induces G1/G0 Growth Arrest. A. 1x105 SCCVII and O12 cells were incubated in 25 cm2 flasks and lactoferrin (250 μM) was added to the medium. At 24 hours, cells were trypsinized, stained, and analyzed using flow cytometry. Lactoferrin induces growth arrest at the G0/G1 checkpoint compared to controls. B. Cells were exposed to lactoferrin (250 μM) for 24 hours. RNA was harvested and Real time quantitative RT-PCR was performed to evaluate for changes in Cyclin D1. Lactoferrin exposure induced a decrease in Cyclin D1. C. Cells were exposed to lactoferrin (250 μM) for 24 hours. RNA was harvested and Real time quantitative RT-PCR was performed to evaluate for changes in P19. Lactoferrin exposure induced an increase in P19. These changes in Cyclin D1 and P19 are the likely mechanism whereby the G1/G0 growth arrest occurs.
Lactoferrin changes the production of proinflammatory cytokines in squamous cell carcinoma
We have previously published that squamous cell carcinomas of the head and neck produce proinflammatory cytokines. To determine whether lactoferrin would alter this production, the supernatant of SCCVII cultured in lactoferrin was collected and a luminex assay was performed. Significant decreases in GM-CSF (p=0.01), IL-6 (p=0.04), TNFα (p=0.05) and IL-8 were observed (Figure 4). Marked increases in IL-10 were noted and as expected, no detectible changes in IL-18. These results demonstrate that lactoferrin can induce changes in the inflammatory mileau of tumor cells and specifically can inhibit the production of proinflammatory and prometastatic cytokines.
Figure 4.
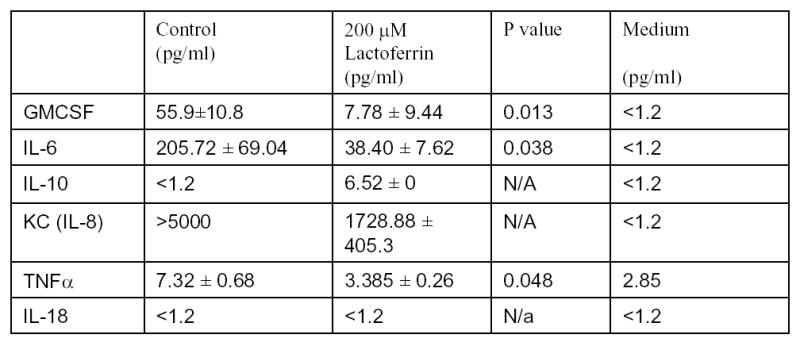
Lactoferrin inhibits squamous cell carcinoma production of proinflammatory cytokines. 1x105 SCCVII cells were incubated in 25 cm2 flasks. Cells were cultured overnight at which time the medium was changed and 250μM of lactoferrin was added. The medium was collected after 24 hours and luminex assay for cytokines was performed. There is anlactoferrin-related decrease in GMCSF, IL-6, IL-2, KC(IL-8), and TNFα over controls and medium. There was an increase in IL-10 in thelactoferrin cells and as expected, no detectable IL-18.
Oral Lactoferrin inhibits the growth of murine squamous cell carcinoma and induces lymphocytic infiltration of tumors
We sought to demonstrate that oral lactoferrin would induce tumor inhibition in the mouse model. It has been previously shown that oral lactoferrin can increase circulating and splenic numbers of CD4+ and CD8+ cells, but it has never been demonstrated that this results in increased infiltration of tumors by these cells. We designed an experiment in which C3H/HeJ mice were anesthetized and 0.1 cc suspension of 5x105 SCCVII cells were implanted on the flank or the floor of mouth. Tumors were allowed to grow for 5 days. Mice were then treated with lactoferrin via gastric gavage for 10 days. The mice with orthotopic tumors were sacrificed and had the tumors measured on day 10 of lactoferrin dosing. Mice with flank tumors were measured daily during lactoferrin dosing. The volume of the orthotopic tumors in mice receiving lactoferrin was 62%–75% reduced compared to controls (P<0.002) (Figure 5a). The volume of flank tumors in lactoferrin treated mice were reduced by 67%–70% of that in control mice (Figure 5b). Mice were also treated with fetal bovine serum (FBS) to evaluate whether the reduction of tumor volume was due to the presence a foreign protein. There was no difference in tumor volume in mice receiving FBS and control mice. Tumors were harvested after 10 days of oral lactoferrin and stained for CD4+ and CD8+ lymphocytes within the tumors. Stained cells were counted per high powered field and there were marked increases in the numbers of both CD4+and CD8+ cells infiltrating the tumors compared to non-lactoferrin treated animals (Figure 5c, 5d). These results demonstrate that oral lactoferrin dramatically inhibits HNSCCA growth in vivo and induces lymphocytic infiltration into the tumors of treated mice.
Figure 5.
