Abstract
CD45, the leucocyte common antigen, is a haematopoietic cell specific tyrosine phosphatase. Human polymorphic CD45 variants are associated with autoimmune and infectious diseases and alter the phenotype and function of lymphocytes, establishing CD45 as an important regulator of immune function. Here we report four patients with diverse diseases with unusual clinical features. All four have the C77G polymorphism of CD45 exon 4, which alters the splicing and CD45RA/CD45R0 phenotype of lymphocytes. We suggest that C77G may be a contributing factor in these unusual cases.
Keywords: abnormal splicing, CD45, immunodeficiency, polymorphism
Introduction
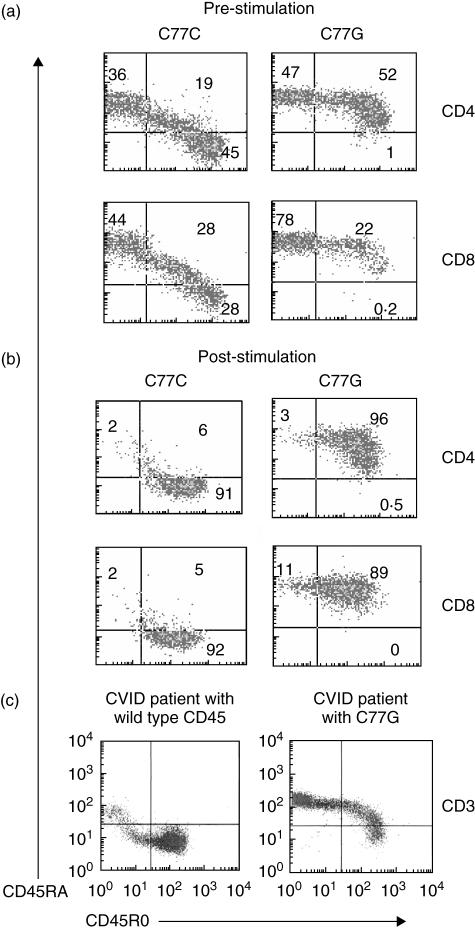
The CD45 (leucocyte common) antigen is a haematopoietic cell specific tyrosine phosphatase essential for efficient T- and B-cell antigen receptor signal transduction [1,2]. Multiple CD45 isoforms can be generated by complex alternative splicing of exons 4 (A), 5 (B) and 6 (C), in the extracellular domain of the molecule. Expression of different CD45 isoforms is dependent on the stage of differentiation and state of activation of haematopoietic cells. In man, naive T lymphocytes express high molecular weight isoforms containing the A exon (‘CD45RA’ cells), but following activation the low molecular weight 180 kDa isoform is expressed (‘CD45R0’cells) (Fig. 1a, b, left hand panels).
Fig. 1.
Flow cytometric analysis of C77G variant individuals. PBMC from two healthy individuals, one C77C (wild-type) homozygote and one C77G heterozygote, were separated by ficoll/hypaque gradient centrifugation and stained with CD45RO-PE, CD45RA-FITC and either CD4 or CD8-APC antibodies pre- (a) and post-stimulation (b) with PHA for 10 days. Analysis was performed on CD4 or CD8 gated cells. Normal expression is characterized by the loss of CD45RA and gain of CD45RO expression upon activation. Variant C77G expression is characterized by the absence of a single CD45RO+ population. Even after stimulation for 10 days C77G cells remain double CD45RA+/CD45RO+. (c) Flow cytometric analysis of CD45 splicing in CVID patients. PBMC were isolated by ficoll/hypaque separation and triple stained with CD3-APC, CD45RA-FITC and CD45-RO antibodies. Analysis of the CD3 gated population is shown. Variant CD45 splicing in the C77G patient with prolonged poliovirus excretion can be identified by the absence of the single CD45RO+ population. The C77C CVID individual shows a depletion of CD45RA (naïve) and increase in CD45R0 (memory/activated) T cells.
