Abstract
Neutrophils and monocytes play a central role in host defence. The invading leucocytes are capable of synthesizing and releasing a variety of proinflammatory mediators including cytokines. Given the importance of cytokines in the progression of chronic and acute inflammatory processes, we aimed to ascertain whether the release of interleukin (IL)-8, IL-1β, tumour necrosis factor (TNF)-α and IL-1ra of neutrophils and monocytes was modified in diabetes. To this end, we measured the release of cytokines in suspensions of cell culture in basal and lipopolysaccharide (LPS)-stimulated conditions. In basal conditions, neutrophils of diabetics release 1·6, 3·2, 1·9 and 1·9-fold higher amounts of IL-8, IL-1β, TNF-α and IL-1ra, respectively, than do healthy controls. Under our experimental conditions, this effect was more evident for neutrophils than for monocytes. Incremental cytokine production was also found to occur when neutrophils were stimulated with LPS. IL-8, IL-1β and TNF-α increased, respectively, by 4·0, 1·7 and 2·8-fold. Although the effect was more marked for neutrophils, monocytes showed a tendency for increased cytokine production. The discovery of this increase in cytokines released by the neutrophils of diabetics contributes towards a clearer understanding of other deficiencies described for neutrophils in diabetes, such as the migration of neutrophils to inflammatory sites, phagocytes, release of lytic proteases, production of reactive oxygen species and apoptosis. The excessive production of cytokines may lead to inappropriate activation and tissue injury and even to increased susceptibility to invasive microorganisms. Thus, the increased responsiveness of neutrophils of diabetics demonstrated in this study may be considered part of the scenario of diabetes physiopathology.
Keywords: cytokines, diabetes, monocytes, neutrophils
Introduction
The immune system can be divided into innate and adaptive humoral systems. In innate immunity, neutrophils and monocytes play a crucial role in host defence by phagocytosing and killing invading microorganisms. In diabetic patients, defects in the microbicidal function of neutrophils and monocytes are a major contributory factor for the development of bacterial infection and an increased rate of morbidity and mortality. Neutrophils of diabetic patients show deficiencies in almost all functions [1–8], such as migration to inflammatory sites [1–3], phagocytosis [4], release of lytic proteases [5], production of reactive oxygen species [6,7] and apoptosis [8]. A similar profile has been observed in monocytes from diabetic patients [9], with impaired chemotaxis [10] and phagocytosis [11].
One important aspect of neutrophils and monocytes is their ability to synthesize pro- and anti-inflammatory cytokines and growth factors that modulate the inflammatory response [12]. In this respect, interleukin (IL)-8, tumour necrosis factor (TNF)-α and interleukin (IL)-1β are proinflammatory cytokines with a central role in inflammatory processes. IL-8 shows angiogenic, chemotactic and stimulatory activities toward blood cells, providing the recruitment of a great number of neutrophils and other immune cells to the inflammatory site and favouring the appearance of neovascularization [13,14]. TNF-α mediates the systemic effects of inflammation, such as fever, the hepatic release of acute phase proteins and haematopoiesis. TNF-α and IL-1β act on leucocytes and the endothelium to induce acute inflammation [15]. IL-1 has several fundamental immunoregulatory, haematological, metabolic and physiological effects. It co-ordinates the different cellular interactions in wound healing and inflammation. An altered production of IL-1 or IL-1 receptor antagonists (IL-1ra), that competitively inhibits binding of IL-1 to cell surface receptors, may cause disturbances in inflammatory process [16]. The same is true for TNF-α and IL-8 production. The clinical features of an inflammatory disease could be explained in part by alterations in cytokine production.
Given the importance of cytokines in the progression of chronic and acute inflammatory processes, we aimed to ascertain whether the release of IL-8, IL-1β, TNF-α and IL-1ra of neutrophils and monocytes was modified in diabetes. To this end, we measured the release of cytokines in suspensions of cell cultures in basal and lipopolysaccharide (LPS)-stimulated conditions.
