Abstract
Maternal transmission of islet autoantibodies to children born to mothers with type 1 diabetes (T1D) has been shown to protect from autoantibodies and diabetes development later in life. However, the factors conferring disease protection are poorly understood. The aim of this study was to evaluate comparatively proinflammatory cytokines, autoantibodies and lymphocyte subsets in cord blood (CB) of children born to mothers with either T1D (n = 13), gestational diabetes (GDM) (n = 32) or healthy mothers (n = 81) in relation to transplacental passage of autoantibodies. The results are consistent with early priming of the fetal immune system only in children born to mothers with T1D. Levels of interleukin (IL)-1β (P = 0·022), tumour necrosis factor (TNF)-α (P = 0·002) and IL-8 (P = 0·0012), as well as the frequency of CD4+ CD25+ T cells (P < 0·01) were significantly increased, and the increased levels correlated positively with anti-GAD65 autoantibody (GADA) levels. Moreover, CD4+ CD25+ T cells of children born to T1D mothers exhibited a more pronounced memory phenotype with increased CCR4 expression and down-regulation of CD62L. These data suggest that early activation of the fetal immune system as a consequence of maternal autoimmunity and transplacental passage of GADA may influence the generation and expansion of fetal regulatory T cells. This might induce an early antigen-specific immunological tolerance that could protect against T1D later in life.
Keywords: cord blood, lymphocyte subsets, regulatory T cells, type 1 diabetes mellitus
Introduction
Type 1 diabetes mellitus (T1D) is a multi-factorial autoimmune disease affecting up to 1% of the general population. T1D develops most often at an early age and always after a complete or partial loss of insulin production due to T cell-mediated destruction of pancreatic islet β-cells. The role of T cells in T1D is well established, and there is a clear association between certain major histocompatibility complex (MHC) alleles and T1D [1]. Moreover, T cells infiltrating pancreatic islets have been identified [2] and T cell clones specific for islet-related autoantigens have been established from T1D patients [3]. However, what is still unclear is when and how these autoreactive T cells are generated.
It has been proposed that T1D autoimmunity might already be initiated in utero. For example, several studies have reported that gestational infections might represent a risk factor for T1D development [4–6]. In contrast, exposure to maternal diabetes in utero has been suggested to represent a protective factor for the offspring during the first two decades of life [5,7]. This finding is reinforced by studies indicating that children born before maternal diabetes onset have a higher risk of developing diabetes than children who are born after the onset of maternal diabetes [7]. Moreover, Koczwara et al. have reported recently that fetal exposure to islet autoantibodies in children born to T1D mothers resulted in protection against future islet autoimmunity and diabetes [8], suggesting that transplacental transmission of autoantibodies could contribute to tolerance induction later in life. Thus, although it appears clear that T1D in mothers may affect the development of the fetal immune system, the factors that may lead to disease protection in the offspring are not elucidated fully.
The present study aims at characterizing the immune systems of children born to T1D mothers directly after delivery in relation to transplacental passage of islet autoantibodies. Our findings suggest that the immune system of children receiving autoantibodies from mothers with T1D is primed during fetal life. This priming may lead to immunological changes in the newborn that result in protection from the development of autoimmunity later in life.
Materials and methods
Subjects
Cord blood samples were collected from a total of 126 children born to healthy mothers (healthy group; n = 81), to mothers with gestational diabetes (GDM group; n = 32) or to mothers diagnosed with T1D (T1D group; n = 13) without evidence of gestational or intrauterine infections. Mothers with emergency delivery or with pathological pregnancies were excluded from the study. All caesarean sections (C-section) were elective and were not due to pathological causes or fetal distress. All diabetic mothers had a level of HbA1c below 7% during the last 4–8 weeks of pregnancy, demonstrating good glycaemic control. None of the mothers with GDM were treated with insulin, excluding the possibility of enrolling women with T1D development during pregnancy and not diagnosed properly. A descriptive summary of all children and their mothers is presented in Table 1.
Table 1.
Demographics of the subjects included in the study.
| Healthy children | Children born to GD mothers | Children born to T1D mothers | |
|---|---|---|---|
| Subjects included in the study | 82 | 32 | 13 |
| Gestational week at birth (mean ± s.d.) | 39·9 ± 1·6 | 39·4 ± 1·0 | 37·4 ± 1·6 |
| Delivery | Vaginal: 69 | Vaginal: 28 | Vaginal: 9 |
| Caesarean section: 13 | Caesarean section: 4 | Caesarean section: 5 | |
| Mean age of mother at delivery (range) | 31·4 (18·2–40·2) | 32·2 (18·3–38·3) | 30·0 (23·0–44·4) |
| Mother’s origin | Sweden: 67 | Sweden: 22 | Sweden: 12 |
| Foreign: 15 | Foreign: 10 | Foreign: 1 |
Cord blood samples were collected into ethylenediamine tetraacetic acid (EDTA) tubes at Malmö University Hospital, Lund University, as well as at the hospitals in Ystad, Helsingborg and Kristianstad, and were processed within 24 h. Filter papers were also blotted with fresh cord blood for human leucocyte antigen (HLA) typing and GAD65 and IA-2 autoantibody analyses. Peripheral blood was obtained from adult healthy volunteers and collected in EDTA tubes. The Lund University Research Ethics Committee approved the study and written informed consent was obtained from participating mothers and healthy adult volunteers.
Quantitative analysis of plasma cytokines
Cord blood plasma was collected and analysed for secreted cytokines [tumour necrosis factor (TNF)-α, interleukin (IL)-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-1β, interferon (IFN)-γ] using a cytometric bead array (CBA; Becton-Dickinson, Franklin Lakes, NJ, USA), according to the manufacturer’s instructions. Samples were analysed by flow cytometry using fluorescence activated cell sorter analysis (FACScalibur) and CBA software (both from Becton-Dickinson).