CD45 is highly polymorphic in many species [3]. The most extensively studied human CD45 polymorphism is the C77G point mutation in a splice silencer region of exon 4, which prevents excision of the exon. Consequently, memory/effector lymphocytes of C77G carriers express both CD45RA and CD45R0 instead of the normal pattern of low molecular weight CD45R0 expression (Fig. 1a, b, right hand panels). The pattern of staining of lymphocytes from C77G individuals is extremely characteristic (Fig. 1a, b, right hand panels), both ex vivo and after mitogen stimulation. C77G heterozygous individuals are relatively rare, with an allele frequency of 0–3·5% in Europe and North America [3,4]. The allele is absent in samples from Africa (Uganda) or in far eastern Orientals [5].
In epidemiological studies the C77G variant has been reported to be associated with multiple sclerosis in German [6], Italian [7] and American [8] patient cohorts, although not in others [9–13]. An increased frequency of C77G has been found in HIV [14], Langerhans cell histiocytosis [15], systemic sclerosis [16], hepatitis C [17] and autoimmune hepatitis [18], but no association with common variable immunodeficiency (CVID), Graves’ disease or diabetes [10,19,20]. Furthermore, C77G individuals show lymphocyte functional abnormalities, including increased IL-2 production by memory CD4 T cells and an altered threshold for signalling through the T-cell receptor [21,22]. Another polymorphism of CD45, A138G in exon 6, is also associated with altered disease susceptibility and immune function [23,24], and absence of CD45 is also a cause of severe combined immunodeficiency [25–27] There is therefore abundant evidence that altered CD45 expression affects the immune function in man, as in experimental animals [28].
We report here four patients with different conditions presenting with unusual features, all of who carry the C77G polymorphism of CD45, in order to draw attention to the possibility that C77G may be a contributing factor in immune-mediated diseases in which other underlying genetic factors play a major role.
Patient 1: CVID with prolonged poliovirus excretion
The patient was a 49-year-old Caucasian male who presented at the age of 17 with hypogammaglobulinaemia on a background of delayed puberty, intermittent diarrhoea associated with Giardia infection and intestinal nodular lymphoid hyperplasia. He had been fully immunized in infancy with triple vaccine (diphtheria, tetanus and pertussis) and with oral polio vaccine. At presentation, IgG was 3·0 g/l, IgA 0·48 g/l and IgM undetectable. The diagnosis of CVID was made. The patient was lost to follow-up between the ages of 17 and 36, when he entered nursing school, and in view of his occupation, treatment with intramuscular immunoglobulin was commenced, although he did not suffer from recurrent infections. At age 37, following a bout of gastroenteritis, stool culture showed the presence of a non-vaccine strain of Type II poliovirus. He had detectable salivary IgA and secretory piece, but despite immunization three times with (Salk) killed polio vaccine intradermally, he made neither salivary antibody nor a cutaneous delayed-type hypersensitivity response to poliovirus. However, 1 year after the first detection of poliovirus, he spontaneously ceased virus excretion. He has been maintained on replacement immunoglobulin, switching from intramuscular to subcutaneous immunoglobulin at age 46 years [29]. Subsequently, he was discovered to carry the C77G polymorphism of CD45 (Fig. 1c). Because wild-type CVID patients often have disturbed ratios of CD45RA:CD45R0 cells (Fig. 1c, left hand panel), the staining pattern of the C77G CVID patient is also slightly atypical (Fig. 1c, right hand panel). In such cases it is wise to confirm the presence of the polymorphism by sequence analysis or PCR and restriction digestion (Fig. 2a, b), as was done in this case.
Fig. 2.
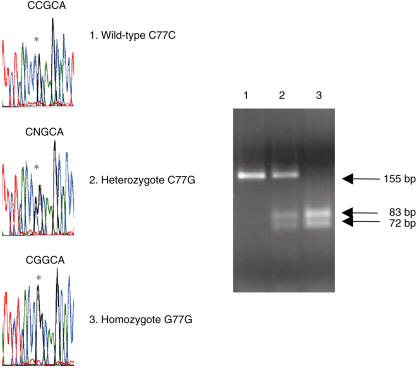
Identification of an exon 4 CD45 G77G homozygote. (a) Sequence analysis of wild-type C77C, heterozygous C77G and homozygous G77G samples. Position 77 of exon 4 is indicated by asterisks. (b) PCR analysis for detection of C77G was performed on wild-type C77C, heterozygous C77G and homozygous G77G genomic DNA, with primers on either side of the site of mutation, amplifying a fragment of 155 bp in wild-type DNA. The C77G transition introduces a new restriction site for MspI, which cleaves the mutant PCR product into two fragments of 72 and 83 bp (34). The absence of an undigested band of 155 bp indicates the presence of homozygous G77G in individual 3.
