Abstract
The cytokine pattern of T lymphocytes has not been characterized in children with combinations of paediatric immunological disorders. We describe cytokine secretion in children with type 1 diabetes, coeliac disease and allergy and combinations of two of these diseases after stimulation with ‘disease-specific’ antigens. Peripheral blood mononuclear cells (PBMC) were collected from 68 children with type 1 diabetes, allergy or coeliac disease, two of these diseases in combination or none of these diseases. Using the enzyme-linked immunospot (ELISPOT) technique, interferon (IFN)-γ and interleukin (IL)-4 were analysed from fresh PBMC spontaneously and after in vitro stimulation with antigens associated with one or more of these diseases (insulin, gluten, birch and cat extract, β-lactoglobulin, ovalbumin and phytohaemagglutinin) in order to divide T helper (Th)1- from Th2-like lymphocytes. Stimulation with birch and cat extract caused increased IL-4 secretion in allergic children. A low IFN-γ response to insulin was found in type 1 diabetic children, whereas allergic children responded to insulin by increased IL-4 secretion. Children suffering from both type 1 diabetes (Th1-prone) and allergy (Th2-prone) reacted distinctly to general mitogen stimulation. Children suffering from two Th1-dominated diseases (type 1 diabetes and coeliac disease) showed hardly any response to either food or inhalation allergens. Our results indicate an important interplay between common immunological diseases in children. The combination of two Th1-deviated diseases is associated with a suppressed immune response, whereas a combination of Th1- and Th2-dominated diseases appears to increase the general immune response.
Keywords: type 1 diabetes, allergy, clinical immunology, coeliac disease, cytokines
Introduction
Type 1 diabetes and coeliac disease are suggested to be of autoimmune origin. In genetically predisposed individuals, type 1 diabetes is caused by the destruction of pancreatic β-cells [1]. The immune profile during the prediabetic phase is probably skewed towards T helper (Th)-1 cells [2], and diabetes has been shown to be transferable by CD4+ T cells expressing a Th1-like cytokine profile in non-obese diabetic (NOD) mice [3]. An increased cellular response, especially by Th1 cytokines, to the autoantigens insulin [4], glutamic acid decarboxylase (GAD65) [5] and tyrosinephosphatase (IA-2) [6] has been found in type 1 diabetic patients.
Coeliac disease is triggered by the ingestion of gluten in genetically susceptible subjects [7], i.e. gluten is the causative agent. The diseased mucosa heals and autoantibodies disappear after gluten withdrawal. Gluten appears to induce a non-proliferative activation of CD4+ lamina propria T cells, especially activated Th1-like cells secreting interferon (IFN)-γ [8].
It has been postulated that newly diagnosed diabetic patients also have a non-specific activation of the immune system directed towards several dietary proteins, e.g. bovine serum albumin (BSA) and β-lactoblobulin (βLG), as a result of defective immune regulation or loss of immunological tolerance to a variety of ingested antigens [9–11]. The same pattern is noted in coeliac disease, where antibodies not only to gluten but also to other dietary proteins are elevated before treatment [12]. Gliadin-specific DQ2-restricted T cells have been isolated from the intestinal mucosa of coeliac patients and gliadin-triggered autoimmune mechanisms might be operative in the pathogenesis of coeliac disease [13]. Tissue transglutaminase (tTG) has been identified as the endomysial autoantigen in coeliac patients [14], and recently a large cohort study showed a high prevalence of IgA tTG autoantibodies (10%) in patients with type 1 diabetes [15].
Allergy is associated with Th2-like immunity to allergens in affected tissues [16]. Such patients often have a hereditary predisposition to produce IgE antibodies against common environmental allergens. Typically, IgE antibodies to the food allergens βLG and ovalbumin (OVA) develop at the beginning of the ‘atopic march’ accompanied by atopic eczema and urticaria, often succeeded by bronchial asthma and allergic rhinoconjunctivitis [17].
Thus, in allergic individuals the immune system appears to be displaced towards an activation of Th2-like cells, while in children with type 1 diabetes or coeliac disease a Th1-like profile seems to dominate. The role of antigen-specific T lymphocytes and their relation to cytokines is not well characterized in children with combinations of common paediatric immunological disorders. In this study, we included children with type 1 diabetes, allergy and coeliac disease or combinations of these diseases in order to study immune responses to antigens associated with these three disorders.
Materials and methods
Children with common immunological disorders
This material includes all children at the paediatric clinic, Linköping University Hospital, Sweden, diagnosed with both type 1 diabetes and coeliac disease. We may presume that these children represent the population, as the prevalence of coeliac disease in Swedish type 1 diabetic patients has been reported to be 4·6% [18] and in our material the prevalence was 4·4% (eight of 180). Eight children with type 1 diabetes and coeliac disease were matched for gender and age, as closely as possible, to children with the following characteristics: nine children diagnosed with type 1 diabetes and allergy, seven with coeliac disease and allergy, 12 with type 1 diabetes, 10 with coeliac disease, 12 with allergy and 10 children with none of these diseases (reference children) (Table 1). These evaluated children were all included, without any selection, for cytokine analysis from fresh cells in all cases.
Table 1.