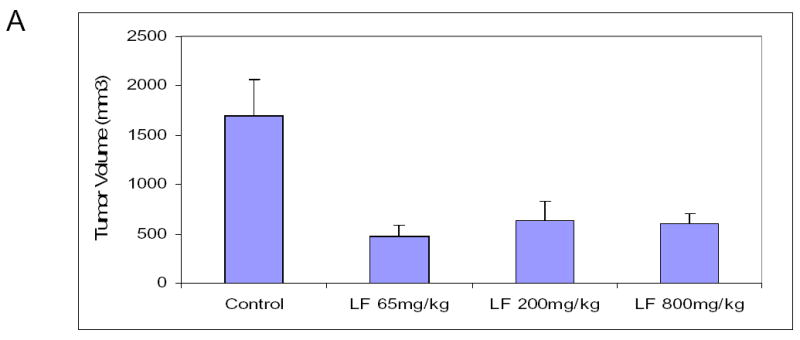
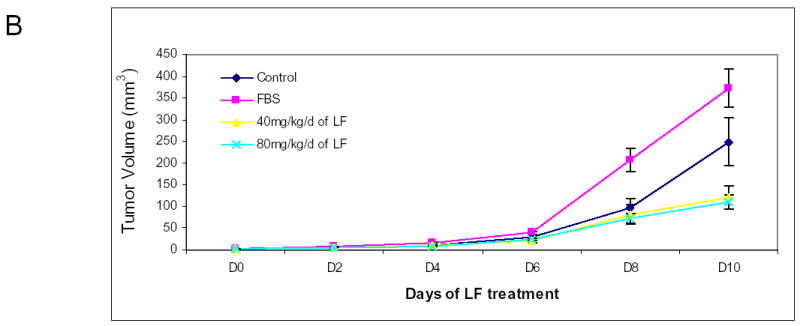
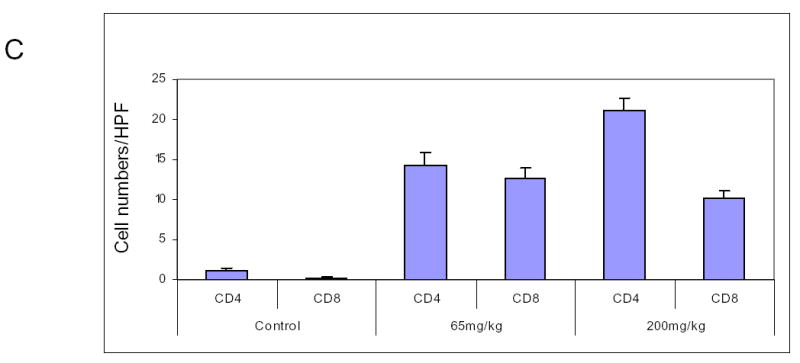
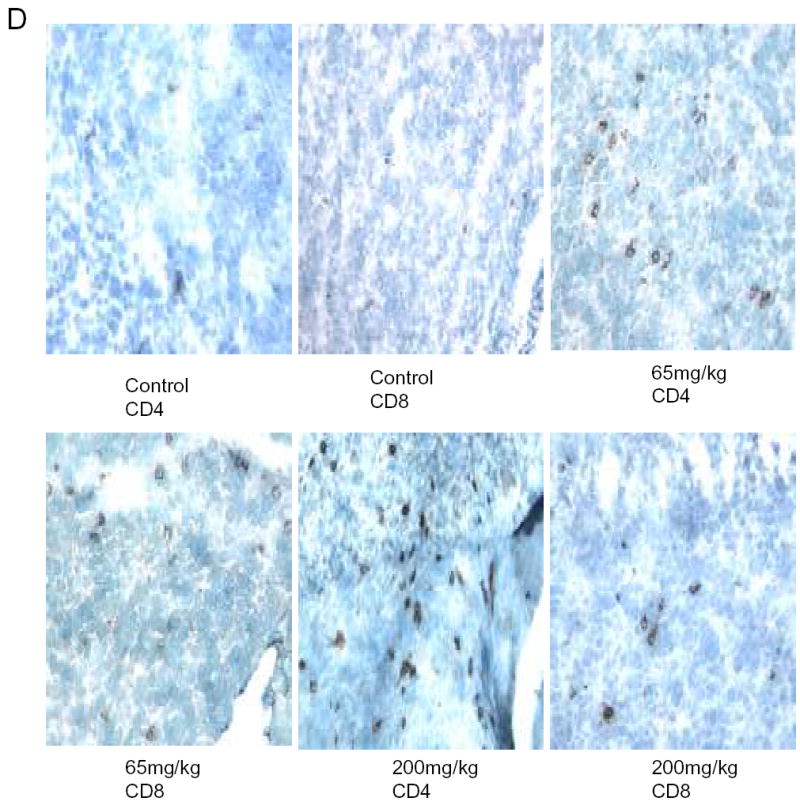
Oral lactoferrin inhibits the growth of SCCVII tumors in the murine model and induces lymphocytic infiltration into tumors. A. 5x106 cells of SCCVII were implanted orthotopically in the floor of mouth of mice. The tumors were allowed to grow for 5 days and the volume was measured. For 10 subsequent days the mice were given varying doses of lactoferrin via oral gavage. After the last lactoferrin dose, mice were sacrificed and the tumors were measured and harvested. After 10 days of lactoferrin, the tumor volumes reduced by 62%–75% compared to control mice. B. To assess evaluate the tumor growth curves, the same model was followed, except that SCCVII were injected on the animal flank. Tumor volumes were measured daily during the 10 days of lactoferrin gavage. Fetal Bovine serum was used as a foreign protein control. After 10 days of lactoferrin, the tumor volumes reduced by 67%–70% compared to serum control mice. C. and D. Tumors were harvested and immunohistochemistry was performed for CD4+ and CD8+ cells within the tumor. Tumors were photographed and positively staining cells were counted per high power field in triplicate. Tumors in lactoferrin treated mice had significant increases in the number of CD4+ and CD8+ lymphocytes within the tumor (P<0.001).