Patient 2: salmonella splenic abscess
A 9-year-old Spanish boy presented with fever (up to 40 °C) of 4 days duration, with left chest and upper abdominal pain. Abdominal ultrasonography disclosed a single splenic lesion of 6·5 cm diameter, enlarged intra-abdominal lymph nodes and free peritoneal fluid. The diagnosis of a splenic abscess was made but despite treatment with i.v. amoxicillin-clavulanic acid and metronidazol, 24 h later he worsened, with increasing abdominal pain. The white blood cell count was 4·2 × 109/l (83% granulocytes), and bilirubin 41 micromol/l (direct bilirubin 29 micromol/l), AST 43 U/l and ALT 44 U/l. At laparotomy a perforated abscess was found in the upper pole of the spleen and free purulent material in the peritoneal cavity. Culture revealed salmonella group D9, sensitive to ampicillin, gentamicin and cotrimoxazol. Subsequent faecal cultures were negative for salmonella. Brucella, EBV, CMV, echinoccocus and HIV were excluded.
Immunoglobulin levels were normal except for IgE (1058 kU/l), and normal numbers of the main lymphocyte subsets (CD3, CD4, CD8, CD19 and CD56) were found. The lymphoproliferative response to mitogens (PHA, Con A, PWM and CD3) was normal compared with healthy controls. IFNγ production was detected by flow cytometry after PMA-Ionomycin stimulation (12·7% of lymphocytes versus 24% in his healthy father).
The patient was discharged and remains asymptomatic except for allergic symptoms. The patient, his mother and brother carry the C77G polymorphism. They share an allergic background and elevated IgE levels.
Patient 3: recurrent myocarditis
A 31-year-old female with no previous history of smoking, alcohol or drug addiction and no known allergies was admitted with fulminant myocarditis. Six years later she suffered a second episode. She had several urticarial episodes during childhood up to the age of 4 years but no signs or symptoms suggestive of autoimmune disease. No severe or recurrent infections were reported except for oral herpes simplex virus.
On both occasions she presented with tachyrhythmia, nausea and vomiting with no dyspnoea or chest pain, rapidly progressing to cardiovascular collapse, shock and pulmonary oedema. The electrocardiogram revealed sinus tachycardia and ST segment changes, echocardiography showed severe left ventricular dysfunction with ejection fraction < 20%. Elevated serum CPK levels were found. The patient required intensive care during both admissions. Shortly after her second discharge, she presented with a brief reactive psychosis that was attributed to prolonged high-dose steroid therapy and was successfully treated with neuroleptic medications.
Extensive viral and bacteriological investigations and tests for serum IgG anti-enterovirus, toxoplasma, HIV, HCV, CMV, EBV, rubella, Q fever, boutenneuse fever and Chlamydia, were all negative. Blood cultures were sterile. Histology of the myocardium following endomyocardial biopsy performed during the first episode, showed a non-specific pattern of cellular polymorphism, isolated lymphocytes without clear inflammatory infiltration, and a degree of periarteriolar fibrosis.