Children with type 1 diabetes (T1D), coeliac disease (CD) or allergy (AD) and combinations of these diseases (T1D + CD, T1D + AD, CD + AD) were matched to each other and to reference (REF) children by age (years) and gender. Duration of disease (in parenthesis d = days, m = months, y = years) is presented for children with T1D and/or CD.
| Gender | T1D + CD | T1D + AD | CD + AD | T1D | CD | AD | REF |
|---|---|---|---|---|---|---|---|
| Boys | 14 (3 y/4 y) | – | 11 (1 m) | 13 (4 y) | – | 13 | 12 |
| 13 (18 m/5 m) | 12 (10 m) | – | 12 (11 y) | 10 (d.n.a.) | 12 | 12 | |
| 12 (8 y/18 m) | 12 (8 y) | – | 12 (2 y) | 10 (1 y) | 9 | 10 | |
| 10 (3 y/9 m) | 9 (4 y) | 10 (6 y) | 9 (5 y) | 8 (6 y) | 9 | 9 | |
| 7 (1 y/9 m) | 6 (4 m) | – | 6 (1 y) | 7 (4 y) | 6 | 8 | |
| – | – | – | 13 (3 m) | – | – | – | |
| Girls | – | 14 (11 y) | 16 (4 m) | 14 (3 y) | 15 (11 y) | 14 | 14 |
| 12 (2 y/1 y) | 12 (5 y) | 14 (12 y) | 12 (7 y) | 14 (11 y) | 12 | 13 | |
| – | 12 (5 y) | 11 (9 y) | 12 (10 y) | 12 (1 d) | 12 | 12 | |
| 10 (5 y/10 m) | 10 (6 y) | 11 (8 y) | 10 (14 m) | 11 (9 y) | 11 | 12 | |
| 8 (2 y/10 m) | 10 (7 y) | 10 (6 y) | 10 (2 y) | 10 (9 y) | 11 | 11 | |
| – | – | – | 10 (9 m) | 10 (7 y) | 10 | – | |
| – | – | – | – | – | 12 | – | |
| N | 8 | 9 | 7 | 12 | 10 | 12 | 10 |
d.n.a. = data not available.
Diagnostic criteria
Coeliac disease was diagnosed according to the modified version of the European Society of Paediatric Gastroenterology and Nutrition (ESPGAN) criteria [19]. Duration of type 1 diabetes was defined from the date of diagnosis and coeliac disease from the date of the first biopsy (Table 1). Allergy was characterized by eczema, bronchial asthma and/or allergic rhinoconjunctivitis, and in all but two the skin prick test (SPT) was positive. Diagnosis of allergy was strictly according to the International Study of Asthma and Allergies in Childhood (ISAAC) protocols for phase I and III (http://isaac.auckland.ac.nz/PhaseThr/phs3Frame.html) (Table 2). The reference children had no signs of type 1 diabetes or other autoimmune diseases, nor of coeliac disease or clinical allergy (in all cases a negative SPT). Furthermore, no signs of these diseases were reported among their first-degree relatives.
Table 2.
Characterization of allergic diseases (atopic dermatitis, bronchial asthma and allergic rhinoconjunctivitis; Y = yes, N = no) for children with exclusively allergy (AD) and allergy in combination with type 1 diabetes (AD + T1D) or coeliac disease (AD + CD).
| Disease | Gender | Age (years) | Atopic dermatitis | Bronchial asthma | Allergic rihno-conjunctivitis | Food allergy | Disease dev. post-sampling | Therapy | Examination (month) |
|---|---|---|---|---|---|---|---|---|---|
| Allergy (AD) | Boys | 13 | Y (13 yrs) | Y (10 yrs) | Y (5 yrs) | I, A, L | Oct | ||
| 12 | Y (11 yrs) | Y (10 yrs) | Y (2 yrs) | I, LA, A, L | Oct | ||||
| 9 | Y (8 yrs) | Y (6 yrs) | N | A. rihnoconj. | A, L | Oct | |||
| 9 | Y (9 yrs) | Y (7 yrs) | N | A. rihnoconj. | I, A, L, SI | Nov | |||
| 6 | Y (5 yrs) | Y (5 yrs) | N | I, A, L, C | Sept | ||||
| Girls | 14 | N | N | Y (d.n.a.) | (d.n.a.) | Oct | |||
| 12 | Y (d.n.a.) | Y (d.n.a.) | N | (d.n.a.) | Nov | ||||
| 12 | N | Y (4 yrs) | Y (6 yrs) | I, A, L | Nov | ||||
| 12 | Y (8 yrs) | Y (d.n.a.) | Y (d.n.a.) | A | Jan | ||||
| 11 | N | Y (7 yrs) | Y (d.n.a.) | (d.n.a.) | Oct | ||||
| 11 | N | Y (d.n.a.) | Y (d.n.a.) | (d.n.a.) | Nov | ||||
| 10 | Y (d.n.a.) | Y (6 yrs) | Y (d.n.a.) | I, A, L | Oct | ||||
| Allergy (AD) + Type 1 diabetes (T1D) | Boys | 12 | N | N | Y (1 yr) | L | Aug | ||
| 12 | N | N | Y (8 yrs) | A | Aug | ||||
| 9 | N | Y (8 yrs) | Y (d.n.a.) | I, A, L | Sept | ||||
| 6 | Y (6 yrs) | Y (5 yrs) | N | A. rihnoconj. | I, A, L | Aug | |||
| Girls | 14 | Y (14 yrs) | Y (9 yrs) | Y (d.n.a.) | B, A | Sept | |||
| 12 | Y (d.n.a.) | N | N | (d.n.a.) | Sept | ||||
| 12 | Y (11 yrs) | N | Y (9 yrs) | B. asthma | I, A | Aug | |||
| 10 | Y (8 yrs) | N | N | A. rihnoconj. | No | Aug | |||
| 10 | Y (d.n.a.) | Y (d.n.a.) | N | No | Aug | ||||
| Allergy (AD) + Coeliac disease (CD) | Boys | 11 | N | N | Y (1 yr) | A, L | Oct | ||
| 10 | N | Y (6 yrs) | Y (d.n.a.) | A | Jan | ||||
| Girls | 16 | Y (d.n.a.) | Y (d.n.a.) | N | No | Oct | |||
| 14 | Y (d.n.a.) | Y (d.n.a.) | Y (d.n.a.) | (d.n.a.) | Dec | ||||
| 11 | Y (10 yrs) | Y (10 yrs) | N | I | Jan | ||||
| 11 | N | N | N | F. allergy | (d.n.a.) | Jan | |||
| 10 | N | N | N | F. allergy | No | Jan |
Duration of allergic diseases is presented in parenthesis (yrs = years).
Food allergy = IgE-mediated food allergy (skin prick test-positive).