Briefly, 50 µl of mixed beads coated with cytokine-specific capture antibodies were added to 50 µl of patient plasma and incubated for 1·5 h at room temperature. After washing, 50 µl of phycoerythrin-conjugated (PE) anti-human cytokine antibodies were added. Simultaneously, 50 µl of standards for each cytokine (0–5000 pg/ml) were treated similarly to generate a standard curve. Two-colour flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson). Data were acquired and analysed using Becton Dickinson CBA software. Forward and side scatter gating was employed to exclude any sample particles other than the 7·5 µm polystyrene beads. A single operator performed flow cytometric analyses and sample cytokine concentrations were determined based on the standard curves using the CBA software. The lower limit of detection for the various cytokines evaluated ranged from 2 to 10 pg/ml depending on the cytokine. For results above the upper limit of detection, serial dilutions of those samples were performed to determine cytokine levels accurately. A level of ≤ 0·1 pg/ml was regarded as being non-detectable.
Flow cytometry of cord blood lymphocytes
The absolute number of lymphocytes in cord blood was estimated using an automated cell counter (AC900EO AutoCounter; Swelab, Stockholm, Sweden). Combining these results with the FACS data the absolute numbers of T cells, B cells and natural killer (NK) cells were calculated. For lymphocyte subset analysis, cord blood was stained with various fluorochrome-conjugated antibodies directed against the following markers: CD3 [PE- or fluorescein isothiocyanate (FITC)-conjugated], CD4 [peridinin chlorophyll (PerCP)-conjugated], CD8 (PE- or PerCP-conjugated), CD25 (FITC-conjugated), CD62L [antigen-presenting cell (APC)-conjugated], CD45RA (PE-conjugated), CD45RO (APC-conjugated), CD19 (PerCP-conjugated), CD16 (PE-conjugated) CD56 (PE-conjugated), CCR4 (PE-conjugated) and irrelevant isotype controls, IgG2a (FITC-conjugated) and IgG1 (PE- and APC-conjugated), all from Becton-Dickinson. After 20 min incubation at room temperature erythrocytes were lysed using lysis buffer (Becton-Dickinson) according to the manufacturer’s protocol, centrifuged at 400 g for 10 min, washed in FACS buffer [0·5% bovine serum albumin (BSA) and 2 mM EDTA in phosphate-buffered saline (PBS)], centrifuged and diluted in appropriate volume of FACS buffer. For forkhead box P3 (FoxP3) expression analysis in cord blood and adult peripheral blood samples, intranuclear staining was performed using a rat monoclonal antibody (APC-conjugated) following the manufacturer’s instructions (clone PCH101; eBioscience, San Diego, CA, USA). The samples were acquired in a four-colour FACScalibur® (Becton-Dickinson) and analysed using CellQuest® software (Becton-Dickinson).
Autoantibody measurement
Autoantibodies against GAD65 or IA-2 in cord blood plasma were analysed using a radio-binding assay as described elsewhere [9,10]. First, a combined analysis was made in which the plasma was considered positive for either or both autoantibodies if the ratio between the internal World Health Organization (WHO) positive control [11] and the mean of the negative control was 1·2 or higher. If the screen for both autoantibodies was considered positive, the sample was analysed for each autoantibody individually. Our laboratory participated as laboratory no. 302 in the Diabetes Antibody Standardization Program (DASP) workshop [12] and was selected as a reference and training laboratory for IA-2 antibody measurement.
Autoantibody levels were expressed in International Units (IU) using the WHO standard as reference. The cut-off level of GAD65 autoantibodies was 32 IU/ml and of IA-2 autoantibodies was 6 IU/ml.
HLA typing
HLA typing was conducted as described [13,14]. In order to obtain the HLA types in a timely fashion we used dried blood spots, punch-out samples in a 96-well format with polymerase chain reaction (PCR) directly from the filters using biotinylated primers for DQB1, streptavidin-coated 96-well plates and lanthanide-labelled probes for the hybridization, essentially as described [13,14]. All reagents were from Wallac Oy, Perkin Elmer Life Sciences (Turku, Finland). The following HLA DQB1 alleles were analysed indicating T1D risk within parentheses: 02 (risk), 0302 (risk), 0301 (protective), 0602 (protective), 0603 (protective) and 0604 (risk when 0302 is also present). The DQB1 genotype was considered to be high-risk if the allele combinations were 02/0302 or 0302/X or 0302/0604; low-risk if the allele combinations were 02/X, 02/0304 or 02/0604. Allele X is neither 0301, 0602 nor 0603. If the DQB1 haplotype contained 02 as an allele, the DQA1 haplotypes were analysed for the following alleles: 05 (risk-associated allele) and 0201 (neutral allele). The combinations of both DQB1 and, in some circumstances DQA1 haplotypes, defined each individual’s genetic risk of developing T1D.
Statistics
Lymphocyte cell counts were expressed as mean ± s.e. Unpaired Student’s t-test was used for statistical differences between groups. CBA and FACS distributions were non-normally distributed and the corresponding median, minimum/maximum and non-parametric Mann–Whitney tests were used instead. Correlation analysis was performed using Pearson’s r-test. Linear regression analysis examined the association between cytokines and HLA risk of offspring after adjusting for diabetes status of the mother. Graphs and analyses were performed using statistical programs prism 4 (GraphPad Software, San Diego, CA, USA) or splus 6·1 (Insightful Corp., Seattle, WA, USA). Unless stated otherwise, P-values less than 0·05 were considered significant. When several groups were compared, a Bonferroni correction was made.
