Abstract
In coeliac disease, gliadin peptides p56–88, p57–68 and p31–49 have been demonstrated to be involved in the pathogenic damage of the small intestine via their immunogenicity or toxicity to epithelial cells. To try to understand the mechanism of their toxicity, we investigated the effect of synthetic peptides (p31–49, p56–88, p57–68, p69–82) and of their deamidated analogues on Caco2 and FHs 74 Int cell toxicity and tissue tranglutaminase activity. Apoptosis, necrosis and cell viability were assessed by flow cytometry, and peptide deamidation was determined indirectly by measuring its capacity to inhibit tTG activity. The results showed that p56–88 and p57–68 reduced cell growth and concomitantly inhibited tTG activity in both cell types. This effect was abolished when Caco2 cells were treated with antibodies to tTG. Deamidated peptide p57–68 (E65) lost practically all of its inhibitory effect on cell growth and on tTG activity. Cellular toxicity was also observed with p31–49, which was not a substrate for tTG. p69–82 was not cytotoxic but became so when glutamine 72 was substituted by glutamic acid. These findings provide evidence for the existence of three types of toxicity among gliadin peptides: (i) peptides that are intrinsically toxic and are not substrates of tTG; (ii) peptides that are non-toxic but become so when they act as substrates of tTG; and (iii) peptides that are non-toxic and are not substrates of tTG but become so when deamidated. A mechanism other than that involving tTG could be responsible for the deamidation of glutamine residues of gliadin in the intestinal tract.
Keywords: coeliac disease, gliadin peptides, transglutaminase
Introduction
Coeliac disease (CD) is an autoimmune enteropathy that manifests itself in genetically susceptible individuals due to their permanent intolerance to wheat gliadins and related proteins contained in cereals [1]. It is characterized by severe villous atrophy, crypt cell hyperplasia and an increase of intraepithelial lymphocytes [2,3]. Studies have clearly suggested a strong genetic predisposing factor, the HLA-DQ2 or DQ8 phenotype, which is able to present to T cells, gliadin-derived peptides deamidated by tissue transglutaminase (tTG) [4].
Tissue transglutaminase (tTG) (protein glutamyl γ-glutamyltransferase, TG2-EC 2·3.2·13), which plays a crucial role in the pathogenesis of CD, is a calcium-dependent enzyme that belongs to a family of protein-modifying enzymes that cross-link proteins by a two-step reaction [5]. This enzyme catalyses the formation of a covalent bond between the carboxamide groups of certain protein-bound glutamines (first substrate) and the ε- amino groups of lysines (second substrate) on the same or different protein chains [6]. This Nε-(γ-glutamyl) lysine isopeptide bond cross-links cellular proteins into an insoluble polymer [5].
Among the different cereal proteins, gliadin, which is rich in glutamine and proline, is a good substrate for tTG. In CD mucosa, the tTG secreted in the lamina propria is thought to act as a deamidase that converts glutamine residues to glutamic acid, thus generating specific negatively charged gliadin peptides that bind with high affinity to coeliac-associated HLA-DQ2 or DQ8 molecules [1,7,8]. Indeed, several deamidated peptides derived from various gluten proteins have been reported to stimulate selectively CD4+ T lymphocytes isolated from the small intestinal mucosa of CD patients [7,9]. There is consensus that the p33 mer peptide is the immunodominant (or unique predominant) peptide of A-gliadin, which resists digestion by gastric and pancreatic enzymes [10]; moreover, it contains two major immunodominant epitopes, PFPQPQLPY and PQPQLPYPQ, which lead to mucosal damage in CD patients via the γ-interferon pathway [11,12].
Among the gliadin peptides involved in the pathogenesis of CD, the p57–68 and p62–75 have been identified as the immunodominant peptides that are able to activate the adaptive immune response [11–13], and the p31–49 is able to stimulate an innate non-T-cell mediated immune response. Discrepancies exist between ‘toxicity’ and ‘immunogenicity’ because the toxic peptides elicit an innate response and therefore are unable to either bind HLA-DQ2/8 molecules or stimulate CD4+ T cells. However, some immunodominant peptides seem to be non-toxic for the intestinal mucosa in vitro, but it cannot be excluded that the same peptide may have both capacities [14–16].
Using different in vitro models or cells derived from intestinal biopsy specimens obtained from CD patients, studies have shown that peptic-digested wheat gliadin displays a direct cytotoxic effect on cells cultured from intestinal biopsy specimens of CD patients. Among the biological properties attributed to gliadin peptides, can be cited: morphological abnormalities, which, in turn, lead to a reduction in cell growth and viability [17–19]; cell agglutination [20,21]; induction of apoptosis in Caco2 cells via the Fas-Fas ligand pathway [22,23]; increase of nitric oxide and alteration of oxidative balance [24,25]; decrease in the synthesis of nucleic acids and proteins in Caco2 [26,27]; reorganization of intracellular actin filaments [25]; and, recently, maturation of dendritic cells [28].
However, the precise molecular mechanism of its effect on enterocytes or cells is unknown [14] and in spite of numerous studies, little is known about the mechanisms by which gliadin fractions damage the intestinal mucosa of CD patients.
Recently, the preferential glutamine residue used as a substrate by tTG was identified in sequence motifs QXP [29,30]. Indeed, the deamidation of glutamine residue at position 65 to glutamic acid in both p57–68 and p62–75 was found to be essential for optimal HLA-DQ2/8 binding and T-cell activation in vivo [7] and in vitro [31,32], but the importance of this deamidation step for toxicity towards the intestinal mucosa is still unknown.
Our aim was to investigate, the effect of synthetic peptides p31–49, p56–88, its N-terminal p57–68, its C-terminal p69–82 regions, and their modified or deamidated equivalents, in vitro on cells in culture to try to understand the role of gliadin in the pathogenesis of CD.
