Abstract
A new bicyclic template has been developed for the synthesis of peptide mimetics. Straightforward synthetic steps, starting from amino acids, allow the facile construction of a wide range of analogs. This system was designed to target the melanocortin receptors (MCRs), with functional group selection based on a known pharmacophore and guidance from molecular modeling to rationally identify positional and stereochemical isomers likely to be active. The functions of hMCRs are critical to myriad biological activities, including pigmentation, steroidogenesis, energy homeostasis, erectile activity, and inflammation. These G-protein-coupled receptors (GPCRs) are targets for drug discovery in a number of areas, including cancer, pain, and obesity therapeutics. All compounds from this series tested to date are antagonists which bind with high affinity. Importantly, many are highly selective for a particular MCR subtype, including some of the first completely hMC5R-selective antagonists reported.
Keywords: Melanocortins, Peptide mimetics, GPCRs
The human melanocortin receptors (hMCRs) comprise a family of five Type I, or rhodopsin-like, G-protein-coupled receptors (GPCRs) to which a wide array of biological functions has been ascribed.1 Some examples include nociception, inflammation, energy balance, and sexual function. From the early understanding of the role MCRs play in pigmentation to recent revelations concerning their relevance to pain, new studies have continually uncovered crucial but previously unknown actions of this receptor system. Beyond advancing our knowledge of basic biology, the understanding and modulation of MCR function also has clinical relevance, with potential therapeutic value for addressing obesity,2 cachexia,3 pain,4 inflammatory diseases,5 and sexual dysfunction,6 as well as the diagnosis and treatment of certain cancers.7
Much research to date has relied on natural and synthetic peptide ligands for these receptors. The MCRs are unique in that both endogenous agonists (α-, β-, γ-MSH, ACTH) and antagonists (agouti, AGRP) for the system have been discovered.8 Each of the agonists contains the His-Phe-Arg-Trp tetrad, the minimum sequence necessary for activation of all melanocortin receptors.9 Both endogenous antagonists contain an Arg-Phe-Phe sequence. Extensive melanotropin peptide structure–activity relationship (SAR) studies by our group and others have identified modifications which enhance potency, stability, or selectivity.10 The value of the ligands generated—particularly the standard agonists NDP-α-MSH and MT-II, and the antagonist, SHU9119, is hard to overestimate.
Nevertheless, with respect to certain applications in biology and medicine, the possession of small molecules with activity at the MCRs and properties complementary to those of peptides would be advantageous. Considerable effort in both academic and industrial laboratories has been directed toward this end.11 Successful examples have come from screening libraries,12 ‘privileged structure’ design strategies,13 and ligand-based rational design using computational chemistry.14
We decided to employ this latter modeling approach to guide our design of small molecule peptide mimetics. Careful consideration of potential molecular scaffolds for β-turn mimetics led us to the bicyclic structure in Figure 1. From a synthetic point of view, compounds of this type are easily constructed from amino acids, allowing us to take advantage both of the naturally available chiral pool and our group's extensive repertoire of unnatural amino acid syntheses.15
Figure 1.
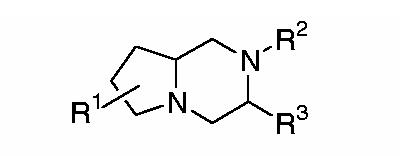
General structure of peptide mimetics.
Interestingly, none of our initial target molecules had been previously reported. Despite the prevalence of piperazine-based structures in medicinal chemistry,16,17 the pyrrolopiperazine moiety remains surprisingly underutilized.18 On the other hand, diketopiperazines, including the cyclodipeptides that are key precursors to our final structures, are found in a wide variety of natural products and synthetic ligands.19-21 As a consequence, a significant body of methodology for the synthesis of these molecules has been developed.22
The functional groups appended to our template were chosen based on SAR both for peptide ligands and previous MCR-targeted small molecules. As noted above, peptides active at all five MCRs contain the His-Phe-Arg-Trp sequence, while the minimum chemical features seemingly common to all active small molecules are the presence of two hydrophobic aromatic groups and a basic nitrogen. In the design of our first set of compounds, we intended to explore the effect of variations in the type and orientation of the hydrophobic groups and of the presence or absence of arginine.
All four stereoisomeric variants of a number of such compounds were modeled and compared to the solution conformations23 of MT-II, a superpotent, nonselective agonist for the MCRs.24 One relatively simple example is shown in Figure 2. Spectroscopy and molecular modeling have suggested that the receptors recognize a β-turn structure in MT-II, probably centered at the histidine and phenylalanine residues.23 The new structures with the best overlap against MT-II were predicted to have the best biological activity. We also hoped to identify structurally distinct features of molecules within this subset which demonstrated greater receptor subtype selectivity. Thus, the synthesis and biological evaluation of multiple analogs, some with better overlap than others, would allow us to simultaneously probe the structural requirements of MCRs and to test the validity of our model.
Figure 2.