Tumor growth inhibition in lactoferrin-treated mice is abrogated by depleting mice of lymphocytes
Although we have shown that lactoferrin will increase the lymphocyte population within the tumors, we hypothesized that this was the mechanism of tumor inhibition. To test this hypothesis, we depleted mice of CD3+ cells and repeated the experiment. C3H/HeJ mice were treated with 3 doses of 200 μg of anti-CD3 monoclonal antibody given intraperitoneally. This protocol was tested in control mice to assure depletion in the serum and spleen (data not shown). In tumor-bearing mice, antibody was delivered for two days prior to SCCVII implantation and on day 3 of lactoferrin dosing. Tumors, serum, and spleens were harvested after 10 days of lactoferrin treatment and flow cytometry for CD3 was performed. There was successful depletion to <1% of cells in both spleen (Figure 6a) and serum (data not shown). Tumor growth curves in CD3 depleted mice revealed no differences in tumor volume in lactoferrin treated mice compared to controls (Figure 6b). To confirm this, the tumors were analyzed for CD3+ cells by immunohistochemistry and there was no difference in tumor staining between the lactoferrin treated group and controls (Figure 6c, 6d).
Figure 6.
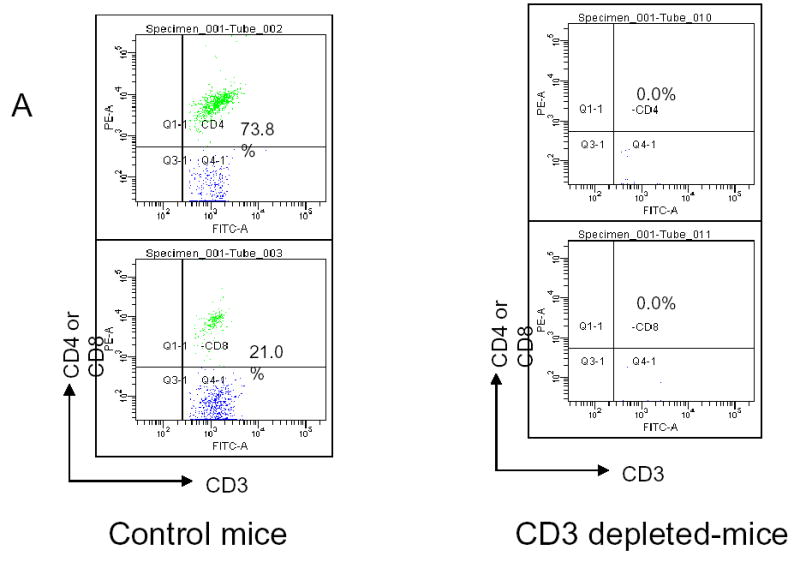
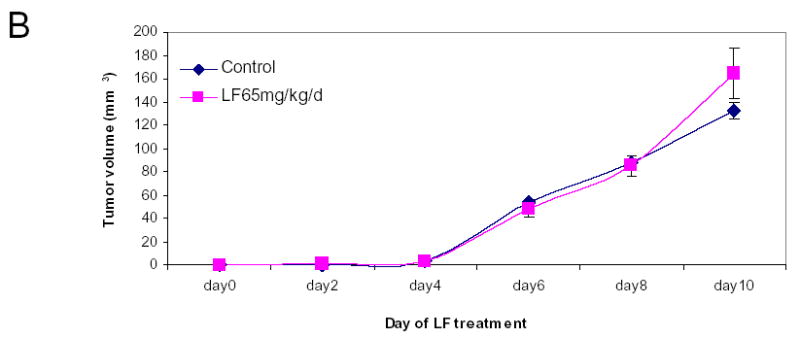
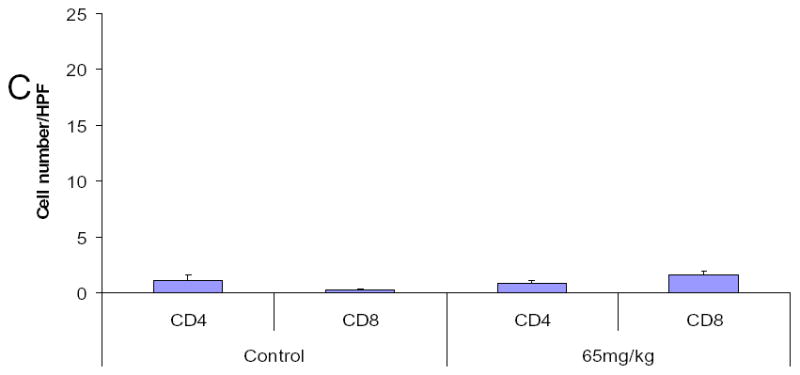
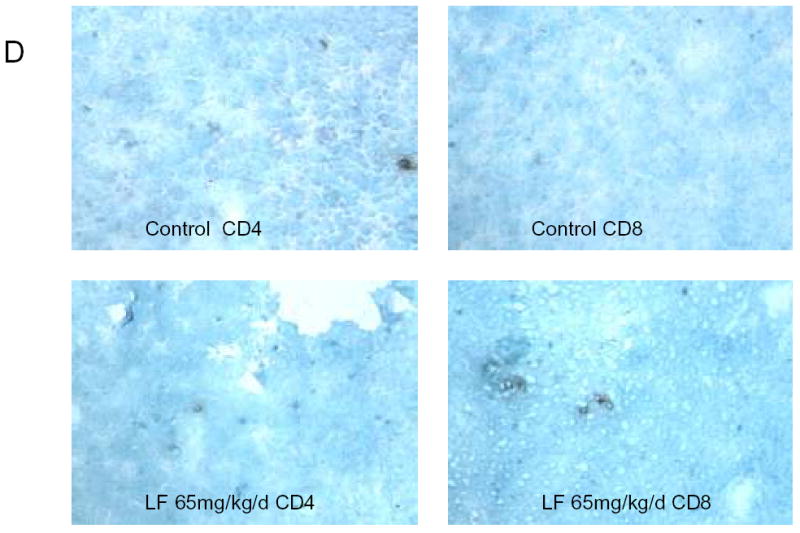
Lactoferrin-induced tumor growth inhibition is abrogated by depleting tumors of CD3. Mice were depleted of CD3+ cells using 200 mg of intraperitoneal anti-CD3 on days −1, 0, and 7 after SCCVII injection. Lactoferrin was given via oral gavage for 10 days starting at day 5 after tumor implantation and tumors were measured daily. Tumors and spleens were harvested after the last lactoferrin dose. A. Spleens were stained for CD3+, CD4+ and CD8+ positive cells and analyzed by flow cytometry. There was complete depletion of splenic CD3+ cells compared to control mice. B. Tumor volumes were measured during the 10 days of lactoferrin dosing. In these CD3+ depleted mice, there was no significant difference in tumor volume between mice receiving lactoferrin and controls. C and D. Tumors were harvested and immunohistochemistry was performed for CD4+ and CD8+ cells within the tumor. Tumors were photographed and positively staining cells were counted per high power field in triplicate. Tumors in lactoferrin treated mice had no difference in the numbers of CD4+ and CD8+ lymphocytes within the tumor.