Diseases developing post-sampling; A. rhinoconj. = allergic rhinoconjunctivitis, B. asthma = bronchial asthma.
Therapy; I = steroids for inhalation, A = anti-histamines, L = local treatment of eyes and/or nose, LA = leucotriene antagonist, SI = specific immune therapy, C = cortisone cream, B = beta-2-agonists without steroids in inhalation.
Examination = month for collection of blood sample, skin prick test and questionnaire.
d.n.a. = data not available.
Examination procedures
Sodium-heparinized venous blood samples were obtained from all 68 children. At the time of blood sampling,none of the children showed signs of colds or other infections. SPTs were performed on all children in duplicate on the volar aspects of the forearms with standardized 10 histamine equivalent potency (HEP) of cat and dog dander extracts, birch, timothy, mite (SoluprickR; ALK, Hørsholm, Denmark) and hen’s egg white. Tests were regarded as positive if skin wheals had a mean diameter of 3 mm or more after 15 min. Histamine dihydrochloride, 10 mg/ml, and a sterile lancet were used as positive and negative controls, respectively.
A validated questionnaire on allergic symptoms, modified from the ISAAC formula (http://isaac.auckland.ac.nz/PhaseThr/Manual.pdf), as well as questions on environmental factors and history of autoimmunity and coeliac disease in the family, were completed by all children together with their parents.
Stimulation of lymphocytes and enumeration of IFN-γ- and interleukin (IL)-4-secreting cells by enzyme-linked immunospot (ELISPOT)
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque density gradient centrifugation (Pharmacia Biotech, Sollentuna, Sweden). The ELISPOT technique was performed as described previously [2] with slight modifications [20]. From all children, aliquots of 100 000 PBMC/well were stimulated with birch and cat extract, insulin, βLG, OVA, wheat gluten (fraction III) [21] and phytohaemagglutinin (PHA) (Table 3) on ELISPOT plates, in all cases less than 4 h after blood sampling.
Table 3.
Concentration and source of antigens used for stimulation of peripheral blood mononuclear cells to induce antigen-specific secretion of interferon-γ and interleukin-4, detected by enzyme-linked immunospot assay.
| Antigen | Concentration | Source |
|---|---|---|
| Birch extract | 10 kSQ-U/ml | Aquagen, ALK-Abell—, Hærsholm, Denmark |
| Cat allergen extract | 10 kSQ-U/ml | “” |
| Insulin | 500 ng/ml | Actrapid, NovoNordisk, Malmö, Sweden |
| β–lactoglobulin | 10 µg/ml | Sigma-Aldrich, Stockholm, Sweden |
| Ovalbumin | 10 µg/ml | “” |
| Gluten | 400 µg/ml | Gift from O Vaarala, Linköping, Sweden |
| Phytohaemagglutinin | 20 µg/ml | Sigma-Aldrich, Stockholm, Sweden |
All variants of differently stimulated and non-stimulated cells were applied in quadruplicate. As a negative control, some wells on each plate were incubated with culture medium only, without cells but otherwise treated as the other wells, while stimulation with PHA was used as a positive control. The plates were blinded for identity to avoid any influence of the outcome of the observation. Plates were counted automatically, under manual supervision, using the AID ELISPOT Reader System (AID, Strassberg, Germany). The median value of the quadruplicates was calculated for each antigen. The value of each specific secretion from in vitro stimulation, after subtraction of spontaneous spots, was calculated. Our laboratory participated in the first ELISPOT workshop as one of the core laboratories, and our Mabtech assay was judged to be sensitive and reproducible [22].
Statistics
As the secretion of IFN-γ and IL-4 was not distributed normally (even after logarithmic transformation), two groups were compared by Mann–Whitney U-test and three or more groups using the Kruskal–Wallis test for unpaired observations. A probability level of < 0·05 was considered statistically significant. Calculations were performed using the statistical package statview 5·0.1 for Macintosh (Abacus Concepts Inc., Berkeley, CA, USA).
Ethics
The study was approved by the Research Ethics Committee of the Faculty of Health Sciences, Linköping University, Linköping, Sweden, and all parents or responsible guardians gave their informed consent on behalf of the children.
Results
High spontaneous IFN-γ and IL-4 secretion in allergic children
Spontaneous and in vitro antigen-induced secretion of IFN-γ and IL-4 was analysed in children with one or two of the immunological disorders type 1 diabetes (T1D), coeliac disease (CD) and allergy (AD) as well as in reference children. The secretion of IFN-γ and IL-4 as markers of induced Th1- and Th2-like responses, respectively, is shown in Table 4. Allergic children showed higher spontaneous secretion of IFN-γ than children with CD (P < 0·05) or T1D (P < 0·05). Children with AD only also secreted higher IFN-γ concentrations than children with AD in combination with CD (P = 0·01) or T1D (P < 0·01). Children with CD secreted less IL-4 spontaneously than children with either AD (P < 0·01) or T1D (P < 0·01) or these two diseases in combination (P < 0·001). In contrast, a combination of AD and CD did not reveal any significant changes in either spontaneous or in vitro-induced cytokine responses (Table 4).
Table 4.