Rheumatoid factor and CRP and anti-nuclear (ANA), anti-double stranded DNA (dsDNA), anti-Sm, anti-SS Ro, anti-SS La, anti-RNP, anti-smooth muscle (ASMA), antimitochondrial (AMA), anticardiolipin (ACA), antib2 glycoprotein, antiliver kidney microsomal (LKM) and antiliver cytosol (LC-1) antibodies were all negative. Serum IgG, IgA, IgM, complement components C3, C4 and factor B levels were normal in the first episode. By the time of the second episode, IgG had dropped to 5 g/l (normal range 5–7·5 g/l), with decreased IgG1 and no response to tetanus toxoid immunization, while levels of IgA, IgM and IgE remained normal. The transient low IgG in this case was secondary to corticosteroid therapy for the myocarditis (high dose i.v. bolus in the hospital intensive care unit, 6 mg oral prednisone for 6 months following her discharge). The antibody response to immunization subsequently returned to normal, with a pre-immunization level of anti-tetanus toxoid specific IgG of 0·0017 g/l and post-immunization level of > 0·07 g/l. Factor B showed a slight decrease (0·14 and 0·16 g/l) but returned to normal levels within months. Normal numbers of CD3, CD4, CD8 and CD56 positive lymphocytes were found, with an increased percentage (23%) and absolute number of B cells (554 per μl). In vitro lymphoproliferative assay showed high spontaneous proliferation (5646 cpm) but a normal response to mitogens compared with healthy controls. Intracellular IFNγ production was detected by flow cytometry after PMA-Ionomycin stimulation (18% of lymphocytes versus 20% in her healthy mother). Both the patient and her healthy father showed the abnormal pattern of CD45RA/CD45RO expression and the C77G polymorphism in exon 4 of CD45. No immunological defect underlying these severe episodes of myocarditis has been identified, with the exception of abnormal CD45 splicing.
Patient 4: recurrent cold abscesses and wound healing disorder
A 47-year-old female presented 8 years ago with nephrolithiasis and following surgical stone removal exhibited post-operative delay in wound healing and recurrent cold abscesses. Since that time, the patient has never been symptom-free and has had multiple superficial and deep tissue abscesses, predominantly located in the thorax and abdomen, but sometimes in the upper and lower limbs. The inflammatory lesions have always necessitated surgical intervention and the patient has undergone a total of 52 surgical interventions. In all cases, the surgical procedures were followed by an unusual delay in wound healing.
The patient suffers from recurrent mild elevation of body temperature, particularly at night. Bacteriological investigation has always been negative, except for a single focus in the right femur about 2 years ago, from which staphylococci and streptococci were cultured. Multiple courses of combined antibacterial therapies had no effect. Recent administration of high-dose intravenous immunoglobulin (2 g/kg body weight) resulted in partial remission. Self-infliction of the wounds was repeatedly excluded.
The personal and family history was negative for any clinical signs related to primary and secondary immunodeficiency. Prior to the onset of the condition, there were no unusual episodes of bacterial, viral, fungal or parasitic infection and vaccinations were tolerated without complications. Extensive immunological investigation showed normal values for total IgG (11·6 g/l), IgM (1·2 g/l) and IgE (115 Ku/l), but total IgA was slightly elevated. IgG subclass analysis revealed elevation of IgG2 (6·8 g/l) and IgG3 (2·1 g/l), but antigen-specific IgG1 (tetanus toxoid) and IgG2 (pneumococcal polysaccharide) antibodies were in the normal range. Immuno-phenotyping of mononuclear cells by flow-cytometry revealed normal numbers of CD14+ monocytes, CD16+ CD56+ NK T cells and CD19 positive B cells. There were normal proportions of αβ and γδ T cells. Although the number of CD8 T cells was normal, CD4 T cells were reduced, resulting in a CD4:CD8 ratio of 0·9. T cells were markedly activated, as shown by the expression of CD25 and HLA-DR. T-cell proliferation in response to the recall antigens tetanus toxoid, candida antigen and PPD was markedly reduced and could not be restored by addition of exogenous IL-2. However, the proliferative response to anti-CD3 or phorbol ester plus ionomycin was normal. Granulocytes showed normal expression of adhesion molecules, while phagocytosis and respiratory burst activity were also within the normal range (assessed by flow cytometry using labelled beads and E. coli). Furthermore, typical integrin activation defects (LAD) were excluded.
Further phenotyping revealed absence of CD45RA-CD45R0+ T cells, typical of carriers of the C77G polymorphism. RFLP analysis confirmed heterozygosity of the 77G allele in the patient.