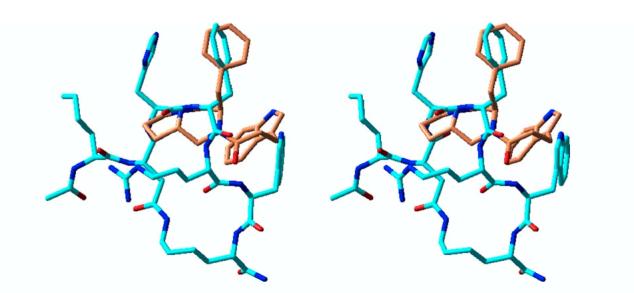
Superimposition of computational model of HMC001 (orange) and NMR-derived structure of MT-II (cyan).23
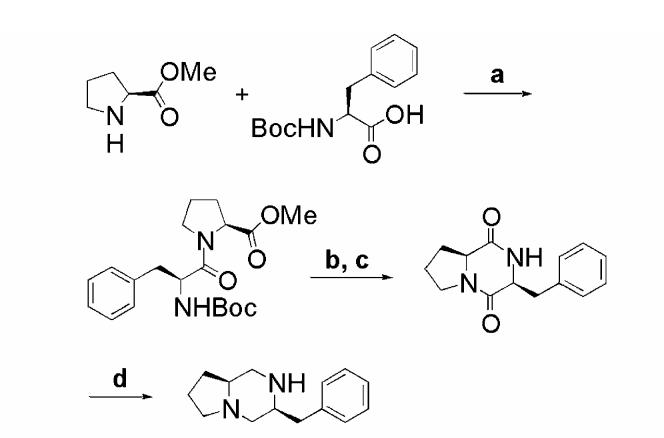
The synthesis of our first set of analogs began with the construction of the core template from l-proline and d-or l-phenylalanine (Scheme 1, l-phenylalanine shown). The dipeptide of these amino acids was easily obtained by BOP-mediated coupling. Deprotection of the Boc-protected amine using TFA was followed by cyclization in triethylamine and methanol to yield the diketopiperazine. Reduction with LAH provided the diamine core structure.
Functionalization of this core structure could be achieved with a variety of acylation chemistries (Scheme 2). Reaction with phenylacetyl chloride provided HMC001 (3S,6S) and HMC002 (3S,6R). A convenient means of introducing a spacer was the reaction with succinic anhydride, followed by amide bond coupling, to generate HMC013 (3S, 6S) and HMC014 (3S,6R). This coupling reaction itself, using BOP, was convenient and effective for functionalizing the core secondary amine, and was used with indolepropionic acid to access HMC009 (3S, 6S) and HMC010 (3S,6R). The same coupling chemistry was used to produce HMC021 and HMC025. All compounds were purified using RP-HPLC.
Biological assays of these compounds were performed using human melanocortin receptors (hMCRs) expressed in whole HEK293 cells.25 Binding was determined by measuring competition with 125I-labeled NDP-α-MSH.26 Binding efficiency refers to the degree of displacement of the radiolabeled ligand by the compound being assayed, relative to the maximum displacement caused by excess amounts of MT-II. Functional activity (activation of adenylate cyclase) was determined by measuring the cAMP produced by the cells after its incubation with ligand.26
Scheme 1.
Reagents: (a) BOP, HOBt, DIEA, DMF; (b) TFA; (c) TEA, MeOH; (d) LiAlH4, THF.
Scheme 2.
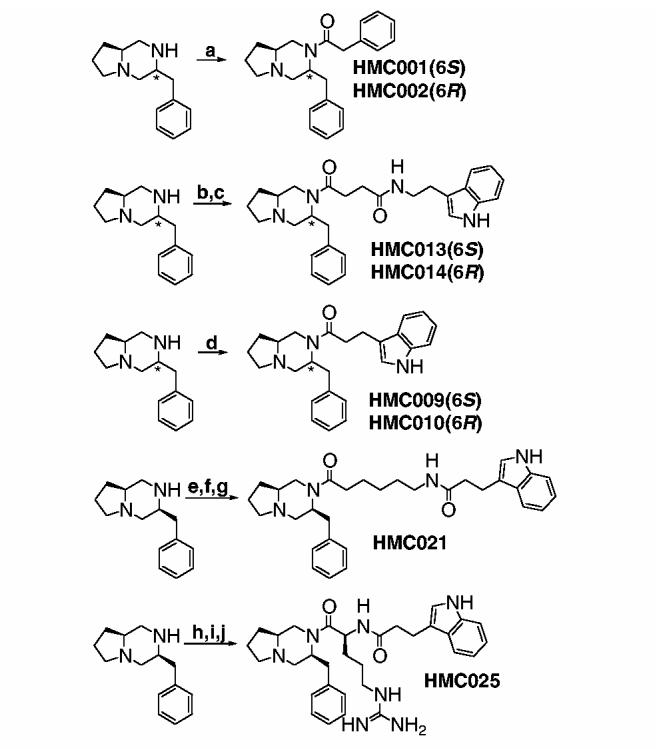
Reagents: (a) Phenylacetyl chloride, DIEA, DMAP, DCM; (b) succinic anhydride, DMAP; (c) tryptamine, BOP, HOBt, DIEA, DMF; (d) indolepropioinic acid, BOP, HOBt, DIEA, DMF; (e) Boc-6-aminohexanoic acid, BOP, HOBt, DIEA, DMF; (f) TFA; (g) indolepropionic acid, BOP, HOBt, DIEA, DMF; (h) Boc-l-arginine, BOP, HOBt, DIEA, DMF; (i) TFA; (j) indolepropionic acid, BOP, HOBt, DIEA, DMF.