To confirm that immunomodulation is a mechanism of lactoferrin-induced tumor inhibition, we repeated oral dosing experiments on Balb/C nude mice. In these animals, the lactoferrin treated animals has significantly larger tumors (p=0.01) after 10 days of lactoferrin (Figure 7). These data demonstrate that immunomodulation is a major mechanism of lactoferrin-induced tumor inhibition and that the G1/G0 growth arrest noted in vitro, does not appear to significantly contribute to tumor inhibition in vivo.
Figure 7.
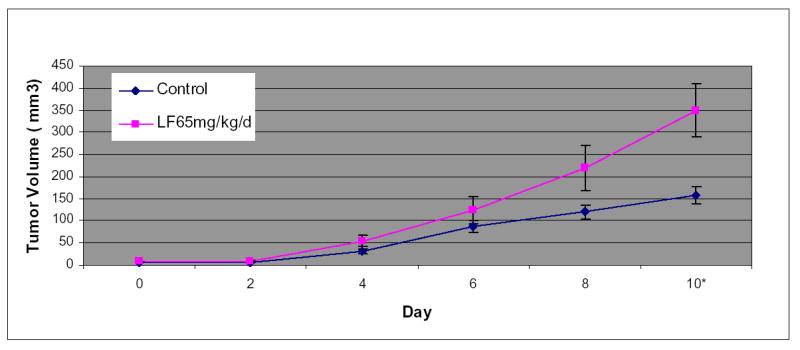
Lactoferrin does not inhibit tumor growth in athymic mice. 5x106 cells of SCCVII were implanted into the flank of Balb/C nude mice. . The tumors were allowed to grow for 5 days and the volume was measured. For 10 subsequent days the mice were given varying doses of lactoferrin via oral gavage. After the last lactoferrin dose, mice were sacrificed and the tumors were measured and harvested. In this athymic model, there were no differences between lactoferrin treated mice and control mice until the 10th day of lactoferrin, when the lf treated mice had larger tumors than control mice(p=0.01). (* denotes statistical significance).
Lactoferrin induces IL-18 production by the intestinal mucosa
To determine whether the increased quantity of intratumoral lymphocytes are related to alteration of intestinal cytokine production in the gut by enteral lactoferrin dosing, we performed isolated intestinal co-culture experiments. The intestines from mice were incised longitudinally and maintained in either complete DMEM or DMEM supplemented with 250μM of lactoferrin. After 1, 4, and 8 hours, supernatants were collected and IL-18 ELISA was performed. The quantity of IL-18 in the lactoferrin-exposed mucosa was 4x greater than control mucosa (Figure 8). There was no difference in concentration of IL-8(KC), or IL-6 in the supernatant when compared to controls (data not shown).
Figure 8.