Materials and methods
Gliadin peptides
The following gliadin peptides: LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF corresponding to α9-gliadin (56–88) (p56–88), the N-terminal peptide QLQPFPQPQLPY (p57–68), corresponding to α9-gliadin (57–68), the peptide QLQPFPQPELPY (p57–68 E65) corresponding to the deamidated equivalent of p57–68, the C-terminal peptide PQPQLPYPQPQLPY (p69–82), corresponding to α9-gliadin (69–82), the peptide (PQPELPYPQPQLPY (p69–82 E72) corresponding to the deamidated equivalent of p69–82 and the peptide p31–49 (LGQQQPFPPQQPYPQPQPF) were all synthesized by Covalab (Lyon, France) and their purity was determined by preparative reversed-phase HPLC and mass spectrometry. An irrelevant peptide was used as an inactive control peptide. All peptides were dissolved in DMSO at a final concentration of 10 mM.
Cell culture and reagents
The human colon adenocarcinoma cell line Caco-2 was obtained from the American Type Culture Collection (ATCC, Salisbury, UK) and maintained in Dulbecco’s Modified Eagle Medium (D-MEM) (Sigma, Taufkirchen, Germany) supplemented with 12·5% fetal calf serum (Biotech Gmbh, Aidenbach, Germany) and 1% penicillin/streptomycin (Sigma). Cells were maintained in a humidified atmosphere of 5% CO2 in air; 5·105−106cells were seeded in 75 cm2 tissue culture flasks (Falcon, New Jersey, USA) and routine renewal of cell stocks was carried out twice weekly by removing cells with a solution containing trypsin–EDTA.
The culture medium was changed every 48 h. For experiments involving flow cytometry, the cells were detached by a solution of 4·5 mM EDTA in PBS for 3–5 min at 37 °C.
Foetal human intestinal cells (FHs 74 Int) (CCL-241) were obtained from the ATCC and grown in DMEM (hybri-care) containing 0·1 mM Hepes, 2 mM glutamine, 0·1 mM non-essential amino acids, 10% NCTC 135 medium, 0·1 mM oxaloacetic acid, 0·2 U/ml human recombinant insulin, 1 mM sodium pyruvate, supplemented with 30 ng/ml human recombinant epidermal growth factor, 10% foetal bovine serum (FBS) and 1% penicillin/streptomicyn.
Treatment of cells with peptides
For each experiment, 104 cells (Caco2 and FHs 74 Int) were seeded as described above in 24-well plates (Falcon, Becton Dickinson, Meylan, France). After 24 h culture, 3 days or 5 days, Caco2 cell monolayers grown in DMEM were washed and then treated with the different gliadin peptides dissolved in DMSO to a final concentration of 25 µM. One series of controls consisted of cells treated with DMSO at a final concentration of 0·25%. Another series consisted of cells treated with camptothecin (25 µM) as a positive control for apoptosis. Cells in the supernatant and adherent cells (monolayer) were removed after 24 and 48 h of treatment and analysed for apoptosis using Annexin V-FITC (Beckman-Coulter, Hialeath, Finland) and for necrosis using propidium iodide (PI) according to the manufacturer’s instructions. To determine the effect of gliadin peptides on cell growth, cell number was evaluated by counting on a haemocytometer (Malassez) or by an absolute count using flow-Count fluoresphere (Beckman-Coulter) according to the manufacturer’s instructions. For flow cytometry (EPICS XL, Beckman-Coulter) analysis, at least 10 000 events were acquired. Data were recorded and analysed statistically. At least five independent experiments were performed for each cell line.
Preparation of tTG from Caco2 and FHs Int cells
Homogenates were prepared from cultured Caco-2 as described previously [33]. Briefly, Caco2 cells were suspensed in 0·14 M NaCl containing 0·1% Triton X100, 1 mM dithiothreitol (DTT), 0·4 mM PMSF and 1 mM EDTA, 0·01 M Tris-HCl pH 7·5, then lysed by three successive cycles of freezing and thawing. The suspensions were centrifuged for 10 min at 600 g and the supernatants dialysed for 30 min against 1 mM EDTA, 0·14 M NaCl, 1 mM DTT and 0·01 M Tris-HCl pH 7·5. Protein content was determined by the Bradford method using BSA as the standard.
Determination of tTG activity
The transamidation activity of tTG in both cell lines was measured by the TG Covtest TCMA (Transglutaminase Colorimetric Microassay; Covalab) using immobilized CBZ-Gln-Gly (approximately 0·3 µmol/well) as the first substrate and biotin cadaverine as a second substrate [34]. Fifty-µl samples containing 200 µg of cellular protein from cells were added after rehydrating microtiter plates with 150 µl of phosphate buffered saline (PBS) containing 0·1% Tween 20 (PBST) at 37 °C for 30 min. For each sample, serial dilutions from 1/2 to 1/32 were made in 0·14 M NaCl. Purified guinea pig tissue tansglutaminase (gp-tTG) was used as a positive control. The different samples of tTG were incubated with 50 µl of biotin-cadaverine dissolved in 25 mM Tris buffered saline (TBS) containing 4 mM CaCl2, 200 mM NaCl and 5 mM dithithreitol (DTT) for 30 min at 37 °C. As the negative control, 10 µl of EDTA (5 mM) were added to the solution of biotin-cadaverine. At the end of the incubation period, plates were washed three times with Tween 20 Tris buffered saline (TTBS). Then, 100 µl of streptavidin-labelled peroxidase (HRP) diluted to 1/2000 in TTBS were added to the wells for 15 min. After washing, peroxidase activity was revealed using 100 µl of 0·01% H2O2 as HRP substrate and (0·1 mg/ml) tetramethyl benzidine as electron acceptor (chromogen). The reaction was stopped by the addition of 50 µl of 2·5 N H2SO4 and the absorbance read on a microplate reader at 450 nm.