The assay results for our first set of compounds (Table 1) are very encouraging. Each of the analogs tested thus far binds to one or more of the receptor subtypes, and most with high selectivity. HMC001, for instance, binds strongly to MC1, with greater than 150-fold selectivity over MC3, and no measurable binding at other subtypes. HMC002, with the opposite stereochemistry at C6, also binds strongly to MC1. The effect of switching the chirality of C6 in the analogs containing an indolepropionyl group is dramatic. HMC009 binds only to MC5, while HMC010 binds to MC1 and MC3. For both HMC009 and HMC010, only 50% displacement of radioligand is observed at each relevant receptor subtype.
Table 1.
Binding and second messenger bioassay results
| hMC1R |
hMC3R |
hMC4R |
hMC5R |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50a (nM) | B.E.b (%) | EC50c (nM) | Act. % | IC50 (nM) | B.E. (%) | EC50 (nM) | Act. % | IC50 (nM) | B.E. (%) | EC50 (nM) | Act. % | IC50 (nM) | B.E. (%) | EC50 (nM) | Act. % | |
| HMC001 | 2.2(±0.4) | 80 | >10,000 | 0 | 350(±60) | 70 | >10000 | 0 | >10,000 | — | 2.7 | 30 | >10,000 | — | >10000 | 0 |
| HMC002 | 1.3(±0.3) | 90 | >10,000 | 0 | 5.0(±0.5) | 30 | >10000 | 0 | 1.2(±0.5) | 30 | >10000 | 0 | >10,000 | — | >10000 | 0 |
| HMC009 | >10,000 | — | >10,000 | 0 | >10,000 | — | >10000 | 0 | >10,000 | — | >10000 | 0 | 0.8 (±0.2) | 50 | >10000 | 0 |
| HMC010 | 4.0(±1.0) | 50 | >10,000 | 0 | 1.2(±0.3) | 50 | >10000 | 0 | >10,000 | — | >10000 | 0 | >10,000 | — | >10000 | 0 |
| HMC013 | >10,000 | — | >10,000 | 0 | >10,000 | — | >10000 | 0 | >10,000 | — | >10000 | 0 | 0.5 (±0.1) | 50 | >10000 | 0 |
| HMC014 | >10,000 | — | >10,000 | 0 | >10,000 | — | >10000 | 0 | >10,000 | — | >10000 | 0 | 0.1(±0.01) | 60 | >10000 | 0 |
| HMC021 | >10,000 | — | >10,000 | 0 | >10,000 | — | >10000 | 0 | >10,000 | — | >10000 | 0 | 0.4 (±0.1) | 65 | >10000 | 0 |
| HMC025 | >10,000 | — | >10,000 | 20 | >10,000 | — | >10000 | 0 | >10,000 | — | >10000 | 0 | 0.1(±0.01) | 60 | >10000 | 0 |
| MT-II | 0.10(±0.01) | 100 | 1.0(±0.2) | 100 | 1.9(±0.3) | 100 | 2.4(±0.4) | 100 | 1.8(±0.4) | 100 | 2.3(±0.5) | 100 | 7.0(±2.2) | 100 | 8.0(±2.4) | 100 |
IC50, concentration of compound at 50% specific binding. Values are means of three experiments done in duplicates; standard deviation is given in parentheses.
Binding Efficiency (maximum radioligand displacement by cmpd/maximum radioligand displacement by MT-II).
EC50, effective concentration of compound that was able to generate 50% maximal intracellular cAMP accumulation. Compounds were tested at a range of concentrations from 10−10 to 10−5 M.
Each of the remaining members of this test set contains an indole ring separated from the bicyclic core by a relatively long linker group. The biological results for all of these compounds are remarkably similar, exhibiting complete MC5 selectivity, with subnanomolar IC50 values and 50–65% binding efficiency. The presence of arginine in HMC025 does not appear to have a significant effect on binding affinity or cAMP accumulation.
The discovery of five new MC5-selective antagonists (HMC009, HMC013, HMC014, HMC021, and HMC025) may prove useful for the study of this relatively unexplored receptor subtype. The most widely expressed of the MCRs, the MC5R has been linked to energy homeostasis27 and thermoregulation.28 Interesting recent work has identified a putative behavioral role in the regulation of aggression related to pheromone signaling in mice.29 Chemical biology using a small molecule may offer advantages over the use of MC5R knockout mice in future studies.