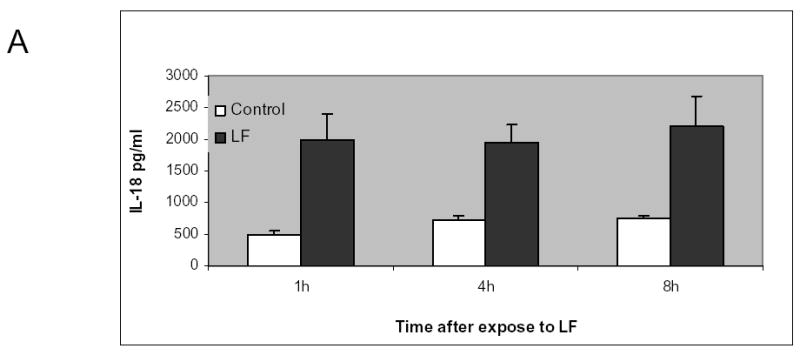
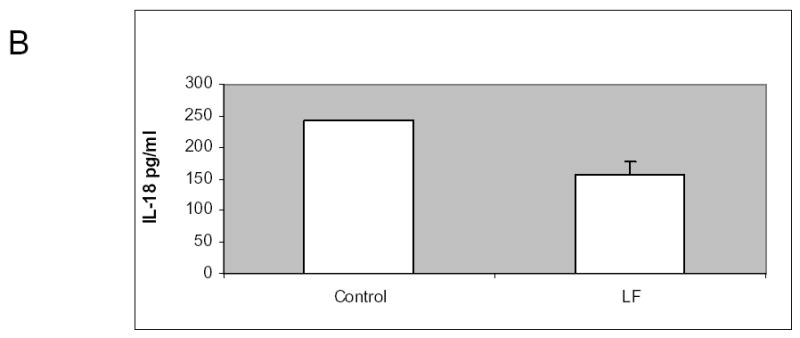
Lactoferrin increases intestinal production of IL-18. A. The intestinal mucosa from C3H/HeJ mice was isolated and co-cultured with 250μM of lactoferrin. Supernatant was collected and measured for IL-18 concentration using ELISA. There was 400% increase in IL-18 in the lactoferrin group compared to control medium (p=0.01). B. 5x106 cells of SCCVII were implanted orthotopically in the floor of mouth of mice. The tumors were allowed to grow for 5 days. For 10 subsequent days the mice were given 65 mg/kg dose of lactoferrin via oral gavage. After the last lactoferrin dose, serum was collected and measured for IL-18. There was no significant difference in serum IL-18 between treated and control mice (p-0.06).
To determine whether increased intestinal production of IL-18 correlated with serum IL-18, serum IL-18 was measured in tumor-bearing C3H/HeJ mice receiving 10 days of oral lactoferrin. There was no significant difference (p=0.06) in measureable serum IL-18 between lactoferrin-treated and control mice (Figure 8).
These data demonstrate that lactoferrin upregulates localized intestinal production of the IL-18, a potent CD4+ chemoattractant, but does not significantly change serum IL-18 concentration.
DISCUSSION
Lactoferrin inhibits the growth of head and neck squamous cell carcinoma. Despite our in vitro studies demonstrating direct growth inhibition via G1/G0 blockade, NF-κB activation, and reduction of cellular proinflammatory cytokines, the mechanism of tumor control in vivo appears to be regulated primarily by the cellular arm of the immune response. Because of the favorable toxicity profile and availability of this drug, our data supports the study of lactoferrin for the treatment of HNSCCA.
Since lactoferrin-induced growth inhibition has been previously attributed to intrinsic and immunologic control of cellular proliferation, we sought to define the relative input of each by using in vitro and in vivo studies. In this current study, we have shown that human recombinant lactoferrin inhibits both human and murine squamous carcinoma cell line proliferation in a dose-dependent manner. This growth inhibition is independent of the iron binding state and is not due to NF-κB downregulation. We have previously demonstrated that inhibition of NF-κB activation in squamous cell carcinoma cell lines decreases cell viability(15, 36), and thus expected this would be a mechanism of lactoferrin. We had hypothesized that growth inhibition may be due directly to downregulation of NF-κB which is constitutively activated in the cell lines used in this study, but our present results show that lactoferrin causes an initial increase in active NF-κB binding followed by return to pretreatment levels(37). The increase in active NF-kb is transient and may explain why we were unable to detect increases in NF-kB dependent cytokines at 24 hours post-treatment.
These findings are in contrast to others published work which demonstrated that lactoferrin inhibits NK-κB activation in monocytes and rat colon cells(13,38). Our data, as well as that of Oh, confirm that lactoferrin upregulates NF-κB in cancer cell lines(39). It is possible that the effects of lactoferrin on NF-κB activity may differ based upon the cell type (monocytes vs. carcinoma cell lines) and it appears from our results that the lactoferrin effect on NF-κB is not directly causative of growth inhibition.
Lactoferrin alters the cytokine mileu of cancer cells lines. This is important in the context that head and neck squamous cell carcinoma lines produce proinflammatory cytokines which allow tumor propagation in vitro and are thought to contribute to the malignant phenotype of head and neck cancer(15–17, 40). We have found shown that lactoferrin decreases the cellular production of GMCSF, IL-6, KC (IL-8), and TNF-α and increases the production of IL-10. Clinically, IL-6, IL-8, GM-CSF, and IL-1-inducible acute phase proteins are all present in significantly higher levels in the serum of head and neck cancer patients than in age-matched controls(17). IL-8 promotes angiogenesis and tumor metastasis in the lung SCCA and GM-CSF promotes metastasis of head and neck SCCA(41, 42). We had hypothesized that downregulation of these cytokines may have contributed to lactoferrin inhibiting the growth of these tumors. It was these in vitro immunomodulatory functions of lactoferrin that enticed us to look at this potential mechanism in vivo.
Once we identified that lactoferrin induced direct cellular growth inhibition, we aimed to determine the mechanism by which this occurred. In all cell lines tested, we found G1/G0 arrest compared to controls. Using microarray and quantitative PCR we specifically identified decreases in cyclin D1 and increases in p19 as the likely mechanism for this cell cycle arrest. This data corresponds with others that have shown lactoferrin induced growth arrest was caused by cell cycle inhibition at the G0–G1 checkpoint(11, 12). Xiao reported this was associated with an increase in p27 protein, accompanied by decreased phosphorylation of retinoblastoma protein, and suppression of cyclin E; however, these were unchanged in our cell lines(11). Using our cell line data, we focused on discerning the relative contribution of this growth inhibition in vivo.