Results
Increased levels of proinflammatory cytokines in cord blood of children born to T1D and GDM mothers
CBA was used to quantify the levels of cytokines in the CB plasma of all the newborns. Children born to T1D mothers (T1D group; n = 13) displayed higher proinflammatory cytokines compared to the healthy group (n = 81), as depicted in Table 2, i.e. IL-1β (median value 45·2 versus 22·5 pg/ml; P = 0·022) and IL-8 (521·8 versus 45·8 pg/ml; P = 0·0012). Although in CB plasma the levels of TNF-α were found to be low, especially in the healthy and GDM group with some values below the detection limit (2 pg/ml), this cytokine was increased consistently in the T1D group with the lower value of 1·8 pg/ml (2·3 versus 1·6 pg/ml; P = 0·002). There was also evidence that GDM children (n = 32) might have higher levels of IL-8 compared to the healthy children, although not statistically significant after Bonferroni correction (median value 103·8 pg/ml versus 45·8 pg/ml; P = 0·047). The levels of the other cytokines analysed, i.e. IL-2, IL-4, IL-6, IL-10, IL-12p70 and IFN-γ, did not differ between the three groups of children (Table 2).
Table 2.
Summary of secreted cytokines in cord blood plasma as analysed by cytometric bead array (CBA). The results are displayed in median pg/ml (minimum/maximum) values.
| TNF-α | IL-1β | IL-8 | IL-12p70 | IL-2 | IL-4 | IL-6 | IL-10 | IFN-γ | |
|---|---|---|---|---|---|---|---|---|---|
| Healthy | 1·6 (0; 8·6) | 22·5 (0; 518) | 45·8 (3·4; 254) | 2·4 (0; 68) | 1·9 (0; 18·1) | 2·6 (0; 11·6) | 4·1 (0; 2871) | 5·7 (1·7; 164) | 14·3 (0; 372) |
| GDM | 2·1 (0; 20·8) | 40·2 (0; 455) | 103·8 (5·4; 1894) | 2·9 (0; 64·6) | 2·0 (0; 9·6) | 2·9 (0; 24) | 4·6 (0; 85·8) | 6·1 (3·1; 80·3) | 15·2 (0; 197) |
| T1D | 2·3** (1·6; 8·8) | 45·2* (10·9; 803) | 521·8** (19·7; 3864) | 2·6 (0; 206) | 2·0 (0; 3·5) | 2·7 (0; 3·5) | 5·0 (2·9; 151) | 6·2 (2·5; 17·5) | 18·9 (13·1; 27) |
P < 0·025;
P < 0·01; P-values less than 0·0167 were considered significant after Bonferroni correction compared to healthy group; Mann–Whitney non-parametric test. GDM: mothers with gestational diabetes; T1D: type 1 diabetes; IFN: interferon; IL: interleukin; TNF: tumour necrosis factor.
Characterization of cord blood lymphocytes
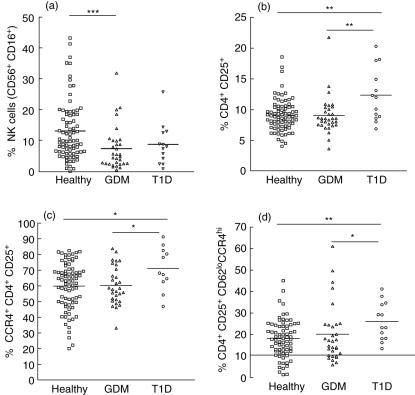
While the absolute number of total leucocytes did not differ between groups (data not shown), the frequency of total cord blood lymphocytes of all white blood cells differed significantly between children born to healthy mothers and to mothers with GDM (mean 35·4 ± 1·2% versus 44·5 ± 2·6%; P = 0·0006), but not to children born to mothers with T1D (mean 40·8 ± 4·5%; P = 0·45). The cytokine analysis in CB revealed an increase of proinflammatory cytokines in children born to T1D mothers, suggesting that the immune system of these newborns might have been stimulated during fetal life. In order to determine if this stimulation could be accompanied by alterations in lymphocyte populations or by the expression of activation markers, we performed FACS analysis of cord blood lymphocyte subsets in all 126 children. No differences were determined in the percentages of total B and T cells, CD4+ and CD8+ T cells as well as between memory (CD45RO+ CD62Llo) and naive (CD45RA+ CD62Lhi) T cells between groups (data not shown). While no significant differences were detected in the frequency of NK cells between healthy and T1D groups, a significant decrease of these cells was observed in the GDM group compared to the healthy group (P = 0·001) (Fig. 1a and Table 3). Interestingly, a significant increase of CD4+ CD25+ T cell numbers was detected in children born to T1D mothers compared to the other two groups (P < 0·01 and P < 0·01, respectively) (Fig. 1b and Table 3) and these cells tended to express more CCR4 (P = 0·034 and P = 0·028) (Fig. 1c and Table 3). In accordance with this finding, a significant increase in the proportion of CD62Llo CCR4hi cells within the CD4+ CD25+ T cell subset was detected in the T1D group (P = 0·010; Fig. 1d and Table 3).
Fig. 1.
Flow cytometric analysis of cord blood lymphocytes. Cumulative analysis of the frequency of natural killer (NK) cells (defined by the expression of both CD16 and CD56 markers) (a), CD4+ CD25+ T cells (b), CD4+ CD25+ CCR4+ T cells (c) and CD4+ CD25+ CD62Llo CCR4hi (d) in cord blood of children born to healthy mothers (healthy, open squares), children born to mothers with gestational diabetes (GDM, open triangles) and with type 1 diabetes (T1D, open circles). Median values for each patient group are marked with a horizontal line. P-values < 0·0167 were considered significant after Bonferroni correction. *P < 0·05, **P < 0·0167, ***P < 0·001.
Table 3.