Materials and methods
Subjects
With the approval of the University of São Paulo Faculdade de Ciências Farmacêuticas (FCF-USP) and University Hospital (HU-USP) Ethics Committee, 18 patients with type 2 diabetes were recruited. All subjects gave written informed consent. Primary exclusion criteria were recent or ongoing infection and/or inflammation, history of cancer disease or treatment with anti-inflammatory drugs. To ensure the correct diagnosis, the World Health Organization (WHO) diagnostic criteria for diabetes were employed [17]. The patients involved in this study had been treated with diet alone or oral hypoglycaemic agents for at least 10 years. The oral hypoglycaemic agents used by type 2 diabetic patients were metformin (insulin sensitizer) and/or glibenclamid (insulin secretory agent). The percentage of patients that had been treated with metformin, glibenclamid or diet alone was 27·8%, 50% and 23·2%, respectively. Twenty non-diabetic healthy control subjects were recruited from the local population. Table 1 shows the general characteristics of the type 2 diabetic patients and the non-diabetic healthy control groups. No statistically significant differences between the groups were found using Student’s t-test with two-sided P-value.
Table 1.
General characteristics of the type 2 diabetic patients and non-diabetic healthy control groups.
| Controls | Type 2 diabetic patients | |
|---|---|---|
| Sex (M : F) | 9 : 11 | 6 : 12 |
| Age (years) | 59 ± 16 | 61 ± 13 |
| Body mass index (kg/m2) | 25·08 ± 2·22 | 26·55 ± 3·51 |
Materials
Bovine fetal serum, HEPES, Histopaque®, LPS (Escherichia coli 026:B6), penicillin, RPMI-1640 supplemented with l-glutamine, sodium bicarbonate and streptomycin were supplied by Sigma Chemical Co. (St Louis, MO, USA).
Cell purification, culture and cytokine determination
Blood samples were collected and experiments were performed within 1 h of venipuncture. Purified neutrophils (98%) and mononuclear (98%) cell preparations were isolated from peripheral blood of healthy donors and diabetes mellitus type 2 patients under endotoxin-free conditions [18]. Neutrophils (2·5 × 106 cells/ml) and monocytes (1·5 × 106 cells/ml) were suspended in RPMI-1640 supplemented with 0·3 g/l glutamine, 2·32 g/l HEPES, 2 g/l sodium bicarbonate, 100 µg/ml streptomycin, 100 UI/ml penicillin and 10% low endotoxin fetal serum and were cultured at 37°C and 5% CO2, with and without LPS (1·0 and 5·0 µg/ml). After 18 h, the supernatants were collected and stored at ≤ −40°C until IL-8, IL-1β, TNF-α and IL-1ra determination by enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was carried out comparing the mean ± standard error (s.e.m.) of the samples of the diabetic group against those of the control group. Statistical analysis involved one-way analysis of variance (anova) and the Student–Newman–Keuls multiple comparisons test.
Results
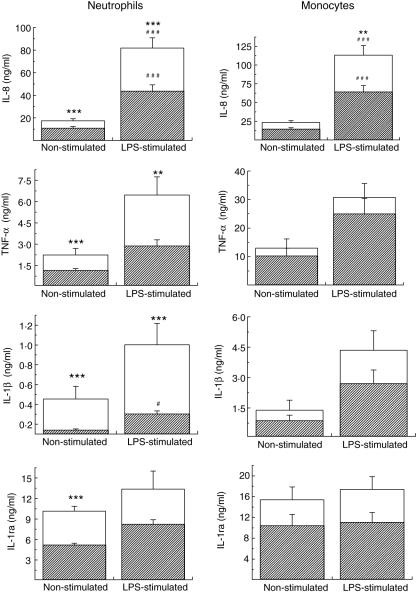
In basal conditions, i.e. without stimulation, neutrophils from type 2 diabetic patients, when compared with healthy controls, release higher amounts of IL-8, IL-1β, TNF-α and IL-1ra, i.e. 1·6; 3·2; 1·9 and 1·9 times higher, respectively (Fig. 1). This effect was restricted to neutrophils, although monocytes showed a tendency for increased cytokine production (Fig. 1).
Fig. 1.