Most importantly, we have developed a new small molecule template which provides compounds recognized by the target receptors. The inability of these ligands to completely displace 125I-NDP-α-MSH, however, suggests the possibility of an allosteric binding mode. This interaction may occur at a site distinct from or partially overlapping the residues which bind standard ligands, with the induction of an alternative receptor conformation having different reactivity toward intracellular components. Additional studies will be needed to clarify this issue, and allow a more clear assessment of the role that β-turn mimicry plays in molecular recognition for this series.
In addition, new analogs are being produced with the goals of achieving selectivity at each receptor subtype and engendering functional agonism through rational substitutions on the core structure. A full paper describing the results of these studies will follow shortly.
References and notes
- 1.(a) Cone RD, editor. The Melanocortin Receptors. The Humana Press, Inc.; New Jersey: 2000. For an introduction, see: [Google Scholar]; (b) Hadley ME, editor. The Melanotropic Peptides. CRC Press; Boca Raton: 1989. [Google Scholar]; (c) Eberle AN, editor. The Melanotropins: Chemistry, Physiology, and Mechanism of Action. Karger; Basel: 1988. [Google Scholar]
- 2.(a) MacNeil DJ, et al. Eur. J. Pharmacol. 2002;450:93. doi: 10.1016/s0014-2999(02)01989-1. [DOI] [PubMed] [Google Scholar]; (b) Harrold J, Pinkney J, Williams G. Drug Discovery Today Ther. Targets. 2004:219. [Google Scholar]; (c) Cheetham SC, Jackson HC, Vickers SP, Dickinson K, Jones RB, Heal DJ. Drug Discovery Today Ther. Targets. 2004:227. [Google Scholar]; (d) Farrigan C, Pang K. Nat. Rev. Drug Disc. 2002;1:257. doi: 10.1038/nrd781. [DOI] [PubMed] [Google Scholar]; (e) Duhl DM, Boyce RS. Annu. Rep. Med. Chem. 2003;38:239. [Google Scholar]; (f) Crowley VEF, Yeo GSH, O'Rahilly S. Nat. Rev. Drug Disc. 2002;1:276. doi: 10.1038/nrd770. [DOI] [PubMed] [Google Scholar]; (g) Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Nature. 1997;385:165. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 3.Scarlett JM, Marks DL. Exp. Opin. Invest. Drugs. 2005;14:1233. doi: 10.1517/13543784.14.10.1233. [DOI] [PubMed] [Google Scholar]
- 4.(a) Vrinten DH, Kalkman CJ, Adan RAH, Gispen WH. Eur. J. Pharmacol. 2001;429:61. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]; (b) Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KVS, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4867. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Starowicz K, Obara I, Przewlocki R, Przewlocka B. Pain. 2005;117:401. doi: 10.1016/j.pain.2005.07.003. [DOI] [PubMed] [Google Scholar]; (d) Vrinten DH, Gispen WH, Kalkman CJ, Adan RAH. Anesthesiology. 2003;99:449. doi: 10.1097/00000542-200308000-00028. [DOI] [PubMed] [Google Scholar]; (e) Bertorelli R, Fredduzzi S, Tarozzo G, Campanella M, Grundy R, Beltramo M, Reggiani A. Behav. Brain Res. 2005;157:55. doi: 10.1016/j.bbr.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Catania A, Gatti S, Colombo G, Lipton JM. Pharmacol. Rev. 2004;56:1. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 6.(a) Andersson K, Hedlund P. Int. J. Impot. Res. 2002;14:S82. doi: 10.1038/sj.ijir.3900797. [DOI] [PubMed] [Google Scholar]; (b) Hafron J, Christ GJ. Drug Discovery Today Ther. Strat. 2004;1:249. [Google Scholar]; (c) Pfaus JG, Shadiack A, Van Soest T, Tse M, Molinoff P. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10201. doi: 10.1073/pnas.0400491101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wessels H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. J. Urol. 1998;160:389. [PubMed] [Google Scholar]; (e) Wessels H, Hruby VJ, Hackett J, Han G, Balse-Srinivasan P, Vanderah TW. Neuroscience. 2003;118:755. doi: 10.1016/s0306-4522(02)00866-7. [DOI] [PubMed] [Google Scholar]