Summary of major cord blood lymphocytes frequencies and phenotypic characterization of CD4+ CD25+ T cells; presented as median values (minimum/maximum) for each group of children.
| Cellular subsets (% positive cells) | Healthy | GDM | T1D | Statistical differences between child groups* |
|---|---|---|---|---|
| Lymphocytes | ||||
| B cells | 13·1 (4·8; 34·1) | 12·0 (1·6; 21·5) | 11·5 (2·1; 30·1) | n.s. |
| NK cells | 9·8 (0·0; 43·1) | 5·0 (0·4; 31·7) | 7·9 (0·8; 25·6) | H versus GDM; P = 0·001 |
| T cells | 58·5 (30·8; 82·3) | 58·6 (10·3; 87·1) | 54·5 (8·4; 71·0) | P > 0·05 |
| T cells (CD3+) | ||||
| CD4+ | 72·3 (52·0; 95·7) | 71·7 (56·9; 83) | 65·7 (54·2; 84·3) | P > 0·05 |
| CD8+ | 26·6 (8·0; 43·4) | 27·8 (15·8; 40·8) | 32·0 (14·7; 43·1) | P > 0·05 |
| CD4+CD25+ | 8·9 (4·1; 18·5) | 8·6 (3·7; 21·7) | 12·0 (6·9; 20·3) | H versus T1D; P = 0·0102 |
| CD4+CD25+ | ||||
| CD45RA+ | 84·3 (25·0; 99·2) | 88·0 (53·3; 100) | 82·7 (60·8; 91·2) | P > 0·05 |
| CD45RO+ | 50·1 (6·9; 99·8) | 53·2 (36·4; 96·9) | 59·2 (47·3; 93·8) | P > 0·05 |
| CD62Lhi | 76·0 (35·2; 95·7) | 78·1 (13·4; 92·9) | 67·6 (52·8; 85·4) | P > 0·05 |
| CD62Llo | 24·1 (4·3; 64·8) | 21·9 (7·1; 86·6) | 32·4 (14·6; 47·3) | P > 0·05 |
| CCR4+ | 63·0 (20·0; 82·4) | 58·0 (33·0; 83·7) | 68·1 (46·9; 91·2) | H versus T1D; P = 0·034 GDM versus T1D; P = 0·028 |
| CD45RO+CCR4lo | 22·0 (0·0; 50·8) | 26·0 (0·0; 40) | 18·2 (8·3; 46·1) | P > 0·05 |
| CD45RO+CCR4hi | 46·8 (3·8; 78·9) | 45·2 (8·6; 83·8) | 51·3 (32·9; 61·8) | P > 0·05 |
| CD45RO–CCR4lo | 19·8 (0·0; 72·6) | 23·2 (0·0; 58·2) | 19·1 (11·6; 26·1) | P > 0·05 GDM versus T1D; P = 0·011 |
| CD62LhiCCR4lo | 41·7 (11·2; 92·1) | 44·7 (6·1; 60) | 36·2 (17·6; 46·5) | H versus T1D; P = 0·035 |
| CD62LhiCCR4hi | 33·6 (2·8; 90·8) | 29·9 (1·1; 89·7) | 38·2 (12·6; 58·2) | n.s. |
| CD62LloCCR4hi | 18·1 (1·4; 45·0) | 16·9 (6·0; 61) | 23·2 (13·5; 41·2) | H versus T1D; P = 0·010 GDM versus T1D; P = 0·032 |
Mann–Whitney non-parametric test. P-values less than 0·05 are reported. P-values less than 0·0167 were considered significant after Bonferroni correction. NK: natural killer; GDM: mothers with gestational diabetes; T1D: type 1 diabetes; H: healthy.
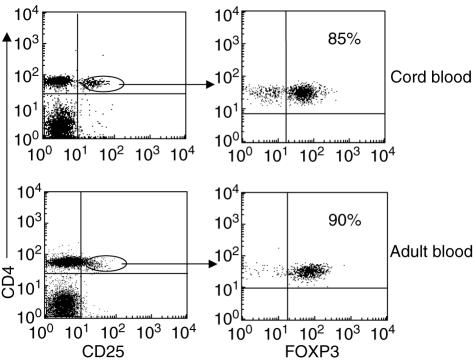
While human peripheral blood CD4+ T cells express variable levels of CD25, it has been reported that the majority of highly suppressive T regulatory cells reside within the CD25hi population [15,16] and they express high levels of FOXP3 (a transcription factor essential for the development and function of CD4+ CD25+ regulatory T cells) [17]. As depicted in Fig. 2 and as reported by others [18–20], cord blood CD4+ CD25+ T cells represent a distinct cell population with high expression of the CD25 and FOXP3 markers and absence of expression of the early activation marker CD69 (data not shown).
Fig. 2.
Expression of forkhead box P3 (FOXP3) in cord blood (CB) and adult CD4+ CD25+ T cells. Representative flow cytometry analysis of CD4+ CD25+ T cells from cord and adult peripheral blood of healthy donors. Cells were stained with peridinin chlorophyll (PerCP)-labelled CD4, fluorescein isothiocyanate (FITC)-labelled CD25 and antigen-presenting cell (APC)-labelled FOXP3 monoclonal antibodies. The oval region depicting CD4+ T cells expressing high levels of CD25 in the dot plot from adult blood is the same used for the cord blood dot plot.
Cord blood autoantibodies and immunological phenotypes
Cord blood plasma was analysed for autoantibodies against GAD65 and IA-2. We found that 46% of the children born to T1D mothers (six of 13) were autoantibody-positive whereas only one child born to a healthy mother (1%; one of 84) and two to GDM mothers (7%; two of 28) had detectable levels of autoantibodies (Table 4). In the T1D group the levels of IA-2 autoantibodies did not correlate with levels of any of the cytokines investigated. However, levels correlated positively with the levels of both IL-12p70 (r = 0·994, P < 0·0001) and IL-1β (r = 0·989, P < 0·0002) in the T1D group (Table 5). Anti-GAD65 autoantibody (GADA) levels in cord blood plasma correlated positively with the percentage of CD4+ CD25+ T cells (r = 0·916, P = 0·001) and with CCR4 expression in these CD4+ CD25+ T cells (r = 0·832, P = 0·020) (Table 5).