Analysis of gliadin peptides as substrates for tTG
tTG activity was assessed in the presence or the absence (control) of gliadin peptides by TCMA. The gliadin peptides (p56–88, p57–68, p69–82, p57–68 E65, p69–82 E72 and p31–49) in solution in DMSO at final concentrations of 0·05 mM, 0·1 mM, 0·2 mM and 0·5 mM were introduced into wells to which had previously been added Caco2 homogenates with predetermined tTG activity or pure human recombinant TG. Incubation was carried out for 30 min at 37 °C, after which the contents of each well were transferred to another well for an additional 30 min incubation at 37 °C in the presence of biotin-cadaverine. The amount of biotin-cadaverine bound to the plate was measured as described above.
Cellular localization of tissue transglutaminase
Surface expression of tTG was verified by flow cytometry using specific mouse monoclonal anti-tTG antibodies AtTG mAbs (CUB7402, DAKO) and rabbit polyclonal anti-tTG antibodies ATG2 (Covalab). For the evaluation of cell surface tTG, EDTA detached cells (106) were incubated with (1 µg/ml) AtTG mAbs, its isotypic control, rabbit IgG anti-TG2 and normal rabbit IgG diluted in PBS. After 30 min, samples and isotypic controls were washed with PBS and stained with fluorescein-labelled goat anti-mouse IgG and anti-rabbit IgG, respectively, for 30 min. After two washes, cells were analysed by flow cytometry (Beckman-Coulter). At least 10 000 events were acquired. Data were recorded and analysed statistically. At least five independent measures were performed for each experiment.
Statistical analysis
Descriptive statistics were summarized by calculating mean values and standard deviations.
The Mann–Whitney U-test (SPSS) was used to compare the statistical significance of the inhibition of cell growth and the TG-activity and the surface expression of tTG with that of the controls. A P-value < 0·05 was considered as statistically significant.
Results
Effect of gliadin peptides on the growth of Caco2 and FHs 74 Int cells
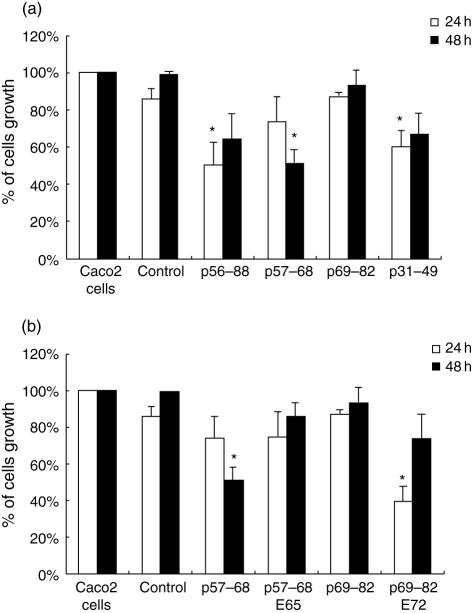
As shown in Fig. 1(a), a significant decrease of Caco2 cell number was seen with p56–88, p57–68 and p31–49 used at 25 µM. Indeed, the percentage of adherent Caco2 cells present after 24 h and 48 h of treatment with p56–88 and p57–68 was reduced to approximately 50% compared with controls. There was no significant difference between the growth of cells exposed to p69–82 and that of controls (P > 0·05). In a comparative study using FHs 74 Int cells, similar growth inhibition (60%) was observed (data not shown).
Fig. 1.
Effect of gliadin peptides on Caco2 cell growth after 24 and 48 h of treatment. (a) Native gliadin peptides (25 µM): p56–88, p57–68, p69–82 and peptide p31–49. (b) Modified gliadin peptides (25 µM): p57–68 (E65) and p69–82 (E72). Bars represent standard deviations and the mean of five independent experiments. *P < 0·05, significant inhibition compared with control.
These results show that p56–88 inhibited cell growth through its N-terminal region (p57–68) (P < 0·05), as its C-terminal region (p69–82) had no effect.
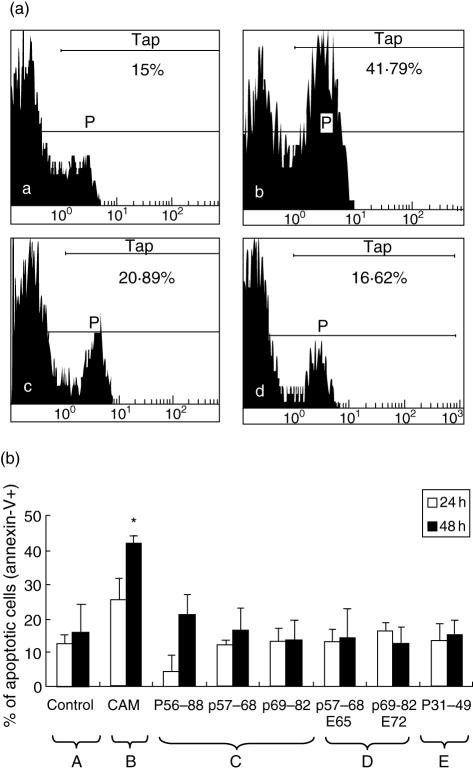
These results obtained with p56–88 confirm those previously published in which it has been demonstrated that this immuno-dominant gliadin peptide inhibits cell growth by inducing enterocyte apoptosis [22]. To try to determine if the growth inhibition seen here with p57–68 was also due to apoptosis/necrosis, these were measured as described in the material and methods section using p56–88 and campothecin as positive controls (Fig. 2a, b). It was found that, here too, there was no significant difference between the percentage of apoptotic or necrotic cells exposed to all the peptides tested compared with that of controls (Fig. 2b). It would therefore appear that the inhibition of cell growth observed with p56–88, p57–68 and p31–49 is due neither to apoptosis nor necrosis and that under the experimental conditions used here, apoptosis was not apparent either with p56–88. Possible reasons for this discrepancy with a previous report in the literature [22] will be discussed later.