Oral delivery of lactoferrin results in decreased tumor growth. Specifically in this study, we showed that oral lactoferrin inhibits tumors while inducing lymphocytic infiltration into tumors. This data is consistent with data that lactoferrin inhibits growth of mouse tumors(25, 26, 43). It has been shown that when given intravenously, orally (via gavage), or directly injected into tumors, the greatest inhibition of tumor growth occurs with oral dosing(26). Oral lactoferrin has been demonstrated to stimulate intestinal IL-18 production by 3–7 fold which increases splenic production of NK cells and serum CD8+ cells(26). It has been shown to reconstitute the circulating and splenic CD4+ and CD8+ cell population in mice treated with cyclophosphamide(21) Our study has elucidated that aside from this increased serum and splenic lymphocytes, oral lactoferrin induces infiltration of these cells within tumors. What is perhaps the most exciting about these data is that that this occurs in the SCCVII cell line, which is poorly immunogenic and rapidly growing(33, 44, 45).
The effect of oral lactoferrin on in vivo tumor growth appears to be largely due to this immunomodulation. Although this has been previously hypothesized, these are the first data to support this hypothesis. We found that by depleting mature lymphocytes with anti-CD3+ antibody, the entire anti-tumor effect is abrogated. We were able to identify no differences in tumor volume or lymphocytic infiltration between control mice and CD3 depleted mice that were treated with lactoferrin. Nude mice (which have intact natural killer cells) actually had slightly increased tumor size in the after ten days of lactoferrin treatment. This supports our premise that it is lymphocytes, and not NK cells, that contribute to tumor inhibition in lactoferrin treated animals. The increased tumor size in LF treated athymic mice after 10 days of treatment may be to improved nutrition from gavage with lactoferrin.
We now know from our work that the tumor inhibition obtained from oral lactoferrin dosing is largely, if not solely, due to immunomodulation resulting in lymphocytic infiltration in tumors. The mere upregulation of the quantity of T lymphocytes does not completely explain the mechanism of lactoferrin; there is a marked increase of lymphocytes within the tumors in the lactoferrin treated group. While lactoferrin upregulates gut production of IL-18, a CD4+ cell chemoattractant, interleukin-18 is not produced the tumors or by cell lines exposed to lactoferrin and lactoferrin is not able to measured in the serum in orally treated animals(26, 46). This brings in to question the mechanism by which the lymphocytes are becoming sensitized to tumor specific antigens. The mechanism by which the immunomodulation occurs will be better elucidated by performing experiments in IL-18 knockout mice, dendritic cell knockout mice and analysis of the tumor-infiltrating lympocytes.
To further understand the potential of lactoferrin as a chemotherapeutic agent, one must examine its safety profile. In published studies, there have been no signs of toxicity in mice or monkeys treated with oral and IV lactoferrin up to 5000 mg/kg. In human studies, no toxicity in intravenous lactoferrin have been reported(47). In a recent non-small cell lung cancer trial, 10 patients were given oral lactoferrin up to 9 grams per day without any hematological, hepatic, or renal toxicities(48). This exceptional safety profile makes lactoferrin attractive as a primary chemotherapeutic agent in addition to a possible adjuvant.
At the oral doses delivered, we were unable to demonstrate a dose-response relationship for oral lactoferrin despite a large dosing range from 40 mg/kg to 800 mg/kg. We hypothesize that there is a low quantity of lactoferrin receptors in the gut, that are entirely saturated with even the lowest dose tested. Teleologically, this is likely due to the moderate concentration of lactoferrin in human breast milk (970 mg/dl) and thus there would be little purpose for high concentration of receptors in the gut(49). The small intestine lactoferrin receptor is rapidly saturated by lactoferrin, which has a small dissociation constant with its receptor(29, 50). Further studies with significantly smaller concentrations of lactoferrin may need to be performed to demonstrate a dose-response relationship or saturation kinetics. With the known concentration of lactoferrin in human breast milk, the anticancer properties that we have demonstrated may be clinically related to the lower rate of malignancies in breastfed infants(8–10)
These data provide insight into the antitumor effect of human recombinant lactoferrin on squamous cell carcinoma of the head and neck and provide a potential strategy for new treatments of head and neck cancer. Lactoferrin can potentially be utilized as a direct agent via intratumoral injection as well as a systemic agent via oral delivery. While more information regarding the mechanism of oral lactoferrin is needed, these early data demonstrates that lactoferrin has potential as a safe and effective means to treat cancers of the head and neck.
Footnotes
Disclosure: Dr. O’Malley is a member of the Scientific Advisory Board and hold equity options in Agennix, Inc.