Table 4.
Individual anti-GAD65 autoantibody (GADA) and IA-2A levels in positive children and their mothers, respectively.
| Children group | GADA CB | GADA mother | IA-2A CB | IA-2A mother |
|---|---|---|---|---|
| T1DM | 1508 | 907 | 33 | 15 |
| T1DM | 40 388 | 15 979 | 43 | 37 |
| T1DM | 532 | 383 | 80 | 68 |
| T1DM | 853 | 455 | 57 | 41 |
| T1DM | 712 | 524 | 30 | 34 |
| T1DM | 103 | 23 | 2 | n.d. |
| GDM | 47 | 22 | 34 | 18 |
| GDM | 118 | n.d. | 1 | n.d. |
| Healthy | 37 | n.d. | 3 | n.d. |
CB: cord blood; n.d.: not determined; T1D: type 1 diabetes.
Table 5.
Statistical correlations between cord blood GAD65 autoantibody levels (U/ml) and immunological phenotypes in children born to type 1 diabetic mothers.
| IL-1β (pg/ml)* | P = 0·0002; r = 0·99 |
| IL-12p70 (pg/ml)* | P = 0·0004; r = 0·995 |
| % CD4+CD25+ T cells | P = 0·001; r = 0·92 (all GADA positive children) |
| % CCR4+ CD4+CD25+ cells* | P = 0·020; r = 0·83 (all GADA positive children) |
Analysed by Pearson’s r-test; GADA: anti-GAD65 autoantibody; IL: interleukin.
HLA genotype analysis
To determine if proinflammatory cytokines in cord blood were associated with high-risk HLA, the children born to all three groups of mothers were HLA typed for the alleles known to affect T1D (Table 6). Although no significant differences in HLA risk alleles between different groups of children were detected (Table 7), an association between high-risk HLA alleles and IL-8 [mean difference (95% CI), + 1·14 (0·30–1·79) log pg/ml, P = 0·008] was noted, even after adjusting for diabetes status of the mother [mean difference, 1·00 (0·21–1·79) log pg/ml, P = 0·014]. In contrast, no significant associations were determined between CB proinflammatory cytokine levels and HLA risk status of the mother. Moreover, no association was evident between HLA risk status of the newborn and the increase in numbers of CD4+ CD25+ T cells, even if the risk allele was derived from the father (data not shown).
Table 6.
Human leucocyte antigen (HLA) DQB1* allele distribution in the newborn population.
| DQB1-allele | ALL | Allele from father | Allele from mother | Unknown (child–mother same genotype) |
|---|---|---|---|---|
| 0602/3/4 | 3 | 0 | 0 | 1 |
| *02 | 42 | 12 | 22 | 8 |
| 0301 | 38 | 11 | 17 | 9 |
| 0302 | 34 | 12 | 13 | 8 |
| 0602 | 28 | 12 | 6 | 9 |
| 0603 | 7 | 1 | 5 | 1 |
| 0604 | 8 | 4 | 3 | 1 |
| X | 66 | 30 | 16 | 16 |
Table 7.
Frequencies of the combination of human leucocyte antigen (HLA) DQB1 and, in some circumstances, DQA1-genotypes linked to type 1 diabetes (T1D) susceptibility (positive samples/samples analysed) in the children enrolled in the study. The definitions for high-, low- and neutral HLA genotypes, respectively, are stated in the Materials and methods section.
| High-risk HLA | Low-risk HLA | Neutral HLA | |
|---|---|---|---|
| Healthy | 0·089 (7/79) | 0·076 (6/79) | 0·835 (66/79) |
| T1D | 0·091 (1/11) | 0·182 (2/11) | 0·727 (8/11) |
| GDM | 0·111 (3/27) | 0·148 (4/27) | 0·741 (20/27) |
GDM: mothers with gestational diabetes.
Discussion
In this study we demonstrate novel immunological differences in children born to mothers with T1D compared to healthy mothers, these being associated with transplacental transmission of GADA. First we detected an increase in the levels of proinflammatory cytokines in CB plasma (IL-1β, TNF-α and IL-8), which suggests that the immune system in neonates born to T1D mothers has responded to the maternal microenvironment in utero. The levels of IL-1β and IL-12p70 correlated positively with GADA titres in CB of positive children. However, whereas the levels of IL-1β were significantly higher in the T1D group of newborns, IL-12p70 levels did not differ between groups if the presence of CB GADA was not taken into consideration. This finding suggests a direct effect of transplacental passage of GADA on the fetal immune system independently of the diabetic status of the mother. However, due to the low numbers of children with detectable GADA in CB born to mothers without T1D, this hypothesis needs to be confirmed in a larger cohort of children born to healthy mothers but GADA positive at birth. These studies are being performed currently.
At the cellular level, a significant increase of CD4+ CD25+ T cells was observed only in CB from children born to T1D mothers, whereas other lymphocyte subsets did not show any difference between groups. It is reasonable to speculate that the subtle proinflammatory response observed at the cytokine levels was not sufficient to induce detectable changes in major lymphocyte populations, but still enough to affect rare subsets of T cells more sensitive to subtle chronic antigen stimulation. Importantly, the observed increased frequency of CB CD4+ CD25+ T cells also correlated positively with GADA titres.