Basal and lipopolysaccharide (LPS)-stimulated release of interleukin (IL)-8, IL-1β, IL-1ra and tumour necrosis factor (TNF)-α from neutrophils (2·5 × 106 cells/ml) and mononuclear cells (1·5 × 105 cells/ml) of type 2 diabetic patients (outlined bars) and healthy control subjects (solid bars). Neutrophils and mononuclear cells were incubated with LPS (1·0 µg/ml for IL-8 and 5 µg/ml for IL-1β, IL-1ra and TNF-α). Cytokines were measured in 18-h culture supernatants by enzyme-linked immunosorbent assay (ELISA). Data are mean ± s.e.m. **P < 0·01 and ***P < 0·001 for comparison between type 2 diabetic patients and controls subjected to the same treatments and #P < 0·05 and ###P < 0·001 for comparison between columns, of the same group, in the presence and absence of LPS.
Incremental cytokine production was also observed when neutrophils were stimulated with LPS. IL-8, IL-1β and TNF-α increased, respectively, to 4·0, 1·7 and 2·8. Monocytes stimulated with LPS showed a tendency for increased cytokine production (Fig. 1).
In both cases, i.e. in spontaneous and LPS-stimulated conditions, neutrophils from type 2 diabetic patients produced higher amounts of cytokines than non-diabetics and were more affected by diabetic conditions than monocytes.
Inflammatory processes, mainly those mediating chronic inflammation, have been implicated as predictors, initiators or contributors to chronic diseases such as diabetes mellitus [19] and obesity [20]. A growing body of evidence has identified adipose tissue as a dynamic endocrine organ that secretes a number of hormones and inflammatory cytokines (particularly TNF-α), contributing to systemic and vascular inflammation. It is important to reinforce that there was no correlation between detected cytokine values and blood glucose levels or body mass index in the groups studied here (data not shown).
Discussion
In both cases, i.e. under spontaneous and LPS-stimulated conditions, neutrophils from type 2 diabetic patients produced higher amounts of cytokines than non-diabetics and were more affected by diabetic conditions than monocytes, although monocytes showed a tendency for increased cytokine production. The finding that neutrophils were more sensitive than monocytes to the diabetic condition was not surprising. Neutrophils and monocytes seem frequently to show dissimilar response patterns [21]. Furthermore, receptors and signalling vary from one cell to another. In diabetes, it has been shown that neutrophils are more vulnerable to oxidative DNA damage than other peripheral blood cells [22].
To our knowledge, three previous studies have investigated the effect of diabetes on cytokine production by isolated human neutrophils or monocytes. These studies found that (i) monocytic TNF-α mRNA production was lower in diabetes type 2 [23]; (ii) IL-12 mRNA was highly expressed in monocytes from type 1 diabetic patients, TNF-α, IL-1 and IL-6 mRNA were elevated in type 1, but especially in type 2 diabetic patients, compared with healthy controls when stimulated by an immune stimulus such as IFN-γ [24]; and (iii) increased production of IL-8, IL-10 and IL-12 for human type 1 diabetic patients neutrophils stimulated by LPS [25].
The augmented production of cytokines in diabetic neutrophils may be associated with a variety of factors including, for instance, the rise in intracellular Ca+2 of the neutrophils triggered by the presence of advanced glycation end products (AGEs) [26] and hyperglycaemia [27]. Additionally, it is known that a prolonged increase in Ca+2 stimulates IL-8 transcription and synthesis [28]. Therefore, in neutrophils, a prolonged increase in intracellular Ca+2 may stimulate cytokine release and the accumulation and activation of phagocytes. Cytokines released by chronically activated phagocytes can also trigger apoptosis [29], formation of reactive oxygen species [30] and increase in intracellular Ca+2, for example. The latter can prime the cells for the release of more cytokine [31].
The combined effect of increments in TNF-α, IL-1β and IL-8 in type 2 diabetic patients may intensify the inflammatory response. The excessive production of cytokines leads to tissue injury and even cell death. In diabetes, increased cytokines levels may lead to inappropriate activation and may contribute towards increased susceptibility to invasive microorganisms [32].
Our findings suggest that part of the clinical picture found in type 2 diabetic patients is associated with excessive release of proinflammatory cytokines by neutrophils and monocytes, because the cytokines determined in this study exert profound effects on the biological signals involved in the regulation of important physiological events impaired in diabetes, such as the wound healing process [33–35] and apoptosis [8].