Supported by NIH 5R03DE015428 (JSW) and the American Academy of Otolaryngology-Head and Neck Surgery/American Head and Neck Society Young Investigators Award (JSW)
References
- 1.Metz-Boutigue MH, Jolles J, Mazurier J, et al. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984;145:659–76. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- 2.Kanyshkova TG, Buneva VN, Nevinsky GA. Lactoferrin and its biological functions. Biochemistry (Mosc) 2001;66:1–7. doi: 10.1023/a:1002817226110. [DOI] [PubMed] [Google Scholar]
- 3.Levay PF, Viljoen M. Lactoferrin: A General Review. Haematologica. 1995;80:252–67. [PubMed] [Google Scholar]
- 4.Masson PL, Heremans JF, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643–58. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward PP, Uribe-Luna S, Conneely OM. Lactoferrin and host defense. Biochem Cell Biol. 2002;80:95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- 6.Ward PP, Paz E, Conneely OM. Multifunctional roles of lactoferrin: a critical overview. Cell Mol Life Sci. 2005;62:2540–8. doi: 10.1007/s00018-005-5369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cumberbatch M, Dearman RJ, Uribe-Luna S, et al. Regulation of Epidermal Langerhans Cell migration by Lactoferrin. Immunology. 2000;100:21–8. doi: 10.1046/j.1365-2567.2000.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breastfeeding and childhood cancer. Br J Cancer. 2001;85:1685–94. doi: 10.1054/bjoc.2001.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur GP, Gupta N, Mathur S, et al. Breastfeeding and childhood cancer. Indian Pediatr. 1993;30:651–7. [PubMed] [Google Scholar]
- 10.Hardell L, Dreifaldt AC. Breast-feeding duration and the risk of malignant diseases in childhood in Sweden. Eur J Clin Nutr. 2001;55:179–85. doi: 10.1038/sj.ejcn.1601142. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Y, Monitto CL, Minhas KM, Sidransky D. Lactoferrin down-regulates G1 cyclin-dependent kinases during growth arrest of head and neck cancer cells. Clin Cancer Res. 2004;10:8683–6. doi: 10.1158/1078-0432.CCR-04-0988. [DOI] [PubMed] [Google Scholar]
- 12.Damiens E, El Yazidi I, Mazurier J, Duthille I, Spik G, Boilly-Marer Y. Lactoferrin Inhibits G1 Cyclin-Dependent Kinases during Growth Arrest of Human Breast Carcinoma. Journal of Cellular Biochemistry. 1999;74:486–98. [PubMed] [Google Scholar]
- 13.Togawa J, Nagase H, Tanaka K, et al. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am J Physiol Gastrointest Liver Physiol. 2002;283:G187–95. doi: 10.1152/ajpgi.00331.2001. [DOI] [PubMed] [Google Scholar]
- 14.Zucali JR, Broxmeyer HE, Levy D, Morse C. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interleukin-1. Blood. 1989;74:1531–6. [PubMed] [Google Scholar]
- 15.Wolf JS, Chen Z, Dong G, et al. IL (Interleukin)-1α Promotes Nuclear Factor-κB and AP-1-induced IL-8 Expression, Cell Survival, and Proliferation in Head and Neck Squamous Cell Carcinomas. Clinical Cancer Research. 2001;7:1812–20. [PubMed] [Google Scholar]
- 16.Ondrey FG, Sunwoo JB, Dong G, Chen Z, Bancroft CC, Van Waes C. Constitutive Expression of Proinflammatory Cytokines and Survival in Head and Neck Squamous Cell Carcinoma Cell Lines. Mol Carcinog. 1999;26:119–29. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Malhotra PS, Thomas GR, et al. Expression of Proinflammatory and Proangiogenic Cytokines in Human Head and Neck Cancer. Clinical Cancer Research. 1999;5:1369–79. [PubMed] [Google Scholar]
- 18.Guillen C, McInnes IB, Baughan DM, et al. Enhanced Th1 Response to Staphylococcus aureus Infection in Human Lactoferrin-Transgenic Mice. The Journal of Immunology. 2002;168:3950–7. doi: 10.4049/jimmunol.168.8.3950. [DOI] [PubMed] [Google Scholar]
- 19.Kuhara T, Iigo M, Itoh T, et al. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL-18 in the intestinal epithelium. Nutrition & Cancer. 2000;38:192–9. doi: 10.1207/S15327914NC382_8. [DOI] [PubMed] [Google Scholar]
- 20.Wang WP, Iigo M, Sato J, Sekine K, Adachi I, Tsuda H. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn J Cancer Res. 2000;91:1022–7. doi: 10.1111/j.1349-7006.2000.tb00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artym J, Zimecki M, Kruzel ML. Reconstitution of the cellular immune response by lactoferrin in cyclophosphamide-treated mice is correlated with renewal of T cell compartment. Immunobiology. 2003;207:197–205. doi: 10.1078/0171-2985-00233. [DOI] [PubMed] [Google Scholar]
- 22.Bezault J, Bhimani R, Wiprovnick J, Furmanski P. Human Lactoferrin inhibits growth of solid Tumors and Development of experimental metastases in Mice. Cancer Research. 1994;54:2310–2. [PubMed] [Google Scholar]
- 23.Masuda C, Wanibuchi H, Sekine K, et al. Chemopreventive effects of bovine lactoferrin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced rat bladder carcinogenesis. Jpn J Cancer Res. 2000;91:582–8. doi: 10.1111/j.1349-7006.2000.tb00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekine K, Watanabe E, Nakamura J, et al. Inhibition of Azoxymethane-initiated Colon Tumor by Bovine Lactoferrin Administration in F344 Rats. Japanese Journal of Cancer Research. 1997;88:523–26. doi: 10.1111/j.1349-7006.1997.tb00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf JS, Li D, Taylor RJ, O’Malley BW., Jr Lactoferrin inhibits growth of malignant tumors of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2003;65:245–9. doi: 10.1159/000075220. [DOI] [PubMed] [Google Scholar]