Discussion
In addition to the disease associations of CD45 alleles described in earlier cohort studies [3], there have been several reports of C77G detected in small numbers of patients with a variety of diseases, for example, four patients with systemic lupus erythematosus (SLE) [16], one with myasthenia gravis [30], and two families with haemophagocytic lymphohistiocytosis or erythrocytic haemophagocytosis [31,32]. So far no living G77G homozygous sample has been reported. The G77G homozygote shown in Fig. 2 was an anonymous thymic specimen from a patient undergoing cardiac surgery [33,34], so we have no information on the CD45RA/R0 phenotype of homozygous peripheral blood lymphocytes. Nor, although there are several syndromes associating cardiac and immunological abnormalities [35,36], do we have any information on the clinical status of the patient, as we could not obtain this for ethical reasons. Discovery of C77G in all these patients may be an incidental finding but the evidence that immune function is abnormal in humans and experimental animals with altered CD45 expression suggests that these polymorphisms may contribute to disease pathogenesis [21–23,28,37,38].
In none of these cases or those reported here do we consider that the C77G mutation is the primary cause of the disease. However, we suggest that it may act as one of several disease-modifying genes. It is increasingly clear that in autoimmune disease, although a few genes have dominant affects, in all cases several other genes also play a role, modifying the severity and phenotype of the condition [39]. Immunodeficiency and autoimmunity overlap in many ways [40] and the phenotype of most immunodeficiencies is extremely variable [41], strongly suggesting that in immunodeficiency as in autoimmunity, although there may be genes with dominant effects, in most cases several other genes will modify the clinical course. CD45 may be one such gene.
The four patients reported here exhibit the C77G polymorphism of CD45 exon 4 [Fig. 1] associated with unusual disease presentations. Prolonged poliovirus excretion is a potentially important problem in polio eradication and although most immunodeficient individuals do not show prolonged excretion [42] one other individual with CVID and prolonged polio excretion but without C77G has been reported [43]. However, CVID is a very heterogeneous disease and although there is no overall increase in the frequency of C77G [44], it may be that C77G is associated with a subtype of disease or an unusual clinical course. As only five CVID patients with C77G were identified in this study [44], this cannot yet be determined. We suggest, however, that the altered cytokine regulation identified in individuals with CD45 polymorphisms might account for our patient’s inability to control poliovirus infection [21–23].
Splenic abscess due to salmonella infection is a very rare condition, most often seen in immunocompromised patients (HIV infection or those undergoing immunosuppressive therapy). Severe salmonella infections can be associated also with polymorphisms of the IL-12/IFN-γ cytokine system but the patient had normal numbers of IFN-γ-producing cells, making it unlikely that this is the cause of the disease [45]. However, as mentioned above, abnormal CD45 expression is associated in animals and in individuals with both C77G and the less well-studied A138G polymorphism, with altered cytokine production [21–23,28,37,38].
The wound healing disorders and immunological abnormalities, including low CD4 T-cell count and reduced in vitro T-cell reactivity to antigens of the fourth patient, could be connected through altered function of fibrocytes, which are a population of blood-borne cells that enter sites of tissue injury and contribute to scar formation. Furthermore, they are potent antigen-presenting cells and express CD45 molecules [46,47]. Altered CD45 expression is known to influence signalling through diverse cell surface molecules, including T- and B-cell antigen receptors, cytokine and toll-like receptors [1,2,48]. Thus, it is plausible that altered CD45 isoform combinations resulting from the mutation in CD45 exon A could affect fibrocyte function in individuals carrying C77G, influencing both wound healing and the induction of antigen-specific T-cell responses. The clinical onset of the phenotype at age 47 is at first sight surprising. However, such a late onset is easily explained if the hypothesis of involvement of many disease-modifying genes in immunodeficiency is accepted. We believe also that the surgical intervention may have been a trigger, perhaps in combination with bacterial infection, starting a cascade of immunological events resulting in the subsequent unusual clinical course.
It will require much larger-scale epidemiological studies to determine whether C77G individuals (and G77G homozygotes) are in general more susceptible to disease and conclusive proof that this polymorphism plays a role in such rare conditions as those reported here will be difficult to obtain, especially as the C77G polymorphism is likely to be only one among many contributing factors in these rare and unusual cases [39]. Nevertheless, to understand the contribution of CD45 polymorphisms to human disease will require documentation of their presence, as well as in the long term a better understanding of their effects on human immune function. It is in this context that we present these unusual cases, in the hope that it will prompt clinical immunologists to screen for the C77G mutation, bearing in mind the possible role of other CD45 polymorphisms as well [3].