Secretion of cytokines (interferon-γ and interleukin-4), spontaneously and after in vitro stimulation with antigens [birch and cat extract, β-lactoglobulin (βLG), ovalbumin (OVA), gluten, insulin and phytohaemagglutinin (PHA)], in children with allergy (AD), coeliac disease (CD), type 1 diabetes (T1D), combinations of these diseases (T1D + CD, T1D + AD, CD + AD) and in reference (REF) children.
| Spontaneous/in vitro stimulation | Cytokines | AD | CD | T1D | CD + AD | T1D + AD | T1D + CD | REF |
|---|---|---|---|---|---|---|---|---|
| Sontaneous | IFN-γ | 157 (19–394) | 68 (19–209) | 71 (23–211) | 41 (13–96) | 48 (21–292) | 100 (15–257) | 41 (5–310) |
| IL-4 | 14 (12–67) | 3 (1–10) | 9 (1–65) | 5 (2–9) | 17 (6–39) | 5 (1–40) | 5 (0–51) | |
| Birch | IFN-γ | 73 (−21–291) | 49 (−32–254) | 73 (−91–402) | 43 (−15–112) | 66 (15–363) | 5 (−123–70) | 77 (−7–283) |
| IL-4 | 22 (−2–62) | 1 (−2–4) | 1 (−6–19) | 2 (−5–7) | 6 (−11–87) | 0 (−36–5) | 0 (−4–4) | |
| Cat extract | IFN-γ | 4 (−79–379) | 32 (−48–148) | 55 (−114–459) | 34 (−21–120) | 88 (22–402) | 8 (−103–56) | 66 (−86–307) |
| IL-4 | 11 (−4–147) | 1 (−8–3) | 1 (−21–9) | 0 (−2–10) | 2 (−7–87) | −1 (−34–11) | 1 (−3–16) | |
| βLG | IFN-γ | 31 (−29–221) | 65 (−34–183) | 99 (−64–295) | 62 (−4–112) | 138 (40–217) | 2 (−116–105) | 153 (6–238) |
| IL-4 | 7 (−14–34) | 6 (2–24) | 8 (−27–19) | 3 (−1–14) | 7 (2–31) | 3 (−9–12) | 14 (−8–61) | |
| OVA | IFN-γ | 43 (−27–184) | 52 (−0–87) | 56 (−33–107) | 40 (−1–81) | 35 (−8–62) | 6 (−53–41) | 27 (−67–128) |
| IL-4 | 0 (−7–10) | −1 (−6–2) | −1 (−29−8) | −1 (−6–0) | 0 (−4–24) | −2 (−33–6) | 0 (−20–5) | |
| Gluten | IFN-γ | −15 (−85–106) | −9 (−94–18) | −11 (−95–48) | 4 (−33–31) | −1 (−25–33) | −22 (−114–39) | 3 (−15–68) |
| IL-4 | 1 (−31–127) | 1 (−6–5) | −1 (−47–10) | 0 (−2–3) | −5 (−25–12) | 1 (−37–5) | −1 (−13–213) | |
| Insulin | IFN-γ | 19 (−31–118) | 5 (−80–20) | 1 (−74–77) | 8 (−9–106) | 0 (−22–7) | 2 (−100–115) | 1 (−17–93) |
| IL-4 | 2 (−1–19) | 0 (−4–6) | −1 (−19–4) | −2 (−3–2) | 0 (−5–27) | 1 (−38–4) | 0 (−15–5) | |
| PHA | IFN-γ | 293 (199–742) | 580 (292–744) | 496 (243–709) | 477 (273–541) | 656 (298–793) | 383 (69–857) | 466 (263–709) |
| IL-4 | 456 (100–551) | 420 (286–514) | 438 (147–671) | 359 (165–409) | 563 (465–637) | 294 (265–743) | 356 (255–584) |
Secretion of cytokines after stimulation with antigens calculated as spots/100 000 peripheral blood mononuclear cells after subtraction of spontaneous cytokine secretion, detected by enzyme-linked immunospot assay and illustrated as median, and range (in parenthesis).
High IL-4 response to birch and cat extract but also to insulin in children with allergy
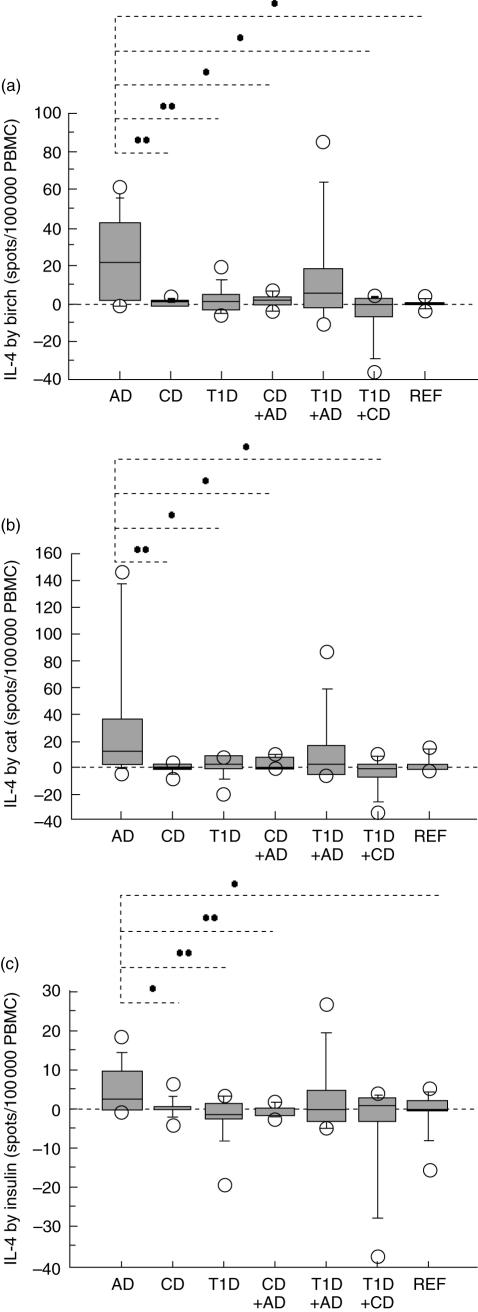
Children with AD showed higher IL-4 secretion from stimulation with birch (Fig. 1a) or cat extract (Fig. 1b) than children with either CD or T1D, children with a combination of T1D and CD or children with AD in combination with CD. Stimulation with birch extract also caused higher secretion of IL-4 in children with AD compared to reference children (Fig. 1a). Further, the insulin-induced IL-4 response (Fig. 1c) was higher in AD children than in children suffering from CD, T1D, a combination of AD and CD and reference children.
Fig. 1.