- 26.Varadhachary A, Wolf JS, Petrak K, et al. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer. 2004;111:398–403. doi: 10.1002/ijc.20271. [DOI] [PubMed] [Google Scholar]
- 27.Shau H, Kim A, Golub SH. Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. Journal of Leukocyte Biology. 1992;51:343–49. [PubMed] [Google Scholar]
- 28.Suzuki YA, Lonnerdal B. Characterization of mammalian receptors for lactoferrin. Biochem Cell Biol. 2002;80:75–80. doi: 10.1139/o01-228. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40:15771–9. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- 30.Talukder MJ, Takeuchi T, Harada E. Characteristics of lactoferrin receptor in bovine intestine: higher binding activity to the epithelium overlying Peyer’s patches. J Vet Med A Physiol Pathol Clin Med. 2003;50:123–31. doi: 10.1046/j.1439-0442.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 31.Hu WL, Mazurier J, Montreuil J, Spik G. Isolation and partial characterization of a lactotransferrin receptor from mouse intestinal brush border. Biochemistry. 1990;29:535–41. doi: 10.1021/bi00454a030. [DOI] [PubMed] [Google Scholar]
- 32.Hu WL, Mazurier J, Sawatzki G, Montreuil J, Spik G. Lactotransferrin receptor of mouse small-intestinal brush border. Binding characteristics of membrane-bound and triton X-100-solubilized forms. Biochem J. 1988;249:435–41. doi: 10.1042/bj2490435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Malley BW, Jr, Cope KA, Johnson CS, Schwartz MR. A new immunocompetent murine model for oral cancer. Arch Otolaryngol Head Neck Surg. 1997;123:20–4. doi: 10.1001/archotol.1997.01900010022003. [DOI] [PubMed] [Google Scholar]
- 34.Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–10. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renard P, Ernest I, Houbion A, et al. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sunwoo JB, Chen Z, Dong G, et al. Novel Proteasome Inhibitor PS-341 Inhibits Activation of Nuclear Factor-kappa B, Cell Survival, Tumor Growth, and Angiogenesis in Squamous Cell Carcinoma. Clin cancer Res. 2001;7:1419–28. [PubMed] [Google Scholar]
- 37.Ondrey FG, Dong G, Sunwoo J, et al. Constitutive activation of transcription factors NF-(kappa)B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinog. 1999;26:119–29. doi: 10.1002/(sici)1098-2744(199910)26:2<119::aid-mc6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 38.Haversen L, Ohlsson BG, Hahn-Zoric M, Hanson LA, Mattsby-Baltzer I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell Immunol. 2002;220:83–95. doi: 10.1016/s0008-8749(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 39.Oh SM, Pyo CW, Kim Y, Choi SY. Neutrophil lactoferrin upregulates the human p53 gene through induction of NF-kappaB activation cascade. Oncogene. 2004;23:8282–91. doi: 10.1038/sj.onc.1208021. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z, Colon I, Ortiz N, et al. Effects of IL-1α, IL-1RA, and Neutralizing Antibody on Proinflammatory Cytokine Expression by Human Squamous Cell Carcinoma Lines. Cancer Research. 1998;58:3668–76. [PubMed] [Google Scholar]
- 41.Smith DR, Polverini PJ, Kunkel SL, et al. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179:1409–15. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young MRI, Wright MA, Lozano Y, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony stimulating factor and contrained CD34+ natural suppressor cells. Int J Cancer. 1997:74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Kawabata K, Kohno H, et al. Chemopreventive Effect on Bovine Lactoferrin on 4-nitroquinoline 1-oxide-induced Tongue Carcinogenesis in Male F344 Rats. Japanese Journal of Cancer Research. 2000;91:25–33. doi: 10.1111/j.1349-7006.2000.tb00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strome SE, Voss S, Wilcox R, et al. Strategies for Antigen Loading of Dendritic Cells to Enhance the Antitumor Immune Response. Cancer Res. 2002;62:1884–9. [PubMed] [Google Scholar]
- 45.Strome SE, Dong H, Tamura H, et al. B7-H1 Blockade Augments Adoptive T-Cell Immunotherapy for Squamous Cell Carcinoma. Cancer Res. 2003;63:6501–5. [PubMed] [Google Scholar]
- 46.Komai-Koma M, Gracie JA, Wei XQ, et al. Chemoattraction of human T cells by IL-18. J Immunol. 2003;170:1084–90. doi: 10.4049/jimmunol.170.2.1084. [DOI] [PubMed] [Google Scholar]
- 47.Ziere B, Van Veen H, Koopman J, Van Berkel P, Nuijens J. Safety, Tolerability, and Pharmacokinetics of I.V. Administered rhLF in Humans. 5th International Conference on Lactoferrin; 2001; Alberta, Canada. Pharming Technologies; 2001. [Google Scholar]
- 48.Hayes TG, Falchook GF, Varadhachary GR, et al. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest New Drugs. 2005 doi: 10.1007/s10637-005-3690-6. [DOI] [PubMed] [Google Scholar]
- 49.Ronayne de Ferrer PA, Baroni A, Sambucetti ME, Lopez NE, Ceriani Cernadas JM. Lactoferrin Levels in Term and Preterm Milk. J Am Coll Nutr. 2000;19:370–3. doi: 10.1080/07315724.2000.10718933. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami H, Lonnerdal B. Isolation and function of a receptor for human lactoferrin in human fetal intestinal brush-border membranes. Am J Physiol. 1991;261:G841–6. doi: 10.1152/ajpgi.1991.261.5.G841. [DOI] [PubMed] [Google Scholar]