Acknowledgments
We are grateful to Barton Haynes and Larry Liu for providing thymic tissue, and the Edward Jenner Institute for Vaccine Research and Deutsche Forschungsgemeinschaft (Schw437/2) for financial support. Local ethical committee approval was obtained for these studies.
References
- 1.Hermiston ML, Zheng X, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 2.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-Dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2(3):89–96. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 3.Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends Immunol. 2006;27:146–53. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Gil J, Ruiz-Tiscar JL, Rodriguez-Sainz C, et al. Prevalence of C77G polymorphism in exon 4 of the CD45 gene in the Spanish population. Med Clin (Barc) 2005;125:10–1. doi: 10.1157/13076408. [DOI] [PubMed] [Google Scholar]
- 5.Tchilian EZ, Dawes R, Ramaley PA, et al. A CD45 polymorphism associated with abnormal splicing is absent in normal African populations. Immunogenetics. 2002;53:980–3. doi: 10.1007/s00251-001-0410-z. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen M, Schweer D, Ziegler A, et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat Genet. 2000;26:495–9. doi: 10.1038/82659. [DOI] [PubMed] [Google Scholar]
- 7.Ballerini C, Rosati E, Salvetti M, et al. Protein tyrosine phosphatase receptor-type C exon 4 gene mutation distribution in an Italian multiple sclerosis population. Neurosci Lett. 2002;328:325–7. doi: 10.1016/s0304-3940(02)00565-7. [DOI] [PubMed] [Google Scholar]
- 8.Vyshkina T, Leist TP, Shugart YY, Kalman B. CD45 (PTPRC) as a candidate gene in multiple sclerosis. Mult Scler. 2004;10:614–7. doi: 10.1191/1352458504ms1115oa. [DOI] [PubMed] [Google Scholar]
- 9.Barcellos LF, Caillier S, Dragone L, et al. PTPRC (CD45) is not associated with the development of multiple sclerosis in US patients. Nat Genet. 2001;29:23–4. doi: 10.1038/ng722. [DOI] [PubMed] [Google Scholar]
- 10.Vorechovsky I, Kralovicova J, Tchilian E, et al. Does 77C-->G in PTPRC modify autoimmune disorders linked to the major histocompatibility locus? Nat Genet. 2001;29:22–3. doi: 10.1038/ng723. [DOI] [PubMed] [Google Scholar]
- 11.Nicholas RS, Partridge J, Donn RP, Hawkins C, Boggild MD. The role of the PTPRC (CD45) mutation in the development of multiple sclerosis in the North West region of the United Kingdom. J Neurol Neurosurg Psychiatry. 2003;74:944–5. doi: 10.1136/jnnp.74.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cocco E, Murru MR, Melis C, et al. PTPRC (CD45) C77G mutation does not contribute to multiple sclerosis susceptibility in Sardinian patients. J Neurol. 2004;251:1085–8. doi: 10.1007/s00415-004-0485-1. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Lira M, Liguori M, Magnani C, et al. CD45 and multiple sclerosis: the exon 4 C77G polymorphism (additional studies and meta-analysis) and new markers. J Neuroimmunol. 2003;140:216–21. doi: 10.1016/s0165-5728(03)00208-x. [DOI] [PubMed] [Google Scholar]
- 14.Tchilian EZ, Wallace DL, Dawes R, et al. A point mutation in CD45 may be associated with HIV-1 infection. AIDS. 2001;15:1892–4. doi: 10.1097/00002030-200109280-00024. [DOI] [PubMed] [Google Scholar]
- 15.Boxall S, McCormick J, Beverley P, et al. Abnormal cell surface antigen expression in individuals with variant CD45 splicing and histiocytosis. Pediatr Res. 2004;55:478–84. doi: 10.1203/01.PDR.0000106803.15344.72. [DOI] [PubMed] [Google Scholar]
- 16.Schwinzer R, Witte T, Hundrieser J, et al. Enhanced frequency of a PTPRC (CD45) exon A mutation (77C – >G) in systemic sclerosis. Genes Immun. 2003;4:168–9. doi: 10.1038/sj.gene.6363894. [DOI] [PubMed] [Google Scholar]