Birch (a) and cat extract (b) induced higher IL-4 secretion in children with allergy (AD) compared to children with coeliac disease (CD) (P = 0·009 and P = 0·008 respectively), type 1 diabetes (T1D) (P = 0·008 and P = 0·03), both T1D and CD (P = 0·01 and P = 0·01), or AD in combination with CD (P = 0·04 and P = 0·04). Stimulation with birch extract (a) also caused higher secretion of IL-4 in AD children compared to reference (REF) children (P = 0·01). Insulin (c) caused a higher IL-4 response in AD children than in children suffering from CD (P = 0·049), T1D (P = 0·007), both AD and CD (P = 0·008), and compared to REF children (P = 0·04). The ELISPOT technique illustrates the number of cytokine-secreting cells/100 000 PBMC by box plots (10th, 25th, 50th, 75th and 90th centiles; outliers are indicated).*, P < 0·05; **, P < 0·01; and *** P < 0·001.
Low IFN-γ response in children with both type 1 diabetes and coeliac disease
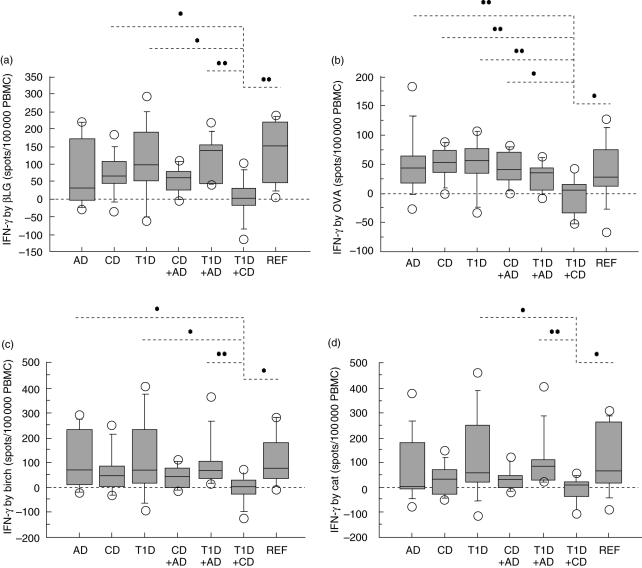
Children suffering from both T1D and CD showed a reduced immunological response to food antigens. They secreted less IFN-γ by in vitro stimulation with βLG (Fig. 2a) and ovalbumin (Fig. 2b) compared to children with either CD or T1D as well as in comparison with reference children. Children suffering from both T1D and CD also showed less IFN-γ secretion with ovalbumin than children with AD alone or AD combined with CD. The βLG-induced IFN-γ response was lower in children with both T1D and CD than in children with a combination of T1D and AD. Further, the combination of T1D and CD caused less IFN-γ response from in vitro stimulation with gluten compared to that observed in reference children (P < 0·05) (Table 4). In contrast, similar levels of food antigen-induced IL-4 were observed in all groups of children (Table 4).
Fig. 2.
Children suffering from both T1D and CD secreted less IFN-γ by in vitro stimulation with β-lactoglobulin (a) and ovalbumin (b) compared to children with either T1D (P = 0·03 and P = 0·007 respectively) or CD (P = 0·03 and P = 0·006) and compared to REF children (P = 0·003 and P = 0·04). The βLG-induced (a) IFN-γ response was lower in children diagnosed with both T1D and CD compared to in children with T1D combined with AD (P = 0·002), while children with both T1D and CD showed less IFN-γ secretion by ovalbumin (b) than children with AD (P = 0·009) or AD in combination with CD (P = 0·02). A reduced IFN-γ response to birch (c) and cat (d) extract was observed in children with T1D and CD compared to children diagnosed with T1D (P = 0·04 and P = 0·02 respectively), T1D and AD (P = 0·007 and P = 0·002), and compared to REF children (P = 0·01 and P = 0·03). Lower IFN-γ secretion from in vitro stimulation with birch extract (c) was also observed in children with both T1D and CD compared to AD children (P = 0·04).
Compared to other children, the children diagnosed with both T1D and CD also secreted less IFN-γ by stimulation with inhalant allergens. A reduced IFN-γ response to birch (Fig. 2c) and cat (Fig. 2d) extract was observed compared to children diagnosed with T1D, children with both T1D and AD, and in comparison with reference children. Lower IFN-γ secretion from in vitro stimulation with birch extract was also observed in children with both T1D and CD compared to AD children.
High IFN-γ and IL-4 response to mitogen in children with a combination of type 1 diabetes and allergy
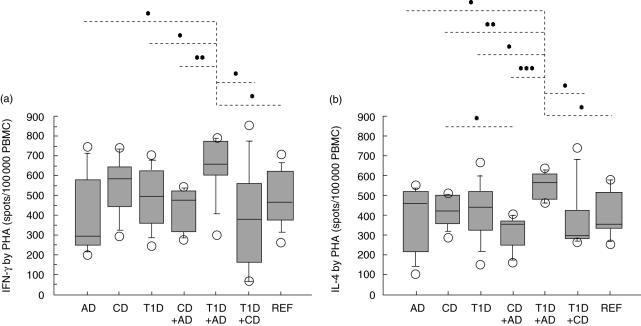
Children with both T1D and AD showed higher secretion of IFN-γ (Fig. 3a) and IL-4 (Fig. 3b) after in vitro stimulation with the mitogen PHA compared to children with exclusively AD or T1D, AD combined with CD, T1D with CD, and compared to reference children. PHA-induced secretion of IL-4 was also higher in children with both T1D and AD than in children with CD only.
Fig. 3.
Children with both T1D and AD showed higher secretion of IFN-γ (a) and IL-4 (b) after in vitro stimulation with the mitogen PHA compared to children with only AD (P = 0·01 and P = 0·01 respectively) or T1D (P = 0·04 and P = 0·03), AD and CD (P = 0·007 and P = 0·0009), T1D and CD (P = 0·03 and P = 0·02) and compared to REF children (P = 0·03 and P = 0·01). PHA-induced secretion of IL-4 (b) was also higher in children with both T1D and AD than in children with CD only (P = 0·006).