- 7.(a) Eves PC, MacNeil S, Haycock JW. Peptides. 2006;27:444. doi: 10.1016/j.peptides.2005.01.027. [DOI] [PubMed] [Google Scholar]; (b) Zwermann O, Schulte DM, Reincke M, Beuschlein F. Eur. J. Endocrinol. 2005;153:435. doi: 10.1530/eje.1.01983. [DOI] [PubMed] [Google Scholar]; (c) Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. J. Nucl. Med. 2004;45:116. [PubMed] [Google Scholar]; (d) McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. J. Med. Chem. 2005;48:2985. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]; (e) Sharma SD, Jiang J, Hadley ME, Bentley DL, Hruby VJ. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13715. doi: 10.1073/pnas.93.24.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Lee TH, Lerner AB. J. Biol. Chem. 1956;221:943. α-MSH: [PubMed] [Google Scholar]; (b) Li CH, Geschwind II, Dixon JS, et al. J. Biol. Chem. 1955;213:171. ACTH: [PubMed] [Google Scholar]; (c) Yalow RS, Berson SA. J. Clin. Endocrinol. Metab. 1973;36:415. doi: 10.1210/jcem-36-3-415. [DOI] [PubMed] [Google Scholar]; (d) Mains RE, Eipper BA, Ling N. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3014. doi: 10.1073/pnas.74.7.3014. β-MSH: [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Roberts JL, Herbert E. Proc. Natl. Acad. Sci. U.S.A. 1977;74:4826. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nakanishi SA, Inoue S, et al. FEBS Lett. 1977;84:105. doi: 10.1016/0014-5793(77)81067-3. [DOI] [PubMed] [Google Scholar]; (g) Nakanishi S, Inoue A, Kita T, Nakamura M, Chang ACY, Cohen SN, Numa S. Nature. 1979;278:423. doi: 10.1038/278423a0. γ-MSH: [DOI] [PubMed] [Google Scholar]; (h) Bultman SJ, Michaud EJ, Woychik RP. Cell. 1992;71:1195. doi: 10.1016/s0092-8674(05)80067-4. agouti: [DOI] [PubMed] [Google Scholar]; (i) Kwon HY, Bultman SJ, Loffler C, Chen W-J, Furdon PJ, Powell JG, Usala A-L, Wilkison W, Hansmann I, Woychik RP. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9760. doi: 10.1073/pnas.91.21.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Wilson BD, Ollmann MM, Kang L, Stoffel M, Bell GI, Barsh GS. Hum. Mol. Gen. 1995;4:223. doi: 10.1093/hmg/4.2.223. [DOI] [PubMed] [Google Scholar]; (k) Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS. Genes Dev. 1993;7:454. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]; (l) Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Genes Dev. 1997;11:593. doi: 10.1101/gad.11.5.593. AGRP: [DOI] [PubMed] [Google Scholar]; (m) Ollmann MM, Wilson BD, Yang Y-K, Kerns JA, Chen Y, Gantz I, Barsh GS. Science. 1997;278:135. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 9.(a) Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, deVaux AE, Dym O, Castrucci AML, Hintz MF, Riehm JP, Rao KR. J. Med. Chem. 1987;30:2126. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]; (b) Castrucci AML, Hadley ME, Sawyer TK, Wilkes BC, Al-Obeidi F, Staples DJ, de Vaux AE, Dym O, Hintz MF, Riehm JP, Rao KR, Hruby VJ. Gen. Comp. Endocrinol. 1989;73:157. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 10.(a) Fung S, Hruby VJ. Curr. Opin. Chem. Biol. 2005;9:352. doi: 10.1016/j.cbpa.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Irani BG, Holder JR, Todorovic A, Wilczynski AM, Joseph CG, Wilson KR, Haskell-Luevano C. Curr. Pharm. Des. 2004;10:3443. doi: 10.2174/1381612043382891. [DOI] [PubMed] [Google Scholar]; (c) Sebhat I, Ye Z, Bednarek M, Weinberg D, Nargund R, Fong TM. Annu. Rep. Med. Chem. 2003;38:31. [Google Scholar]; (d) Holder JR, Haskell-Luevano C. Med. Res. Rev. 2004;24:325. doi: 10.1002/med.10064. [DOI] [PubMed] [Google Scholar]; (e) Bednarek MA, Fong TM. Exp. Opin. Ther. Patents. 2004;14:327. [Google Scholar]; (f) Hruby VJ, Cai M, Grieco P, Han G, Kavarana M, Trivedi D. Ann. N.Y. Acad. Sci. 2003;994:12. doi: 10.1111/j.1749-6632.2003.tb03157.x. [DOI] [PubMed] [Google Scholar]