- 17.Dawes R, Hennig B, Irving W, et al. Altered CD45 expression in C77G carriers influences immune function and outcome of hepatitis C infection. J Med Genet. 2006;8:678–84. doi: 10.1136/jmg.2005.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel A, Strassburg CP, Manns MP. 77 C/G mutation in the tyrosine phosphatase CD45 gene and autoimmune hepatitis: evidence for a genetic link. Genes Immun. 2003;4:79–81. doi: 10.1038/sj.gene.6363918. [DOI] [PubMed] [Google Scholar]
- 19.Wood JP, Bieda K, Segni M, et al. CD45 exon 4 point mutation does not confer susceptibility to type 1 diabetes mellitus or Graves’ disease. Eur J Immunogenet. 2002;29:73–4. doi: 10.1046/j.1365-2370.2002.00262.x. [DOI] [PubMed] [Google Scholar]
- 20.Thude H, Rosenhahn S, Hunger-Dathe W, Muller UA, Barz D. A transmembrane protein-tyrosine phosphatase receptor type C (CD45) exon A point mutation (77 C to G) is not associated with the development of type 1 diabetes mellitus in a German population. Eur J Immunogenet. 2004;31:245–7. doi: 10.1111/j.1365-2370.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 21.Do HT, Baars W, Schwinzer R. Functional significance of the 77C – >G polymorphism in the human CD45 gene: enhanced T-cell reactivity by variantly expressed CD45RA isoforms. Transplant Proc. 2005;31:35–8. doi: 10.1016/j.transproceed.2005.01.075. [DOI] [PubMed] [Google Scholar]
- 22.Do HT, Baars W, Borns K, Windhagen A, Schwinzer R. The 77C->G mutation in the human CD45 (PTPRC) gene leads to increased intensity of TCR signaling in T cell lines from healthy individuals and patients with multiple sclerosis. J Immunol. 2006;176:931–8. doi: 10.4049/jimmunol.176.2.931. [DOI] [PubMed] [Google Scholar]
- 23.Boxall S, Stanton T, Hirai K, et al. Disease associations and altered immune function in CD45 138G variant carriers. Hum Mol Genet. 2004;13:2377–84. doi: 10.1093/hmg/ddh276. [DOI] [PubMed] [Google Scholar]
- 24.Ward V, Hennig BJ, Hirai K, et al. Geographical distribution and disease associations of the CD45 exon 6 138G variant. Immunogenetics. 2006;58:235–9. doi: 10.1007/s00251-006-0099-0. [DOI] [PubMed] [Google Scholar]
- 25.Byth KF, Conroy LA, Howlett S, et al. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996;183:1707–18. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung C, Pingel JT, Heikinheimo M, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat Med. 2000;6:343–5. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 27.Tchilian EZ, Wallace DL, Wells RS, Flower G, Morgan G, Beverley PC. A deletion in the gene encoding the CD45 antigen in a patient with SCID. J Immunol. 2001;166:1308–13. doi: 10.4049/jimmunol.166.2.1308. [DOI] [PubMed] [Google Scholar]
- 28.Dawes R, Petrova S, Liu Z, Wraith D, Beverley PC, Tchilian EZ. Combinations of CD45 isoforms are crucial for immune function and disease. J Immunol. 2006;176:3417–25. doi: 10.4049/jimmunol.176.6.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misbah SA, Lawrence PA, Kurtz JB, Chapel HM. Prolonged faecal excretion of poliovirus in a nurse with common variable hypogammaglobulinaemia. Postgrad Med J. 1991;67:301–3. doi: 10.1136/pgmj.67.785.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tackenberg B, Nitschke M, Willcox N, et al. CD45 isoform expression in autoimmune myasthenia gravis. Autoimmunity. 2003;36:117–21. doi: 10.1080/0891693031000084369. [DOI] [PubMed] [Google Scholar]
- 31.Bujan W, Schandene L, Ferster A, De Valck C, Goldman M, Sariban E. Abnormal T-cell phenotype in familial erythrophagocytic lymphohistiocytosis. Lancet. 1993;342:1296. doi: 10.1016/0140-6736(93)92385-7. [DOI] [PubMed] [Google Scholar]