Discussion
During an immune response low numbers of resting naive and memory cells, which do not secrete cytokine clonally, expand and acquire a cytokine-expressing phenotype. The secretion of IFN-γ and IL-4, as markers of induced Th1- and Th2-like responses, respectively, was analysed in children with one or two of the immunological diseases type 1 diabetes, coeliac disease and allergy as well as in reference children. As expected, allergic children secreted high levels of IL-4 spontaneously, and a strong IL-4 response was also observed to inhalation allergens. Unexpectedly, allergic children also showed a high number of spontaneous IFN-γ-producing cells. This is in accordance with the recent report of a mixed Th1- and Th2-like response in allergic children. Asthmatic patients, in addition to the well-known Th2 (IL-4) inflammation, show signs of an increased frequency of IFN-γ producing CD4+ and CD8+ T cells, possibly controlled by IL-12 [23,24]. Allergen-induced (house dust mite and rye grass pollen) cytokine response in children with allergic disease has also been found to be associated with both increased Th1 (IFN-γ) and Th2 (IL-5 and IL-13) cytokine production [25].
The cytokine response to the autoantigen insulin was found to be low in type 1 diabetic children, as described earlier [26–28]. Surprisingly, the group of children with allergy responded in a Th2-like manner to in vitro stimulation with insulin. This may be due to the fact that allergic children also respond to types of antigens other than allergens in a Th2-like manner. In NOD mice it has been observed that administration of insulin, or the insulin B-chain, primes Th2 cellular and humoral immunity to insulin, shifting the predominant insulin response towards a Th2 phenotype [29]. Further, BDC2·5 transgenic mice, which express IL-4 under the control of the insulin promoter, increase islet antigen presentation by IL-4 [30]. A shift towards production of Th2-like cytokines has also been observed in high-risk individuals and newly diagnosed type 1 diabetic patients by in vitro challenge with insulin [31]. Further, it has been found recently that T cell reactivity to preproinsulin in CD45RA (both memory and naive or recently primed) subsets is Th2-dominated in subjects with detectable diabetes-associated autoantibodies and a genetic risk of type 1 diabetes [32].
Patients with manifest type 1 diabetes may have had latent coeliac disease, which is activated parallel to the anti-islet immune reactivity during the development of type 1 diabetes. This is explained at least partly by the shared genetic risk determined by human leucocyte antigen (HLA)-DQB1*0201 allele in type 1 diabetes and coeliac disease. Class II HLA genes in the HLA-D region code for cell-surface molecules which bind and present antigenic peptides to T cells, thus playing a major role in the pathogenesis of many autoimmune disorders. T cells in the gut mucosa of coeliac patients [33] and pancreatic islets of type 1 diabetic patients [34] have been reported to represent particularly Th1 cells producing IFN-γ. Th1-like profile cytokines, IL-2, IFN-γ and tumour necrosis factor (TNF)-α, have been observed to correlate with the degree of mucosal affection in type 1 diabetic patients [35]. Administration of IL-2, resulting in accumulation of this cytokine, has been observed in the bowel of all studied patients with coeliac disease and in the pancreas of all prediabetic individuals [36]. A recent study has reported signs of increased mucosal inflammation by a higher density of intraepithelial CD3+ and lamina propria CD25+ in jejunal biopsies from type 1 diabetic patients compared to control subjects [37]. Taken together, these results indicate a Th1-like dominance.
The most notable results in our cohort were in patients with both type 1 diabetes and coeliac disease showing a diminished Th1-like profile. These children showed hardly any IFN-γ secretion after in vitro stimulation with either food (βLG, OVA and gluten) or inhalation allergens (birch and cat extract). This result was very distinct from all the other groups studied, including reference children, and has not been described previously. It has been shown that the islet-infiltrating, autoreactive cells express gut-associated homing receptor α4β7-integrin in both NOD mice and type 1 diabetic patients [38], suggesting that lymphocytes may recirculate between the gut and pancreas [35]. However, after introduction of a gluten-free diet in coeliac patients as well as in type 1 diabetic patients who have lost all their β-cells, the access to diabetes-associated antigens is markedly reduced. Interestingly, immune aberrancies in the gut observed in children who are prone to type 1 diabetes suggest poor development of oral tolerance [35]. This may influence the immune process in general and also reduce the immunological process towards other antigens. Thus, we speculate that the low IFN-γ response to antigens observed in children suffering from two Th1-dominated diseases is a sign of a suppressed immune system.
Most studies still support an inverse association between Th1- (type 1 diabetes) and Th2- (allergy) related diseases [39,40]. However, in recent years the discussion concerning the balance of Th1 and Th2 has included the suggestion of an immune dysregulation, allowing both Th1 and Th2 responses to go unimpeded [41]. There is a significant increase in the risk of presenting with a Th1-mediated autoimmune condition in patients with a history of allergic diseases, and similarly, presentation with either eczema or allergic rhinitis is significantly more common in subjects with a history of Th1-mediated disease [42,43]. We found that children suffering from a combination of a Th1-characterized disease (type 1 diabetes) and a Th2-like disease (allergy) showed a significantly stronger Th1- and Th2-like response to the mitogen PHA compared to all the other groups studied. Because there seem to be shared risk factors for the two diseases, one possible hypothesis suggests that Th1- and Th2-related diseases are associated positively within individuals. Further, the simultaneous rise in several Th1- and Th2-related diseases at the population level supports a common aetiology [43]. Thus, the immune system of children with both type 1 diabetes and allergy may react more distinctly to stimulators in general. It has been suggested that Th1- and Th2-mediated diseases are significantly associated, supporting the proposal that autoimmune and allergic diseases share risk factors that increase the propensity of the immune system to generate both Th1- and Th2-mediated inappropriate responses to non-pathological antigens [42–44].