Fig. 2.
(a) Typical example of flow cytometric analysis of Caco2 cells treated with different gliadin peptides (25 µM) and stained with Annexin V-FITC for apoptotic cells. Positive control with Camptothecin. a. DMSO (negative control). P. Percentage of total Caco2 cells. T. Percentage of apoptotic cells stained with Annexin V-FITC. b. Camptothecin (positive control), Tap in b-Tap in a = 26·79% of apoptotic cells induced by CAM (*P < 0·05, compared with control). c. p56–88, Tap in c-Tap in a = 5·89% of apoptotic cells induced by p56–88. d. p57–68, Tap in d-Tap in a = 1·62% of apoptotic cells induced by p57–68. (b) Percentage of apoptotic Caco2 cells treated with different compounds. A. DMSO (negative control). B. Camptothecin (positive control). *P < 0·05, compared with control. C. Native gliadin peptides p56–88, p57–68, p69–82. D. Modified gliadin peptides: p57–68 (E65) and p69–82 (E72). E. p31–49.
Effect of modified gliadin peptides on cell growth
To determine the importance of the glutamine residues in p57–68 for the inhibition of cell growth, gln 65 was changed to glu in p57–68 E65. The analysis of cells treated with this modified peptide during 24 h and 48 h showed that, contrary to the native peptide, cell growth was no longer inhibited (P < 0·05) (Fig. 1b). In the case of p69–82, the opposite effect was observed, in that the native peptide, which was not growth-inhibitory, became so when gln72 was changed to glu in p69–82 E72. These results suggest that the substitution of glu for gln in peptides has a profound influence on their effect on cells, but is dependent on the position of the glutamine residues and on the peptide itself. Analyses by flow cytometry showed that, here too, there was no effect of these two modified peptides on apoptosis and necrosis of Caco2 cells (Fig. 2b).
Effect of gliadin peptides on the activity of tTG in Caco2 and in FHs74 Int cell homogenates
In view of the importance of glutamine 65 in p57–68 to cytotoxicity, the well-documented action of tTG as a deamidation enzyme [31,35] and the apparent correlation between antibodies to tTG in CD patients and disease severity, we decided to determine if cytotoxic peptides were modulators or substrates of TG.
Before doing so, the basal activity of tTG in cells was first measured.
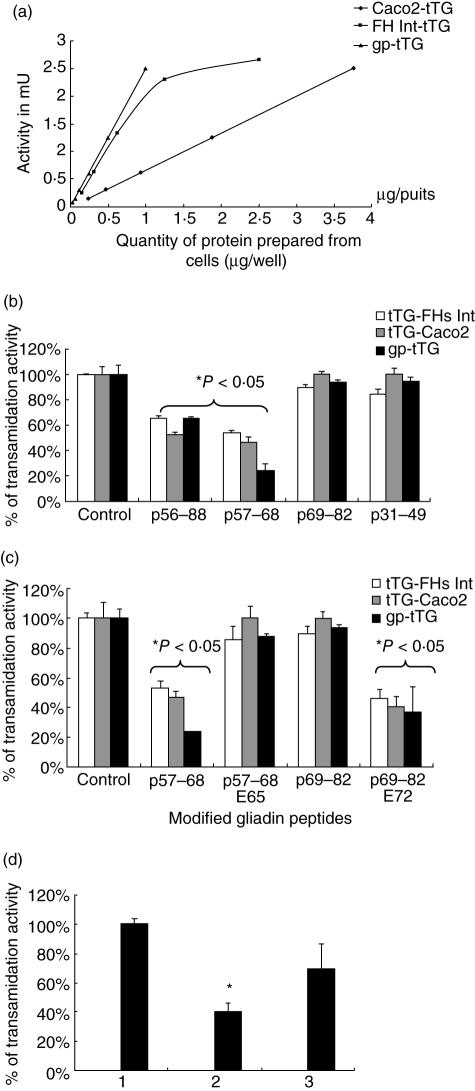
The results (Fig. 3a) show that there was a linear increase in tTG activity as the amount of cellular protein increased with FHs74 Int cells, showing a higher specific activity than Caco2 cells. Hence, 0·75–1 µg of Caco2 cell-homogenate were used to investigate the effect of the peptides.
Fig. 3.
(a) Measurement of the transamidation activity of TG prepared from Caco2 cells and FHs 74 Int cells. gp-tTG was used as a standard. (b) and (c) Inhibition of the transamidation activity of Caco2-tTG, FHs 74 Int-tTG cells and gp-tTG by different gliadin peptides at 2 mM. (b) Native gliadin peptides: p56–88, p57–68 and p69–82 and p31–49. (c) Modified gliadin peptides p57–68 (E65) and p69–82 (E72). Results represent the mean of three experiments, each done in duplicate. (d) P57-68 mediated inhibition of the activity of tTG extracted from caco2 cells. (1) Control DMSO, (2) p57–68, and (3) reversal of inhibition on transferring the contents of (2) to wells without p57–68.
Effect of native gliadin peptides on tTG activity
When this was examined as described in the methods section it was found that p56–88 and its N-terminal peptide, p57–68, both inhibited the activity of Caco2 TG as well as that of guinea pig liver TG, whereas p69–82, the C-terminal moiety, had no effect. P31-49, which was growth inhibitory, had no significant effect on TG activity (Fig. 3b).
These results suggest that for peptide p57–68, its growth inhibitory effect may be correlated with its capacity to serve as a substrate for tTG, whereas for p 31–49, its toxic effect must be linked with a mechanism other than TG-mediated deamidation.