- 32.Wagner R, Morgan G, Strobel S. A prospective study of CD45 isoform expression in haemophagocytic lymphohistiocytosis; an abnormal inherited immunophenotype in one family. Clin Exp Immunol. 1995;99:216–20. doi: 10.1111/j.1365-2249.1995.tb05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao H-X, Montefiori DC, Patel DD, et al. Linkage of the CCR5delta32 mutation with a functional polymorphism of CD45RA. J Immunol. 2000;165:148–57. doi: 10.4049/jimmunol.165.1.148. [DOI] [PubMed] [Google Scholar]
- 34.Tchilian EZ, Wallace DL, Imami N, et al. The exon A (C77G) mutation is a common cause of abnormal CD45 splicing in humans. J Immunol. 2001;166:6144–8. doi: 10.4049/jimmunol.166.10.6144. [DOI] [PubMed] [Google Scholar]
- 35.Portnoi MF, Lebas F, Gruchy N, et al. 22q11.2 duplication syndrome: two new familial cases with some overlapping features with DiGeorge/velocardiofacial syndromes. Am J Med Genet A. 2005;137:47–51. doi: 10.1002/ajmg.a.30847. [DOI] [PubMed] [Google Scholar]
- 36.Shah M, Bogucki B, Mavers M, deMello DE, Knutsen A. Cardiac conduction abnormalities and congenital immunodeficiency in a child with Kabuki syndrome: case report. BMC Med Genet. 2005;6:28. doi: 10.1186/1471-2350-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subra JF, Cautain B, Xystrakis E, et al. The balance between CD45RChigh and CD45RClow CD4 T cells in rats is intrinsic to bone marrow-derived cells and is genetically controlled. J Immunol. 2001;166:2944–52. doi: 10.4049/jimmunol.166.5.2944. [DOI] [PubMed] [Google Scholar]
- 38.Xystrakis E, Cavailles P, Dejean AS, et al. Functional and genetic analysis of two CD8 T cell subsets defined by the level of CD45RC expression in the rat. J Immunol. 2004;173:3140–7. doi: 10.4049/jimmunol.173.5.3140. [DOI] [PubMed] [Google Scholar]
- 39.Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584–9. doi: 10.1038/nature03723. [DOI] [PubMed] [Google Scholar]
- 40.Campagnoli MF, Garbarini L, Quarello P, et al. The broad spectrum of autoimmune lymphoproliferative disease: molecular bases, clinical features and long-term follow-up in 31 patients. Haematologica. 2006;91:538–41. [PubMed] [Google Scholar]
- 41.Goldacker S, Warnatz S. Tackling the heterogeneity of CVID. Curr Opin Allergy Clin Immunol. 2005;5:504–9. doi: 10.1097/01.all.0000191888.97397.b3. [DOI] [PubMed] [Google Scholar]
- 42.Halsey NA, Pinto J, Espinosa-Rosales F, et al. Search for poliovirus carriers among people with primary immune deficiency diseases in the United States, Mexico, Brazil, and the United Kingdom. Bull World Health Organ. 2004;82:3–8. [PMC free article] [PubMed] [Google Scholar]
- 43.MacLennan C, Dunn G, Huissoon AP, et al. Failure to clear persistent vaccine-derived neurovirulent poliovirus infection in an immunodeficient man. Lancet. 2004;363:1509–13. doi: 10.1016/S0140-6736(04)16150-3. [DOI] [PubMed] [Google Scholar]
- 44.Vorechovsky I, Kralovicova J, Tchilian E, et al. Does 77C – >G in PTPRC modify autoimmune disorders linked to the major histocompatibility locus? Nat Genet. 2001;29:22–3. doi: 10.1038/ng723. [DOI] [PubMed] [Google Scholar]
- 45.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11:321–33. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 46.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–25. [PubMed] [Google Scholar]
- 48.Piercy J, Petrova S, Tchilian EZ, Beverley PCL. CD45 negatively regulates TNF and IL-6 production in dendritic cells. Immunology. 2006;118:250–6. doi: 10.1111/j.1365-2567.2006.02363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]