Our study describes the co-analysis of cytokines in children with three common paediatric immunological disorders and possible combinations of two of these diseases. We have used antigens relevant for each disorder for studies of the immunological response in all children regardless of disease. Importantly, the sensitive ELISPOT technique allowed detection of low concentrations of cytokines exclusively in fresh cell samples. In summary, we found that allergic children showed an induced Th2-like response to insulin, and furthermore children with both allergy and type 1 diabetes responded with a high Th1- and Th2-like response to a mitogen. In contrast, children suffering from the two Th1-deviated diseases, type 1 diabetes and coeliac disease, showed hardly any response to food antigens and allergens. We speculate that the increased response in patients suffering from type 1 diabetes and allergy is a result of an interplay between the two sides of the Th-scale, producing a strong combined Th1- and Th2-like response. In contrast, children with type 1 diabetes and coeliac disease showed a low Th1-like response that may be due to dysregulation or suppression of the immune system.
Acknowledgments
This study has been generously supported by the Child Diabetes Foundation (Barndiabetesfonden), Schelins Foundation, the County of Östergötland and Linköping University and the Research Foundation of the Swedish Asthma and Allergy Association. The sources of funding had no role in the study, including the collection, analysis, interpretation of data, writing of the report and the decision to submit for publication. We would like to thank the research nurses Kicki Helander and Eva Isacsson for their skilful assistance with skin prick testing and blood sampling, Professor Outi Vaarala for providing gluten and Professor Johnny Ludvigsson for valuable comments on the manuscript.
References
- 1.Bach J-F. Insulin-dependent diabetes mellitus as an autoimmune disease. Endoc Rev. 1994;15:516–42. doi: 10.1210/edrv-15-4-516. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson MGE, Sederholm Lawesson S, Ludvigsson J. Th1-like dominance in high-risk first-degree relatives of type 1 diabetic patients. Diabetologia. 2000;43:742–9. doi: 10.1007/s001250051372. [DOI] [PubMed] [Google Scholar]
- 3.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–8. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 4.Keller RJ. Cellular immunity to human insulin in individuals at high risk for the development of type 1 diabetes mellitus. J Autoimmun. 1990;3:321–7. doi: 10.1016/0896-8411(90)90150-q. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson MA, Kaufman DL, Campbell L, et al. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992;339:458–9. doi: 10.1016/0140-6736(92)91061-c. [DOI] [PubMed] [Google Scholar]
- 6.Ellis TM, Schatz DA, Ottendorfer EW, et al. The relationship between humoral and cellular immunity to IA-2 in IDDM. Diabetes. 1998;47:566–9. doi: 10.2337/diabetes.47.4.566. [DOI] [PubMed] [Google Scholar]
- 7.Shan L, Molberg Ø, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 8.Nilsen EM, Jahnsen FL, Lundin KEA, et al. Gluten induces an intestinal cytokine response strongly dominated by interferon-γ in patients with celiac disease. Gastroenterology. 1998;115:551–63. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson MA, Bowman MA, Kao KJ, et al. Lack of immune responsiveness to bovine serum albumin in insulin-dependent diabetes. N Engl J Med. 1993;329:1853–8. doi: 10.1056/NEJM199312163292505. [DOI] [PubMed] [Google Scholar]
- 10.Åkerblom HK, Vaarala O. Cow milk proteins, autoimmunity and Type 1 diabetes. Exp Clin Endocrinol Diabetes. 1997;105:83–5. doi: 10.1055/s-0029-1211731. [DOI] [PubMed] [Google Scholar]
- 11.Kolb H, Pozzilli P. Cow’s milk and type 1 diabetes: the gut immune system deserves attention. Immunol Today. 1999;20:108–10. doi: 10.1016/s0167-5699(98)01425-x. [DOI] [PubMed] [Google Scholar]
- 12.Fälth-Magnusson K, Jansson G, Stenhammar L, Magnusson K-E. Serum food antibodies analysed by enzyme-linked immunosorbent assay (ELISA) and diffusion-in-gel (DIG)-ELISA methods in children with and without celiac disease. J Pediatr Gastroenterol Nutr. 1994;18:56–62. doi: 10.1097/00005176-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Mäki M. Coeliac disease and autoimmunity due to unmasking cryptic epitopes. Lancet. 1996;348:1046–7. doi: 10.1016/S0140-6736(05)64411-X. [DOI] [PubMed] [Google Scholar]
- 14.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 15.Barker JM, Yu J, Yu L, et al. Autoantibody ‘subspecificity’ in type 1 diabetes. Diabetes Care. 2005;28:850–5. doi: 10.2337/diacare.28.4.850. [DOI] [PubMed] [Google Scholar]
- 16.Romagnani S. Regulation of the development of type 2 T-helper cells in allergy. Curr Opin Immunol. 1994;6:838–46. doi: 10.1016/0952-7915(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 17.Kay AB. Allergy and allergic diseases (part 1) N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 18.Cronin CC, Shanahan F. Insulin-dependent diabetes mellitus and coeliac disease. Lancet. 1997;349:1096–7. doi: 10.1016/S0140-6736(96)09153-2. [DOI] [PubMed] [Google Scholar]