Effect of modified gliadin peptides on tTG activity
To try to obtain additional evidence for the importance of gln 65 in p57–68 in the inhibition of TG activity, the effect of p57–68 E65 was investigated. It was found (Fig. 3c) that p57–68 E65 had no effect. On the contrary, p69–82 E72 inhibited TG activity, reaching up to about 60% at 0·5 mM (Fig. 3c). It is noteworthy that native p69–82, which did not inhibit TG activity (Fig. 3c), acquired this inhibitory capacity when gln 72 was replaced by glu. Possible implications of this observation will be discussed later.
This inhibition of TG activity by gliadin peptides p56–88, p57–68 and p69–82 E72 (Fig. 3b, c) could have been competitive due to a greater affinity of the enzyme for the peptides than for the immobilized first substrate (CBZ-Gln-Gly). It could also have been due to a direct inhibitory effect of the peptides on the enzyme itself. To examine these two possibilities, the reversibility of inhibition was investigated.
Reversibility of cellular TG inhibition
Accordingly, the reaction mixture containing tTG and inhibitory gliadin peptides, which had served for measuring enzyme activity, was transferred to new wells containing fresh first substrate and second substrate (biotin-cadaverine) but no additional peptide. Incubation was continued for a further 30 min at 37 °C. The results obtained (Fig. 3d) show that the majority (70%) of TG activity was recovered under these conditions, suggesting then that inhibition by peptides was due not to inactivation of the enzyme itself but to competition with the first substrate.
All the experiments carried out so far on enzyme activity have been done on total cell homogenates containing intracellular and possibly membrane TG. For the observations made to be of pathogenic relevance, evidence had to be obtained for the existence of TGs at the cell surface. This was sought using two approaches: flow cytometry on intact cells incubated with antibodies AtTG specific for tTG; and inhibition of peptide-induced cytotoxicity of intact cells by AtTG.
Localization of tTG on the cell surface
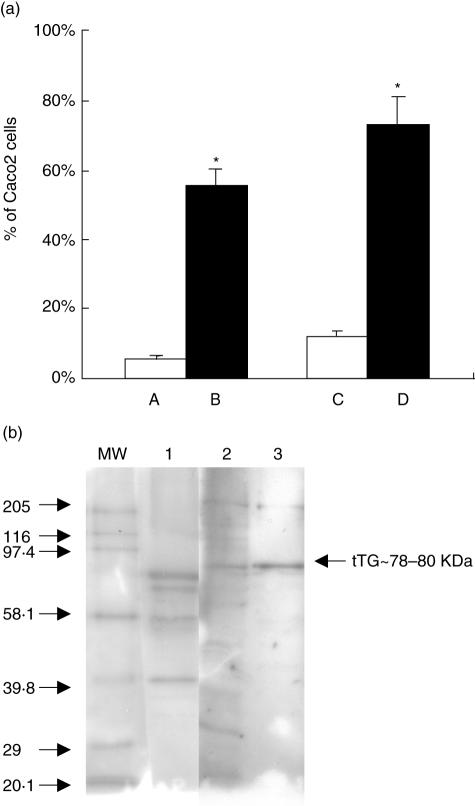
The results (Fig. 4a) show that 50% of non-permeabilized Caco2 cells express tTG at their membrane in comparison with an isotypic control. Confirmation of the presence of tTG was also obtained by immunoblots (Fig. 4b) on TG purified from the membranes of Caco2 and FHs74 Int cells in which a 78 kDa band corresponding to tTG can be clearly seen. To investigate whether growth inhibition was due to the interaction of peptide p57–68 with tTG at the cell surface, the peptide was added to Caco2 cells pre-incubated or not with AtTG.
Fig. 4.
(a) Analysis by flow cytometry of the expression of TG on Caco2 cell membrane. A. Normal rabbit IgG. B. Rabbit IgG anti-TG2. C. Isotypic control (mouse IgG). D. mAb anti-tTG. *P < 0·05, significant detection of tTG on the membrane of cells compared with isotypic control. (b) Detection of tTG by immunoblot using mAb anti-tTG. MW, molecular weight standard; line1, gp-tTG; line 2, protein extract of Caco2 cells; line 3, protein extract of FHs74 Int cells.
Effect of AtTG on cellular TG activity and on the inhibition of cell growth by gliadin peptides
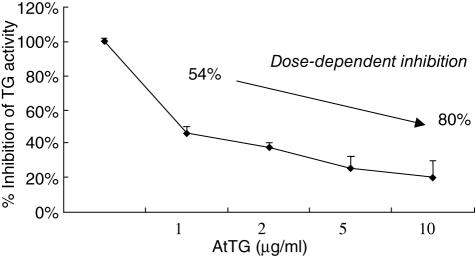
Figure 5 shows clearly that the AtTG inhibits cellular TG activity from Caco2 cells and that the inactivation of the cellular enzyme is dose dependent, passing from 54% inhibition in the presence of 1 µg AtTG to 80% in the presence of 10 µg.
Fig. 5.
Effect of mAb antit-TG on Caco2-TG activity. Dose-dependent inhibition of TG activity measured by TG-covtest.
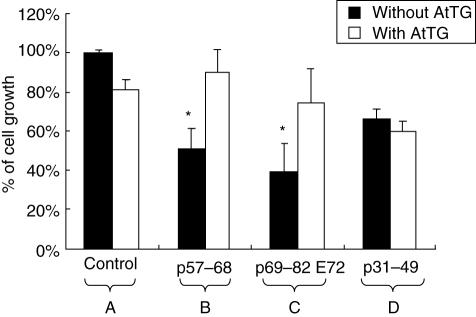
Results (Fig. 6) show that the AtTG protects cells from the growth-inhibitory effect of peptides p57–68 and p69–82 E72 (Fig. 6), whereas it does not protect cells from the toxic effect of p31–49 (Fig. 6). In this latter case, as also shown (Fig. 6), there is no significant difference between the number of Caco2 cells treated with peptides in the presence or absence of AtTG.