- 11.Todorovic A, Haskell-Luevano C. Peptides. 2005;26:2026. doi: 10.1016/j.peptides.2004.11.024. See Ref. 10; also: [DOI] [PubMed] [Google Scholar]
- 12.(a) Arasasingham PN, Fotsch C, Ouyang X, Norman MH, Kelly MG, Stark KL, Karbon B, Hale C, Baumgartner JW, Zambrano M, Cheetham J, Tamayo NA. J. Med. Chem. 2003;46:9. doi: 10.1021/jm0255522. [DOI] [PubMed] [Google Scholar]; (b) Tian X, Field T, Mazur AW, Ebetino FH, Wos JA, Crossdoersen D, Pinney BB, Sheldon RJ. Bioorg. Med. Chem. Lett. 2005;15:2819. doi: 10.1016/j.bmcl.2005.03.120. [DOI] [PubMed] [Google Scholar]; (c) Pan K, Scott MK, Lee DHS, Fitzpatrick LJ, Crooke JJ, Rivero RA, Rosenthal DI, Vaidya AH, Zhao B, Reitz AB. Bioorg. Med. Chem. 2003;11:185. doi: 10.1016/s0968-0896(02)00428-5. [DOI] [PubMed] [Google Scholar]
- 13.(a) Sebhat IK, Martin WJ, Ye Z, Barakat K, Mosley RT, Johnston DBR, Bakshi R, Palucki B, Weinberg DH, MacNeil T, Kalyani RN, Tang R, Stearns RA, Miller RR, Tamvakopoulos C, Strack AM, McGowan E, Cashen DE, Drisko JE, Hom GJ, Howard AD, MacIntyre DE, van der Ploeg LHT, Patchett AA, Nargund RP. J. Med. Chem. 2002;45:4589. doi: 10.1021/jm025539h. [DOI] [PubMed] [Google Scholar]; (b) Herpin TF, Yu G, Carlson KE, Morton GC, Wu X, Kang L, Tuerdi H, Khanna A, Tokarski R, Lawrence M, Macor J. J. Med. Chem. 2003;46:1123. doi: 10.1021/jm025600i. [DOI] [PubMed] [Google Scholar]; (c) Fisher MJ, Backer RT, Husain S, Hsiung HM, Mullaney JT, O'Brian TP, Ornstein PL, Rothhaar RR, Zgombick JM, Briner K. Bioorg. Med. Chem. Lett. 2005;15:4459. doi: 10.1016/j.bmcl.2005.07.035. [DOI] [PubMed] [Google Scholar]; (d) Richardson TI, Ornstein PL, Briner K, Fisher MJ, Backer RT, Biggers CK, Clay MP, Emmerson PJ, Hertel LW, Hsiung HM, Husain S, Kahl SD, Lee JA, Lindstrom TD, Martinelli MJ, Mayer JP, Mullaney JT, O'Brien TP, Pawlak JM, Revell KD, Shah J, Zgombick JM, Herr RJ, Melekhov A, Sampson PB, King CR. J. Med. Chem. 2004;47:744. doi: 10.1021/jm0304109. [DOI] [PubMed] [Google Scholar]
- 14.Fotsch C, Smith DM, Adams JA, Cheetham J, Croghan M, Doherty EM, Hale C, Jarosinski MA, Kelly MG, Norman MH, Tamayo NA, Xi N, Baumgartner JW. Bioorg. Med. Chem. Lett. 2003;13:2337. doi: 10.1016/s0960-894x(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 15.(a) Zhang JY, Wang W, Xiong CY, Hruby VJ. Tetrahedron Lett. 2003;44:1413. For selected examples, see: [Google Scholar]; (b) Wang W, Cai MY, Xiong CY, Zhang JY, Trivedi D, Hruby VJ. Tetrahedron. 2002;58:7365. [Google Scholar]; (c) Xiong CY, Wang W, Cai CZ, Hruby VJ. J. Org. Chem. 2002;67:1399. doi: 10.1021/jo010860d. [DOI] [PubMed] [Google Scholar]; (d) Soloshonok VA, Tang XJ, Hruby VJ. Tetrahedron. 2001;57:6375. [Google Scholar]; (e) Han GX, Lewis A, Hruby VJ. Tetrahedron Lett. 2001;42:4601. [Google Scholar]; (f) Tamaki M, Han GX, Hruby VJ. J. Org. Chem. 2001;66:3593. doi: 10.1021/jo005625u. [DOI] [PubMed] [Google Scholar]; (g) Li GG, Patel D, Hruby VJ. Tetrahedron: Asymmetry. 1993;4:2315. [Google Scholar]; (h) Boteju LW, Hruby VJ. Tetrahedron Lett. 1993;34:1757. [Google Scholar]; (i) Valle G, Kazmierski WM, Crisma M, Bonora GM, Toniolo C, Hruby VJ. Int. J. Pept. Protein Res. 1992;40:222. [PubMed] [Google Scholar]
- 16.(a) Bhatt V, Trivedi AC, Narula RC. Chem. Eng. World. 1990;25:75. For reviews, see: [Google Scholar]; (b) Murasaki M, Miura S. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1992;16:833. doi: 10.1016/0278-5846(92)90103-l. [DOI] [PubMed] [Google Scholar]; (c) Ye L. Huagong Shikan. 1997;11:33. [Google Scholar]; (d) Coudert P, Rubat C, Bastide M, Malhuret R, Chopineau J. J. Pharm. Belg. 1995;50:445. [PubMed] [Google Scholar]; (e) Li R, Chen H, Yang J. Huaxue Tongbao. 1992;3:7. [Google Scholar]; (f) Popescu M. Farmacia. 1972;20:535. [Google Scholar]; (g) Arbasino M, Corona GL. Boll. Chim. Farmac. 1964;103:226. [PubMed] [Google Scholar]; Dolle RE. Mol. Divers. 1998;3:199. doi: 10.1023/a:1009699413828. Also note the frequency with which piperazine structures are found in biologically active molecules from chemical libraries: [DOI] [PubMed] [Google Scholar]