Regulatory T cells in the periphery most probably control autoreactive T cells that have escaped thymic selection. Indeed, several reports indicate that breaking the suppression maintained by this regulatory cell population leads to development of autoimmune diseases [21,22]. During recent years a population of T cells expressing CD25 (IL-2Rα-chain) detectable in human thymus, peripheral blood and cord blood has emerged as having important immunoregulatory properties in vitro as well as in vivo [18,23–25]. Although in our study we did not test directly the immunosuppressive function of cord blood CD4+ CD25+ T cells, based on their high expression of CD25 and FOXP3 (Fig. 2 and [19,20]) and the absence of CD69 expression as a marker of recent activation (data not shown), it is likely that the majority of these cells represent a regulatory T cell population rather than recently activated T cells. Indeed, it has been demonstrated previously that human T regulatory cells are characterized by high-level CD25 and FOXP3 expression in peripheral blood [17,26]. Further phenotypic characterization of CB CD4+ CD25+ T cells in the T1D group revealed a significant increase of cells displaying a concomitant lower expression of the memory marker CD62L (peripheral lymph node-homing ligand) and high expression of the chemokine receptor CCR4 (CD62Llo CCR4hi), compared to the equivalent cells in the healthy group (P = 0·010), with a tendency to also be increased when compared with the GDM group (P = 0·032). In accordance with this finding we also observed a tendency to a decrease of CD4+ CD25+ T cells co-expressing high levels of CD62L and low levels of CCR4 (CD62Lhi CCR4lo) between the T1D and the healthy group (P = 0·035), which was significant between the T1D and GDM group of neonates (P = 0·010). Indeed, CCR4 is a receptor highly expressed in peripheral primed CD4+ CD25+ regulatory T cells [27,28], which binds primarily to the chemokines CCL17/TARC (thymus and activation-regulated chemokine) [29] and CCL22/macrophage-derived chemokine (MDC) [30]. CCR4 has been implicated directly in the regulation of immune responses by mediating binding of antigen-primed T cells to dendritic cells [31] and therefore a role in the suppression function of regulatory T cells was also suggested. Taken together, these data indicate that immunological priming of the CB CD4+ CD25+ T cell population might have already occurred during pregnancy.
It would have been of interest to study the suppressive function of CB CD4+ CD25+ T cells in these newborns, which could not be performed here due to the insufficient amount of blood received. It is important to note that it has been shown clearly by others [19,20,32,33] and confirmed by us (data not shown) that total CB CD4+ CD25+ T cells exert comparable, if not enhanced, suppressive function as CD4+ CD25hi T cells in adult blood which, together with the high expression of FOXP3, indicate less contamination of non-regulatory T cells and less heterogeneity within the CD4+ CD25+ T cell population in CB compared to adult blood.
Therefore, as we do not suggest that in children born to T1D mothers these cells might have gained a general increase of function but rather have been expanded in utero. We believe that the lack of these experiments do not alter dramatically the conclusions of this study. Nevertheless, we cannot exclude that CB CD4+ CD25+ T cell in children receiving GADA from their T1D mothers are more efficient in suppressing GAD65-specific T cell responses. This possibility is currently under investigation.
As demonstrated here and in previous studies of our own [34,35], as well as of others [36–38], GADA in the fetus arise mainly because of the transplacental passage. We therefore propose the hypothesis that the immunological changes observed in the cord blood of neonates born to mothers with T1D may be due to GAD65 autoantibodies per se. Alternatively, but not exclusively, they may be due to maternal pathogenic factors that are associated with GAD65 autoantibody formation. In support of this hypothesis, the observed increase in CD4+ CD25+ T cells in cord blood of neonates born to mothers with T1D was correlated with the levels of GAD65 autoantibodies. These results are of high relevance when considering the recent finding of Koczwara et al. [8]. When studying a large cohort of offspring who were GAD65 or IA-2 autoantibody-positive at birth, these authors reported that the children had a significantly lower risk of developing multiple islet autoantibodies and diabetes after 10 years than offspring who were islet autoantibody-negative at birth [8].
Other groups have investigated peripheral lymphocyte profiles in cord blood of children born to diabetic mothers and the impact that this might have on the risk of developing T1D, albeit with contrasting results [39–41]. While no differences were observed in the population studied by Di Mario and colleagues, Roll et al. demonstrated decreased frequencies of lymphocytes, in particular T and B cells in children born to diabetic mothers [40], whereas Lapolla et al. [41] recorded an increase of total lymphocytes in neonates born to both T1D and GDM mothers. Although the lymphocyte alterations reported by Roll et al. could not be confirmed in our study, the frequency of autoantibody-positive children born to T1D mothers was almost identical (nine of 22 children or 41% versus six of 13, or 46% in our study, respectively).
That proinflammatory cytokine levels in cord blood plasma are increased in children born to mothers with T1D implies that there is a systemic response of the fetal immune system. This early immune response could have resulted in higher recruitment of recent thymic emigrants and new generation or expansion of a regulatory T cell population. In concordance with this possibility, it has been demonstrated that administration of TNF-α in young non-obese diabetic (NOD) mice can modulate the levels of CD4+ CD25+ regulatory T cells [42].
Importantly, GAD65 autoantibody levels in cord blood plasma also correlated positively with the levels of proinflammatory cytokines, supporting our hypothesis that transplacental transfer of autoantibodies is associated with immunological changes in the fetal immune system.
Furthermore, although there was a correlation between the high-risk HLA DQB1 genotype and the plasma levels of IL-8 (involved in the homing of T cells to inflammatory sites [43]), HLA did not appear to have a major impact on the immunological changes observed in children born to T1D mothers, due most probably to the fact that most T1D patients share the same risk alleles.