Fig. 6.
Effect of mAb antit-TG on the gliadin-induced inhibition of the growth of Caco2. A. Control (DMSO), with AtTG, without AtTG. B. Inhibition of cell growth by p57–68; neutralization of the effect of p57–68 by AtTG. C. Inhibition of cell growth by p69–82 (E72); neutralization of the effect of p69–82 (E72) by AtTG. D. Inhibition of the cell growth by p31–49 in the absence and presence of AtTG. *P < 0·05, significant compared with control without AtTG.
Discussion
The results presented provide evidence that the glutamine-containing gliadin peptides inhibit the growth of Caco2 and FHs 74 Int cells by mechanisms dependent on and independent of their interaction with cell-surface tTG.
One example of a cell-surface tTG-dependent mechanism is that involving p57–68 (N terminal of p56–88), which loses its toxicity when intact Caco2 cells are pre-treated with AtTG (Fig. 6) before adding peptide. It must be noted though, that, in addition to tTG at the cell surface, results from flow cytometry have also provided evidence for the presence of membrane TG (TG1) (data not shown). Hence, the loss of the gliadin-induced growth inhibition with AtTG pre-treated cells clearly implicates tTG present at the cell surface but does not eliminate the possibility that membrane TG1 may also be involved. This will be clarified when similar experiments are performed with ATG1.
With regard to the particular glutamine residue of p57–68, which is recognized by tTG, we have been able to obtain unequivocal evidence that it is gln 65 because its replacement by glu 65 in p57–68 E65 brought about a loss of both the growth inhibition (Fig. 1b) and TG inhibitory effects (Fig. 3c). A closer look at the amino acids surrounding gln 65 shows that this PQL sequence is present not only in p57–68 but also in p69–82 at two positions: 71–73 and 78–80. Yet p69–82 (C terminal of p56–88) was not toxic (Fig. 1a) and did not inhibit tTG activity (Fig. 3b). This finding clearly shows that the mere presence of the PQL sequence is insufficient for classifying a gliadin peptide as growth inhibitory and suggests that accessibility of the PQL sequence to tTG may also be a contributing factor. Evidence in support of this latter concept is provided by the results obtained with p69–82, which, as said before, inhibited neither cell growth nor tTG activity (Figs 1a and 3b) but acquired both characteristics when gln72 was changed to glu in p69–82 E72 (Figs 1b and 3c). A factor that could have contributed to the recognition of p69–82 E72 by tTG is the presence on the glu of E72 of a negative charge that could have changed the configuration of p69–82 E72 and thus exposed the 78–80 PQL sequence. This replacement of gln72 by glu and the ensuing inhibitory effect of the deamidated peptide p69–82 E72 on both cell growth and on tTG activity provides a second example of TG-dependent growth inhibition. However, the real question is whether this putative deamidation of gln 72 takes place in vivo. Our results do not permit us to answer this question, as the approach used here was restricted to cells in culture. Nevertheless, it must be remembered that the γ-carboxamide of gln is readily cleaved enzymically by glutaminases and chemically at an alkaline pH [36]. These two reactions could be mediated by the intestinal flora.
With regard to the mechanism of inhibition of tTG (Fig. 3), the inhibitory peptides contain no lysine residues. Therefore, TG as a two substrate enzyme using peptide-bound gln residues as first substrates (amine acceptors) and protein-bound lys residues as second substrates (amine donors) could be inserting the synthetic amine donor, biotin-cadaverine, into the PQL sequence of the peptides and not into the immobilized first substrate CBZ-LGln-LGly. Were this to be the case, then TG inhibition should be reversible in the absence of peptides. Indeed, TG activity recovered to 70% when the reaction mixture from the first incubation was reincubated in fresh wells with immobilized CBZ-LGln-LGly and free biotin-cadaverine but in the absence of additional peptides.
Should a similar TG-mediated reaction take place when peptides are added to intact cells, then peptide-bound lysine residues of membrane proteins must serve as amine donors [37,38]. Their identities are at present unknown but one cell-surface protein that can act as an amine donor is TG itself [39]. This would result in the formation of tTG-gliadin complexes linked together by isopeptide bonds. There is evidence in the literature for the existence of these complexes [39]. As a result, changes take place in the configuration of the complex, resulting in the exposure of formerly cryptic epitopes on both TG and gliadin. Such a mechanism offers one plausible explanation for the origin of the immunogenicity of endogenous TG in CD [7,12,40]. These deamidation and transamination steps, which are an integral part of TG function, also offer a rationale for the formation of deamidated gliadin peptides in CD [12,31], as they could be formed if lysine residues of membrane proteins were not available as second substrates because, for example, of their pre-engagement in isopeptide bond formation with other proteins. It must be recalled that the binding of negatively charged deamidated gliadin peptides to HLA-DQ2 or D8 with high affinity is a characteristic of CD [1,8,41]. On the contrary, should the lysines of membrane proteins on neighbouring cells serve as amine donors, then this would explain the cell agglutination seen in the presence of gliadin peptides described by other authors [20,21].