- 19.Walker-Smith J, Guandalini S, Schmitz J, Shmerling D, Visakorpi J. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson Faresjö MGE, Ludvigsson J. Diminished Th1-like response to autoantigens in children with a high risk of developing type 1 diabetes. Scand J Immunol. 2005;61:173–9. doi: 10.1111/j.0300-9475.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 21.Klemetti P, Savilathi E, Ilonen J, Åkerblom HK, Vaarala O. T-cell reactivity to wheat gluten in patients with insulin-dependent diabetes mellitus. Scand J Immunol. 1998;47:48–53. doi: 10.1046/j.1365-3083.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 22.Schloot NC, Meierhoff G, Karlsson Faresjö M, et al. Comparison of cytokine ELISpot assay formats for the detection of islet antigen autoreactive T cells. Report of the Third Immunology of Diabetes Society T-cell Workshop. J Autoimmun. 2003;21:365–76. doi: 10.1016/s0896-8411(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 23.Magnan AO, Mély LG, Camilla CA, et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Am J Respir Crit Care Med. 2000;161:1790–6. doi: 10.1164/ajrccm.161.6.9906130. [DOI] [PubMed] [Google Scholar]
- 24.Cho SH, Stanciu LA, Begishivili T, Bates PJ, Holgate ST, Johnston SL. Peripheral blood CD4+ and CD8+ T cell type 1 and type 2 cytokine production in atopic asthmatic and normal subjects. Clin Exp Allergy. 2002;32:427–33. doi: 10.1046/j.1365-2222.2002.01281.x. [DOI] [PubMed] [Google Scholar]
- 25.Smart JM, Kemp AS. Increased Th1 and Th2 allergen-induced cytokine response in children with atopic disease. Clin Exp Allergy. 2002;32:796–802. doi: 10.1046/j.1365-2222.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- 26.Durinovic-Belló I, Hummel M, Ziegler AG. Cellular immune response to diverse islet cell antigens in IDDM. Diabetes. 1996;45:795–800. doi: 10.2337/diab.45.6.795. [DOI] [PubMed] [Google Scholar]
- 27.Schloot NC, Roep BO, Wegmann D, et al. Altered immune response to insulin in newly diagnosed compared to insulin-treated diabetic patients and healthy control subjects. Diabetologia. 1997;40:564–72. doi: 10.1007/s001250050716. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson Faresjö M, Ernerudh E, Ludvigsson J. Cytokine profile in children during the first 3 months after the diagnosis of type 1 diabetes. Scand J Immunol. 2004;59:517–26. doi: 10.1111/j.0300-9475.2004.01420.x. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Chau C, Kaufman DL. Insulin selectively primers Th2 responses and induces regulatory tolerance to insulin in pre-diabetic mice. Diabetologia. 1998;41:237–40. doi: 10.1007/s001250050896. [DOI] [PubMed] [Google Scholar]
- 30.Falcone M, Yeung B, Tucker L, Rodriguez E, Krahl T, Sarvetnick N. IL-4 triggers autoimmune diabetes by increasing self-antigen presentation within the pancreatic islets. Clin Immunol. 2001;98:190–9. doi: 10.1006/clim.2000.4979. [DOI] [PubMed] [Google Scholar]
- 31.Kretowski A, Mysliwiec J, Szelachowska M, Kinalski M, Kinalska I. Insulin increases in vitro production of Th2 profile cytokines in peripheral blood cultures in subjects at high risk of diabetes type 1 and patients with newly diagnosed IDDM. Horm Metab Res. 1999;31:289–92. doi: 10.1055/s-2007-978736. [DOI] [PubMed] [Google Scholar]
- 32.Durinovic-Bello I, Schlosser M, Riedl M, et al. Pro- and anti-inflammatory cytokine production by autoimmune T cells against preproinsulin in HLA-DRB1*04, DQ8 type 1 diabetes. Diabetologia. 2004;47:439–50. doi: 10.1007/s00125-003-1315-1. [DOI] [PubMed] [Google Scholar]
- 33.Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995;37:766–76. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foulis AK, McGill M, Farquharson MA. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man − macrophages, lymphocytes, and interferon-γ containing cells. J Pathol. 1991;165:97–103. doi: 10.1002/path.1711650203. [DOI] [PubMed] [Google Scholar]
- 35.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–95. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 36.Signore A, Picarelli A, Annovazzi A, et al. 1123I-Interleukin-2: biochemical characterization and in vivo use for imaging autoimmune diseases. Nucl Med Commun. 2003;24:305–16. doi: 10.1097/00006231-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Auricchio R, Paparo F, Maglio M, et al. In vitro-deranged intestinal immune response to gliadin in type 1 diabetes. Diabetes. 2004;53:1680–3. doi: 10.2337/diabetes.53.7.1680. [DOI] [PubMed] [Google Scholar]
- 38.Paronen J, Klemetti P, Kantele JM, et al. Glutamate decarboxylase-reactive peripheral blood lymphocytes from patients with IDDM express gut-specific homing receptor α4β7-integrin. Diabetes. 1997;46:583–8. doi: 10.2337/diab.46.4.583. [DOI] [PubMed] [Google Scholar]
- 39.Braae Olesen A, Juul S, Birkebaek N, Thestrup-Pedersen K. Association between atopic dermatitis and insulin-dependent diabetes mellitus: a case–control study. Lancet. 2001;357:1749–52. doi: 10.1016/S0140-6736(00)04896-0. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbauer J, Herzig P, Giani G. Atopic eczema in early childhood could be protective against type 1 diabetes. Diabetologia. 2003;46:784–8. doi: 10.1007/s00125-003-1108-6. [DOI] [PubMed] [Google Scholar]
- 41.Caffarelli C, Cavagni G, Pierdomenico R, Chiari G, Spattini A, Vanelli M. Coexistence of IgE mediated allergy and type 1 diabetes in childhood. Int Arch Allergy Immunol. 2004;134:288–94. doi: 10.1159/000079166. [DOI] [PubMed] [Google Scholar]
- 42.Kero J, Gissler M, Hemminki E, Isolauri E. Could Th1 and Th2 disease coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108:781–3. doi: 10.1067/mai.2001.119557. [DOI] [PubMed] [Google Scholar]
- 43.Simpson CR, Anderson WJA, Helms PJ, et al. Coincidence of immune-mediated diseases driven by Th1 and Th2 subsets suggests a common aetiology: a population-based study using computerized General Practice data. Clin Exp Allergy. 2002;32:37–42. doi: 10.1046/j.0022-0477.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh A, Smeeth L, Hubbard R. There is no evidence of an inverse relationship between Th2-mediated atopy and Th1-mediated autoimmune disorders. J Allergy Clin Immunol. 2003;111:131–5. doi: 10.1067/mai.2003.8. [DOI] [PubMed] [Google Scholar]