In contrast to the T1D group, neonates born to mothers with GDM displayed a different phenotype, albeit with some similarities to the T1D neonates. For instance, newborns to GDM mothers had an increase of IL-8 in their cord blood plasma. However, the immunological changes observed in cord blood lymphocytes differed among the children born to T1D or GDM mothers. Besides the observed increase of total white blood cells, we determined a significant decrease of NK cells in the GDM cohort as also reported previously [41,44], but not in the T1D group or children born to healthy mothers. These differences may reflect the response of the fetal immune system to two different maternal environments, both of which share the deranged diabetic metabolic state, with only the T1D mothers also suffering from autoimmunity. In the present study the mother’s metabolic status was not analysed fully and it is therefore possible that some of the changes that were observed both in neonates born to T1D and GDM mothers might have been associated with the degree of metabolic control. However, this possibility is unlikely, as all mothers had HbA1c levels below 7% at the end of pregnancy, and there are currently no data suggesting that GADA levels are related to HbA1c levels. In addition, no associations were determined between the types of delivery, the length of pregnancy, the age of mothers or the country of origin, indicating that these factors did not account for the observed differences and therefore excluding possible bias during analysis and interpretation of our data.
In concordance with the hypothesis that maternal factors such as transplacental passage of islet autoantibodies could confer protection in children born to mothers with T1D, it has been reported that children born to fathers with T1D are at higher risk of developing the disease [45,46].
Although we realize that it would have been important to study such a group of newborns, the primary aim of this study was to investigate the impact of maternal autoimmunity on the newborn immune system. Analysis of HLA status of the child did not reveal significant associations with most proinflammatory cytokines (except for IL-8) and more importantly no association was evident between HLA risk alleles and the increase of CD4+ CD25+ T cells, even if the risk allele was inherited from the father. These data suggest that the immunological changes observed here in children born to mothers with T1D are related most probably more to maternal autoimmunity than to the influence of the HLA status of the father. Preliminary analysis of CB of three neonates born to fathers with T1D did not reveal any differences in plasma cytokine levels or in the frequency of CD4+ CD25+ T cells compared to newborns of healthy mothers (data not shown), indicating that the immunological phenotype observed in children born to T1D mothers is related more to the maternal autoimmunity and transplacental passage of GADA than the diabetic status of the father.
Our results demonstrate that neonates born to T1D mothers have an increase of proinflammatory cytokines, probably reflecting a response of the fetal immune system to maternal autoimmunity. Interestingly, we observed a significantly higher proportion of potential regulatory CD4+ CD25+ T cells in cord blood of these children, who also displayed a more pronounced memory phenotype. It is possible to speculate that the observed increase in regulatory CD4+ CD25+ T cells could result in early immunological tolerance and therefore operate potentially as protective factor later in life. These observations might also suggest a novel mechanism by which transplacental passage of autoantibodies might protect the offspring from diabetes development. Because of the prospective design of this study, future follow-up studies of these children will reveal whether these immunological changes represent a risk or a protective factor for T1D development.
Acknowledgments
The other members of the DiPiS study group are: Barbro Lernmark (Department of Clinical Sciences, Malmö University Hospital, Malmö, Sweden), Björn Jönsson and Karin Larsson (Department of Paediatrics, Kristianstad Hospital, Kristianstad, Sweden), Helena Elding-Larsson and Bengt Lindberg (Department of Paediatrics, Malmö University Hospital, Malmö, Sweden) Elisabeth Cederwall (Department of Paediatrics, Ängelholm Hospital, Ängelholm, Sweden), Karin Kockum (Department of Paediatrics, Ystad Hospital, Ystad, Sweden), Jan Neiderud (Department of Paediatrics, Helsingborg Hospital, Helsingborg, Sweden) and Sture Sjöblad (Department of Paediatrics, Lund University Hospital, Lund, Sweden). We would like to thank Robert Harris and Francesco Colucci for critical reading of the manuscript as well as all the midwives from the above-mentioned hospitals for their dedicated help in collecting cord blood from neonates as part of the DiPiS study. This work was supported by the Swedish Research Council (grants 2002–6535 to CMC, 72X- 14064 to ÅL), the Juvenile Diabetes Research Foundation (grant 11-2004-38 to CMC), the Swedish Childhood Diabetes Research Fund, the Swedish Diabetes Association, the Novo Nordisk Fund, the Skane Council Foundation for Research and Development and the MAS Funds. B. C. Holm is supported by a postdoctoral fellowship from the Juvenile Diabetes Research Foundation (grant 3-2004-578).