With regard to the TG-independent growth-inhibitory activity shown by p31–49, it too can be explained on the one hand, by the absence of the PQL sequence and of lys that are recognized by TG, and on the other, by the presence of poly gln repeats at 33–35 and 40–41. Poly gln sequences have been shown to be toxic to cells in model cultures of Huntington disease [42]. Mauiri and coworkers showed that p31–43 is apoptogenic on T84 cells and binds to the cell membrane as measured by immunofluorescence. Peptide binding, as well as apoptosis, was inhibited by mAbs to TG2 and Fas [35]. In our case, rhodamine labelled p31–49 was found to bind to the membrane of Caco2 cells (data not shown), bringing about growth inhibition but without any evidence for apoptosis (Fig. 2b), or to binding to TG2. There is an obvious discrepancy between the results obtained by Mauiri and us that can be ascribed only to the difference in peptide length. The six amino acids that are present in our peptide but absent in that used by Mauiri could have influenced the folding of the p31–49 peptide, thus rendering its gln residues inaccessible to tTG.
The precise mechanism of the TG-mediated growth inhibitory effect of p57–68 and p69–82 E2 requires further study, as it apparently involves neither apoptosis nor necrosis. This is contrary to the findings of other authors showing that Caco2 cells undergo apoptosis when they are treated with the p57–68 gliadin peptides [22,23]. The difference between their results and ours may be linked to differences in experimental design. Indeed, in that report cells were treated with peptic-tryptic digests of gliadin and therefore contained not only p57–68 but also all the other gliadin peptides of unknown composition and activity. In our case, synthetic peptides with known sequences were used. It may therefore mean that the apoptosis/necrosis seen by those authors could have been due to the other unidentified peptides.
Regardless of the cellular mechanism responsible for inhibiting cell growth, our results provide good evidence for the key role played by cell-surface TG2 in the cytotoxic action of glutamine-containing gliadin peptides, be they substrates of TG directly or indirectly after putative enzymic or chemical deamidation in the intestinal tract.
Acknowledgments
This work was supported by CMCU (Comité Mixte Franco-Tunisien pour la Coopération Universitaire) and by OSEO ANVAR, Lyon, France (grant #A0312390V).
References
- 1.Sollid M. Molecular basis of coeliac disease. Ann Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Walker Smith JA, Guandalini S, Schmitz J, Sherling DH, Visakorpi JK. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Pediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Vader W, Stepniak D, Kooy Y, et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc Natl Acad Sci. 2003;100:12390–5. doi: 10.1073/pnas.2135229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folk JE, Finlayson JS. The Nε-(γ-glutamyl) lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- 6.Birckbichler PJ, Dowben RM, Matacic S, Loewy AG. Isopeptide bonds in membrane proteins from eukaryotic cells. Biochim Biophys Acta. 1973;291:149–55. doi: 10.1016/0005-2736(73)90070-9. [DOI] [PubMed] [Google Scholar]
- 7.Molberg O, McAdam S, Lundin KE, et al. T cells from coeliac disease lesions recognise gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur J Immunol. 2001;31:1317–23. doi: 10.1002/1521-4141(200105)31:5<1317::AID-IMMU1317>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Quarsten H, Molberg O, Fugger L, McAdam SM, Sollid LM. HLA binding and T cell recognition of a tissue transglutaminase-modified gliadin epitope. Eur J Immunol. 1999;29:2506–14. doi: 10.1002/(SICI)1521-4141(199908)29:08<2506::AID-IMMU2506>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Novak P, Man P, Tuckova L, Tlaskalova-Hogenova H, Bezouska K, Havlicek V. Monitoring of in vitro deamidation of gliadin peptic fragment by mass spectrometry may reflect one of the molecular mechanisms taking place in coeliac disease development. J Mass Spectrom. 2002;37:507–11. doi: 10.1002/jms.305. [DOI] [PubMed] [Google Scholar]
- 10.Hausch F, Shan L, Santiago NA, Gray GM, Khosla C. Intestinal digestive resistance of immunodominant gliadin peptides. Am J Physiol Gastrointest Liver Physiol. 2002;283:996–1003. doi: 10.1152/ajpgi.00136.2002. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AVS. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med. 2000;6:337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 12.Arentz-Hansen H, Korner R, Molberg O, et al. The intestinal T cell response to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J Exp Med. 2000;191:603–12. doi: 10.1084/jem.191.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundin KE, Scott H, Hansen T, et al. Gliadin-specific, HLA-DQ (a1*0501,b1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993;178:187–96. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan L, Molberg O, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–9. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 15.Martucci S, Fraser JS, Biagi F, Corazza GR, Ciclitira PJ, Ellis HJ. Characterizing one of the DQ2 candidate epitopes in coeliac disease: A-gliadin 51–70 toxicity assessed using an organ culture system. Eur J Gastroenterol Hepatol. 2003;15:1293–8. doi: 10.1097/00042737-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Fraser JS, Engel W, Ellis HJ, et al. Coeliac disease: in vivo toxicity of the putative immunodominant epitope. Gut. 2003;52(12):1698–702. doi: 10.1136/gut.52.12.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson DA, Cornell HJ, Purdham DR, Rolles CJ. Non-specific cytotoxicity of wheat gliadin components towards cultured human cells. Lancet. 1976;14(1):339–41. doi: 10.1016/s0140-6736(76)90089-1. [DOI] [PubMed] [Google Scholar]