- 17.(a) Bakshi RK, Guo L, Hong Q, Nargund RP, Pollard PG, Sebhat IK, Ujjainwalla F, Ye Z. U.S. Patent Application 0204398. 2004 The melanocortin field is no exception. For a partial summary, see Ref. 11. A number of examples may be found in the patent literature, as well. Some include:; (b) Fotsch CH, et al. U.S. Patent Application 0220324 2003; (c) Sharma SD, Shi Y-Q, Wu Z, Rajpurohit R. U.S. Patent Application 0157264 2004; (d) Sharma SD, Shi Y-Q, Rajpurohit R, Wu Z, Purma P, Shadiack AM, Burris KD. U.S. Patent Application 0224957 2004; (e) Sharma SD, Shi Y-Q, Wu Z, Rajpurohit R. U.S. Patent Application 0152134 2004
- 18.(a) Long Y-Q, Mou Q-Y, Qiu D-P, Wu R-Q. Chin. J. Chem. 2002;20:1073. The pyrrolopiperazine core structure has been used in the synthesis of potential κ-opioid agonists. [Google Scholar]; (b) Peng H, et al. J. Med. Chem. 2004;47:6218. doi: 10.1021/jm0494321. This moiety is also employed in substituents to other scaffolds in a library of adenosine A2A receptor antagonists: [DOI] [PubMed] [Google Scholar]; (c) Omura S, Hirano A, Iwai Y, Masuma R. J. Antibiot. 1979;32:786. doi: 10.7164/antibiotics.32.786. The structure also appears in the natural product Herquline A, isolated from both terrestrial and marine fungi: [DOI] [PubMed] [Google Scholar]; (d) Kagata T, Shigemori H, Mikami Y, Kobayashi J. J. Nat. Prod. 2000;63:886. doi: 10.1021/np000034k. [DOI] [PubMed] [Google Scholar]
- 19.(a) Prasad C. Peptides. 1995;16:151. doi: 10.1016/0196-9781(94)00017-z. Cyclic dipeptides have been shown to exhibit a range of interesting biological activities: [DOI] [PubMed] [Google Scholar]; (b) Walter R, Ritzmann RF, Bhargava HN, Flexner LB. Proc. Natl. Acad. Sci. U.S.A. 1979;76:518. doi: 10.1073/pnas.76.1.518. Intriguingly, these effects include the inhibition of the development of physical dependence on morphine in mice by cyclo(Pro-Phe): [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ienaga K, Nakamura K, Kurohashi M, Nakanishi T, Ichii T. Phytochemistry. 1990;29:35. The actions of cyclic dipeptides are also not confined to animals, as illustrated by the protective effect conferred to rice plants against low-temperature and dehydrating conditions by cyclo(Hyp-Pro) and cyclo(Hyp-Leu): [Google Scholar]
- 20.(a) Cui C-B, Kakeya H, Osada H. Tetrahedron. 1996;52:12651. Some examples of more complex natural products containing the diketopiperazine structure are: Spirotryprostatin A and B: [Google Scholar]; (b) Cui C-B, Kakeya H, Okada G, Onose R, Ubukata M, Takahashi I, Isono K, Osada H. J. Antibiot. 1995;48:1382. doi: 10.7164/antibiotics.48.1382. Tryprostatins A and B: [DOI] [PubMed] [Google Scholar]; (c) Yamazaki M, Suzuki S, Miyaki K. Chem. Pharm. Bull. 1971;19:1739. doi: 10.1248/cpb.19.1739. Fumitremorgin A,B, and C: [DOI] [PubMed] [Google Scholar]; (d) Yamazaki M, Sasago K, Miyaki K. J. Chem. Soc. Chem. Comm. 1974:408. [Google Scholar]; (e) Cole RJ, et al. J. Agric. Food Chem. 1977;25:826. doi: 10.1021/jf60212a015. [DOI] [PubMed] [Google Scholar]; (f) Hino T, Kawate T, Nakagawa M. Tetrahedron. 1989;45:1941. [Google Scholar]; (g) Funabashi Y, Horiguchi T, Iinuma S, Tanida S, Harada S. J. Antibiot. 1994;47:1202. doi: 10.7164/antibiotics.47.1202. TAN-1496(A-E)/sirodesmin A: [DOI] [PubMed] [Google Scholar]