References
- 1.Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3:235–49. doi: 10.1038/sj.gene.6363875. [DOI] [PubMed] [Google Scholar]
- 2.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–22. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peakman M, Wen L, McNab GL, Watkins PJ, Tan KC, Vergani D. T cell clones generated from patients with type 1 diabetes using interleukin-2 proliferate to human islet antigens. Autoimmunity. 1994;17:31–9. doi: 10.3109/08916939409014656. [DOI] [PubMed] [Google Scholar]
- 4.Dahlquist GG, Ivarsson S, Lindberg B, Forsgren M. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM. A population-based case-control study. Diabetes. 1995;44:408–13. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 5.Dahlquist G, Blom L, Lonnberg G. The Swedish Childhood Diabetes Study – a multivariate analysis of risk determinants for diabetes in different age groups. Diabetologia. 1991;34:757–62. doi: 10.1007/BF00401524. [DOI] [PubMed] [Google Scholar]
- 6.Hyoty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes. 1995;44:652–7. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 7.Warram JH, Martin BC, Krolewski AS. Risk of IDDM in children of diabetic mothers decreases with increasing maternal age at pregnancy. Diabetes. 1991;40:1679–84. doi: 10.2337/diab.40.12.1679. [DOI] [PubMed] [Google Scholar]
- 8.Koczwara K, Bonifacio E, Ziegler AG. Transmission of maternal islet antibodies and risk of autoimmune diabetes in offspring of mothers with type 1 diabetes. Diabetes. 2004;53:1–4. doi: 10.2337/diabetes.53.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Schranz DB, Bekris L, Landin-Olsson M, et al. A simple and rapid microSepharose assay for GAD65 and ICA512 autoantibodies in diabetes. Diabetes Incidence Study in Sweden (DISS) J Immunol Meth. 1998;213:87–97. doi: 10.1016/s0022-1759(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 10.Grubin CE, Daniels T, Toivola B, et al. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia. 1994;37:344–50. doi: 10.1007/BF00408469. [DOI] [PubMed] [Google Scholar]
- 11.Mire-Sluis AR, Gaines Das R, Lernmark A. The World Health Organization International Collaborative Study for islet cell antibodies. Diabetologia. 2000;43:1282–92. doi: 10.1007/s001250051524. [DOI] [PubMed] [Google Scholar]
- 12.Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52:1128–36. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 13.Ilonen J, Reijonen H, Herva E, et al. Rapid HLA-DQB1 genotyping for four alleles in the assessment of risk for IDDM in the Finnish population. The Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care. 1996;19:795–800. doi: 10.2337/diacare.19.8.795. [DOI] [PubMed] [Google Scholar]
- 14.Sjoroos M, Iitia A, Ilonen J, Reijonen H, Lovgren T. Triple-label hybridization assay for type-1 diabetes-related HLA alleles. Biotechniques. 1995;18:870–7. [PubMed] [Google Scholar]
- 15.Baecher-Allan CM, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin Immunol. 2005;117:192. doi: 10.1016/j.clim.2005.08.008. discussion 193. [DOI] [PubMed] [Google Scholar]
- 16.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 18.Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–9. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, Rudin A. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516–25. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–8. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Kuniyasu Y, Toda M, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 22.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S. Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol. 2000;12:1145–55. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]
- 23.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–18. [PubMed] [Google Scholar]
- 24.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 26.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp. 2003;252:67–88. doi: 10.1002/0470871628.ch6. discussion 88–91, 106–14. [DOI] [PubMed] [Google Scholar]
- 27.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iellem A, Colantonio L, D’Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur J Immunol. 2003;33:1488–96. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- 29.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–42. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 30.Imai T, Chantry D, Raport CJ, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–8. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 31.Wu MT, Fang H, Hwang ST. Cutting edge: ccr4 mediates antigen-primed t cell binding to activated dendritic cells. J Immunol. 2001;167:4791–5. doi: 10.4049/jimmunol.167.9.4791. [DOI] [PubMed] [Google Scholar]
- 32.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 33.Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622–9. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg B, Ivarsson SA, Landin-Olsson M, Sundkvist G, Svanberg L, Lernmark A. Islet autoantibodies in cord blood from children who developed type I (insulin-dependent) diabetes mellitus before 15 years of age. Diabetologia. 1999;42:181–7. doi: 10.1007/s001250051137. [DOI] [PubMed] [Google Scholar]
- 35.Novak EJ, Ortqvist E, Nord E, et al. Stability of disease-associated antibody titers in pregnant women with type 1 diabetes with or without residual beta-cell function. Diabetes Care. 2000;23:1019–21. doi: 10.2337/diacare.23.7.1019. [DOI] [PubMed] [Google Scholar]
- 36.Hamalainen AM, Ronkainen MS, Akerblom HK, Knip M. Postnatal elimination of transplacentally acquired disease-associated antibodies in infants born to families with type 1 diabetes. The Finnish TRIGR Study Group. Trial to Reduce IDDM in the Genetically at Risk. J Clin Endocrinol Metab. 2000;85:4249–53. doi: 10.1210/jcem.85.11.6987. [DOI] [PubMed] [Google Scholar]
- 37.Naserke HE, Bonifacio E, Ziegler AG. Prevalence, characteristics and diabetes risk associated with transient maternally acquired islet antibodies and persistent islet antibodies in offspring of parents with type 1 diabetes. J Clin Endocrinol Metab. 2001;86:4826–33. doi: 10.1210/jcem.86.10.7931. [DOI] [PubMed] [Google Scholar]
- 38.Stanley HM, Norris JM, Barriga K, et al. Is presence of islet autoantibodies at birth associated with development of persistent islet autoimmunity? The Diabetes Autoimmunity Study in the Young (DAISY) Diabetes Care. 2004;27:497–502. doi: 10.2337/diacare.27.2.497. [DOI] [PubMed] [Google Scholar]
- 39.Di Mario U, Dotta F, Gargiulo P, et al. Immunology in diabetic pregnancy: activated T cells in diabetic mothers and neonates. Diabetologia. 1987;30:66–71. doi: 10.1007/BF00274573. [DOI] [PubMed] [Google Scholar]
- 40.Roll U, Scheeser J, Standl E, Ziegler A-G. Alterations of lymphocyte subsets in children of diabetic mothers. Diabetologia. 1994;37:1132–41. doi: 10.1007/BF00418377. [DOI] [PubMed] [Google Scholar]
- 41.Lapolla A, Sanzari MC, Zancanaro F, et al. A study on lymphocyte subpopulation in diabetic mothers at delivery and in their newborn. Diabetes Nutr Metab. 1999;12:394–9. [PubMed] [Google Scholar]
- 42.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sims-Mourtada JC, Guzman-Rojas L, Rangel R, et al. In vivo expression of interleukin-8, and regulated on activation, normal, T-cell expressed, and secreted, by human germinal centre B lymphocytes. Immunology. 2003;110:296–303. doi: 10.1046/j.1365-2567.2003.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapolla A, Dalfra MG, Sanzari M, et al. Lymphocyte subsets and cytokines in women with gestational diabetes mellitus and their newborn. Cytokine. 2005;31:280–7. doi: 10.1016/j.cyto.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Tillil H, Kobberling J. Age-corrected empirical genetic risk estimates for first-degree relatives of IDDM patients. Diabetes. 1987;36:93–9. doi: 10.2337/diab.36.1.93. [DOI] [PubMed] [Google Scholar]
- 46.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–52. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]