- 18.Dolfini E, Elli L, Dasdia T, et al. In vitro cytotoxic effect of bread wheat gliadin on the LoVo human adenocarcinoma cell line. Toxicol Vitro. 2002;16:331–7. doi: 10.1016/s0887-2333(02)00017-6. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini C, Maiuri L, De Vincenzi M. Cytotoxic effect of prolamin-derived peptides on in vitro cultures of cell line Caco-2: implications for coeliac disease. Toxicol In Vitro. 1994;9:251–5. doi: 10.1016/0887-2333(94)00212-d. [DOI] [PubMed] [Google Scholar]
- 20.Auricchio S, De Ritis G, De Vincenzi M, et al. Agglutinating activity of gliadin-derived peptides from bread wheat: implications for coeliac disease pathogenesis. Biochem Biophys Res Commun. 1984;121:428–33. doi: 10.1016/0006-291x(84)90200-6. [DOI] [PubMed] [Google Scholar]
- 21.De Vincenzi M, Stammati A, Luchetti R, Silano M, Gasbarrini G, Silano V. Structural specificities and significance for coeliac disease of wheat gliadin peptides able to agglutinate or to prevent agglutination of K562 (S) cells. Toxicology. 1998;127:97–106. doi: 10.1016/s0300-483x(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 22.Giovannini C, Sanchez M, Straface E, Scazzocchio B, Silano M, De Vincenzi M. Induction of apoptosis in caco-2 cells by wheat gliadin peptides. Toxicology. 2000;145(1):63–71. doi: 10.1016/s0300-483x(99)00223-1. [DOI] [PubMed] [Google Scholar]
- 23.Giovannini C, Matarrese P, Scazzocchio B, et al. Wheat gliadin induces apoptosis of intestinal cells via an autocrine mechanism involving Fas-Fas ligand pathway. FEBS Lett. 2003;540(1–3):117–24. doi: 10.1016/s0014-5793(03)00236-9. [DOI] [PubMed] [Google Scholar]
- 24.Rivabene R, Mancini E, De Vincenzi M. In vitro cytotoxic effect of wheat gliadin-derived peptides on the Caco-2 intestinal cell line is associated with intracellular oxidative imbalance: implications for coeliac disease. Biochim Biophys Acta. 1999;1453:152–60. doi: 10.1016/s0925-4439(98)00095-7. [DOI] [PubMed] [Google Scholar]
- 25.Clemente MG, De Virgiliis S, Kang JS, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218–23. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giovannini C, Lucchetti R, De Vincenzi M. The activities of peptides ‘31–43’, ‘44–55’ and ‘56–68’ of A-gliadin on in vitro cultures of Caco-2 cells. ATLA. 1997;25:437–43. [Google Scholar]
- 27.Giovannini C, Mancini E, De Vincenzi M. Inhibition of the cellular metabolism of Caco-2 cells by prolamin peptides from cereals toxic for coeliacs. Toxicol In Vitro. 1996;10:533–8. doi: 10.1016/s0887-2333(96)00042-2. [DOI] [PubMed] [Google Scholar]
- 28.Nikulina M, Habich C, Floh B, Scott F, Kolb H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol. 2004;173:1925–33. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- 29.Vader W, Kooy Y, van Veelen P, et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology. 2002;122:1729–37. doi: 10.1053/gast.2002.33606. [DOI] [PubMed] [Google Scholar]
- 30.Sugimura Y, Hosono M, Wada F, Yoshimura T, Maki M, Hitomi K. Screening for the preferred substrate sequence of transglutaminase using a phage-displayed peptide library: identification of peptide substrates for TGASE 2 and Factor XIIIA. J Biol Chem. 2006;281:17699–706. doi: 10.1074/jbc.M513538200. [DOI] [PubMed] [Google Scholar]
- 31.Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut derived T cells in celiac disease. Nat Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 32.Elli L, Dolfini E, Bardella MT. Gliadin cytotoxicity and in vitro cell cultures. Toxicol Lett. 2003;146:1–8. doi: 10.1016/j.toxlet.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.El Alaoui S, Legastelois S, Roch AM, Chantepie J, Quash G. Transglutaminase activity and N epsilon (gamma glutamyl) lysine isopeptide levels during cell growth: an enzymic and immunological study. Int J Cancer. 1991;48(2):221–6. doi: 10.1002/ijc.2910480212. [DOI] [PubMed] [Google Scholar]
- 34.Thomas V, El Alaoui S, Massignon D, Clement S, Simonet F, Quash G. Development and evaluation of a modified colorimetric solid-phase microassay for measuring the activity of cellular and plasma (Factor XIII) transglutaminases. Biotechnol Appl Biochem. 2006;43:171–9. doi: 10.1042/BA20050161. [DOI] [PubMed] [Google Scholar]
- 35.Maiuri L, Ciacci C, Ricciardelli I, et al. Unexpected role of surface transglutaminase type II in celiac disease. Gastroenterol. 2005;129:1400–13. doi: 10.1053/j.gastro.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 36.Fleckenstein B, Molberg O, Qiao SW, et al. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation reactions. J Biol Chem. 2002;277:34109–16. doi: 10.1074/jbc.M204521200. [DOI] [PubMed] [Google Scholar]
- 37.Jones RA, Nicholas B, Mian S, Davies PJ, Griffin M. Reduced expression of tissue transglutaminase in a human endothelial cell line leads to changes in cell spreading, cell adhesion and reduced polymerisation of fibronectin. J Cell Sci. 1997;110(19):2461–72. doi: 10.1242/jcs.110.19.2461. [DOI] [PubMed] [Google Scholar]
- 38.Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000;41:1–27. doi: 10.3109/03008200009005638. [DOI] [PubMed] [Google Scholar]
- 39.Fleckenstein B, Qiao SW, Larsen MR, Jung G, Roepstorff P, Sollid LM. Molecular characterization of covalent complexes between tissue transglutaminase and gliadin peptides. J Biol Chem. 2004;279:17607–16. doi: 10.1074/jbc.M310198200. [DOI] [PubMed] [Google Scholar]
- 40.Van de Wal Y, Kooy YMC, van Veelen PA, et al. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc Natl Acad Sci USA. 1998;95:10050–4. doi: 10.1073/pnas.95.17.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim C-H, Quarsten H, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci USA. 2004;101:4175–9. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper JK, Schilling G, Peters MF, et al. Truncated N-terminal fragments of huntingtin with expanded glutamine repeats form nuclear and cytoplasmic aggregates in cell culture. Hum Mol Genet. 1998;7:783–90. doi: 10.1093/hmg/7.5.783. [DOI] [PubMed] [Google Scholar]