- 21.(a) Goodfellow VS, Lauderman CP, Gerrity JI, Burkhard M, Strobel E, Zuzack JS, McLeod DA. Mol. Divers. 1996;2:97. doi: 10.1007/BF01718706. Selected applications of diketopiperazines in medicinal chemistry include: Bradykinin antagonists. [DOI] [PubMed] [Google Scholar]; (b) Lazarus LH, Bryant SD, Cooper PS, Guerrini R, Balboni G, Salvadori S. Drug Discovery Today. 1998;3:284. Opioid antagonists: [Google Scholar]; (c) Baures PW, Ojala WH, Gleason WB, Mishra RK, Johnson RL. J. Med. Chem. 1994;37:3677. doi: 10.1021/jm00048a003. D2-binding modulators: [DOI] [PubMed] [Google Scholar]; (d) Baures PW, Ojala WH, Costain WJ, Ott MC, Pradhan A, Gleason WB, Mishra RK, Johnson RL. J. Med. Chem. 1997;40:3594. doi: 10.1021/jm970328b. [DOI] [PubMed] [Google Scholar]; (e) Lopez-Rodriguez ML, Morcillo MJ, Fernandez E, Porras E, Orensanz L, Beneytez ME, Manzanares J, Fuentes JA. J. Med. Chem. 2001;44:186. doi: 10.1021/jm000929u. Mixed 5-HT1A/D2 antagonists: [DOI] [PubMed] [Google Scholar]; (f) Cody WL, et al. Bioorg. Med. Chem. Lett. 1999;9:2497. doi: 10.1016/s0960-894x(99)00418-7. Thrombin inhibitors: [DOI] [PubMed] [Google Scholar]; (g) Cody WL, et al. Bioorg. Med. Chem. Lett. 1999;9:2503. doi: 10.1016/s0960-894x(99)00419-9. [DOI] [PubMed] [Google Scholar]; (h) Szardenings AK, et al. J. Med. Chem. 1999;42:1348. doi: 10.1021/jm980475p. Matrix Metalloproteinase/Collagenase-1 inhibitors: [DOI] [PubMed] [Google Scholar]; (i) Szardenings AK, et al. J. Med. Chem. 1998;41:2194. doi: 10.1021/jm980133j. [DOI] [PubMed] [Google Scholar]
- 22.(a) Fischer PM. J. Pept. Sci. 2003;9:2003. doi: 10.1002/psc.446. [DOI] [PubMed] [Google Scholar]; (b) Dinsmore CJ, Beshore DC. Tetrahedron. 2002;58:3297. [Google Scholar]; (c) Horton DA, Bourne GT, Smythe ML. Mol. Divers. 2000;5:289. doi: 10.1023/a:1021365402751. [DOI] [PubMed] [Google Scholar]; (d) Robinson JA. Synlett. 1999;4:429. [Google Scholar]; (e) Li W-R, Yang JH. J. Comb. Chem. 2002;4:106. doi: 10.1021/cc010055c. [DOI] [PubMed] [Google Scholar]; (f) Bianco A, Sonksen CP, Roepstorff P, Briand J-P. J. Org. Chem. 2000;65:2179. doi: 10.1021/jo991818+. [DOI] [PubMed] [Google Scholar]; (g) Perrotta E, Altamura M, Barani T, Bindi S, Giannotti D, Harmat NJS, Nannicini R, Maggi CA. J. Comb. Chem. 2001;3:453. doi: 10.1021/cc0000904. [DOI] [PubMed] [Google Scholar]; (h) Krchnak V, Weichsel AS, Cabel D, Flegelova Z, Lebl M. Mol. Divers. 1996;1:149. doi: 10.1007/BF01544953. [DOI] [PubMed] [Google Scholar]; (i) Nefzi A, Giulianotti MA, Houghten RA. Tetrahedron. 2000;56:3319. [Google Scholar]; (j) Wang D-X, Liang M-T, Tian G-J, Lin H, Liu H-Q. Tetrahedron Lett. 2002;43:865. [Google Scholar]
- 23.Ying J, Kover KE, Gu X, Han G, Trivedi DB, Kavarana MJ, Hruby VJ. Biopolymers (Pept. Sci.) 2003;71:696. doi: 10.1002/bip.10596. [DOI] [PubMed] [Google Scholar]
- 24.Al-Obeidi F, Hadley ME, Pettitt BM, Hruby VJ. J. Am. Chem. Soc. 1989;111:3413. [Google Scholar]
- 25.(a) Haskell-Luevano C, Miwa H, Dickinson C, Hruby VJ, Yamada T, Gantz I. Biochem. Biophys. Res. Commun. 1994;204:1137. doi: 10.1006/bbrc.1994.2581. [DOI] [PubMed] [Google Scholar]; (b) Cai M, Cai C, Mayorov AV, Xiong C, Cabello CM, Soloshonok VA, Swift JR, Trivedi D, Hruby VJ. J. Pept. Res. 2004;63:116. doi: 10.1111/j.1399-3011.2003.00105.x. [DOI] [PubMed] [Google Scholar]; (c) Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, Delvalle J, Yamada T. J. Biol. Chem. 1993;268:15174. [PubMed] [Google Scholar]
- 26.Cai M, Mayorov AV, Cabello C, Stankova M, Trivedi D, Hruby VJ. J. Med. Chem. 2005;48:1839. doi: 10.1021/jm049579s. [DOI] [PubMed] [Google Scholar]
- 27.Chagnon YC, Chen WJ, Perusse L, Chagnon M, Nadeau A, Wilkison WO, Bouchard C. Mol. Med. 1997;3:663. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Cell. 1997;91:789. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 29.(a) Morgan C, Thomas RE, Cone RD. Horm. Behav. 2004;45:58. doi: 10.1016/j.yhbeh.2003.08.004. [DOI] [PubMed] [Google Scholar]; (b) Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD. Chem. Senses. 2004;29:111. doi: 10.1093/chemse/bjh011. [DOI] [PubMed] [Google Scholar]