Neutrophils play a key role in the inflammation phase of wound repair by secreting cytokines and a great variety of growth factors [34,35]. In diabetes, the failure of neutrophils to control the inflammatory healing phase impairs the normal process [33]. Neutrophil apoptosis is a component of inflammation and its resolution. Cytokines are important signallers for the apoptotic process. Neutrophils from type 2 diabetic patients show decreased functional longevity and increased clearance from infectious sites [8], possibly contributing to the increased susceptibility and severity of infections in diabetic patients.
Acknowledgments
The authors are indebted to the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their financial support. The authors also thank the diabetes patients and volunteers for providing the samples used in this work.
References
- 1.Sawant JM. Biochemical changes in polymorphonuclear leucocytes in diabetic patients. J Postgrad Med. 1993;39:183–6. [PubMed] [Google Scholar]
- 2.Pereira MA, Sannomiya P, Leme JG. Inhibition of leukocyte chemotaxis by factor in alloxan-induced diabetic rat plasma. Diabetes. 1987;36:1307–14. doi: 10.2337/diab.36.11.1307. [DOI] [PubMed] [Google Scholar]
- 3.Sannomiya P, Pereira MAA, Garcia Leme J. Inhibition of leukocyte chemotaxis by serum factor in diabetes-mellitus − selective depression of cell responses mediated by complement-derived chemoattractants. Agents Actions. 1990;30:369–76. doi: 10.1007/BF01966301. [DOI] [PubMed] [Google Scholar]
- 4.Krol E, Agueel R, Banue S, Smogorzewski M, Kumar D, Massry SG. Amlodipine reverses the elevation in [Ca2+]i and the impairment of phagocytosis in PMNLs of NIDDM patients. Kidney Int. 2003;64:2188–95. doi: 10.1046/j.1523-1755.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 5.Marhoffer W, Stein M, Schleinkofer L, Federlin K. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res Clin Prac. 1993;19:183–8. doi: 10.1016/0168-8227(93)90112-i. [DOI] [PubMed] [Google Scholar]
- 6.Marhoffer W, Stein M, Schleinkofer L, Federlin K. Monitoring of polymorphonuclear leukocyte functions in diabetes mellitus − a comparative study of conventional radiometric function tests and low-light imaging systems. J Biolumin Chemilumin. 1994;9:165–70. doi: 10.1002/bio.1170090310. [DOI] [PubMed] [Google Scholar]
- 7.Sudhir V, Wallin JD, Eilen SD. Chemiluminescence and superoxide anion production by leukocytes from diabetic patients. J Clin Endocrinol Metab. 1983;57:402–9. doi: 10.1210/jcem-57-2-402. [DOI] [PubMed] [Google Scholar]
- 8.Tennenberg SD, Finkenauer R, Dwivedi A. Absence of lipopolysaccharide-induced inhibition of neutrophil apoptosis in patients with diabetes. Arch Surg. 1999;134:1229–33. doi: 10.1001/archsurg.134.11.1229. [DOI] [PubMed] [Google Scholar]
- 9.Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1994;26:259–65. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 10.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–90. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- 11.Katz S, Klein B, Elian I, Fishman P, Djaldetti M. Phagocytotic activity of monocytes from diabetic patients. Diabetes Care. 1983;6:479–82. doi: 10.2337/diacare.6.5.479. [DOI] [PubMed] [Google Scholar]
- 12.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 13.Baggliolini M, Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992;307:97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro FP, Furlaneto CJ, Hatanaka E, et al. mRNA expression and release of interleukin-8 induced by serum amyloid A in neutrophils and monocytes. Med Inflamm. 2003;12:173–8. doi: 10.1080/0962935031000134897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatanaka E, Furlaneto CJ, Ribeiro FP, Souza GM, Campa A. Serum amyloid A-induced MRNA expression and release of tumor necrosis factor-alpha (TNF-α) in human neutrophils. Immunol Lett. 2004;91:33–7. doi: 10.1016/j.imlet.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetes Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;5:9–15. [PubMed] [Google Scholar]
- 19.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 20.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–93. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 21.Silva SO, Rodrigues MR, Ximenes VF, et al. Neutrophils as a specific target for melatonin and kynuramines: effects on cytokine release. J Neuroimmunol. 2004;156:146–52. doi: 10.1016/j.jneuroim.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Pitozzi V, Giovannelli L, Bardini G, Rotella CM, Dolara P. Oxidative DNA damage in peripheral blood cells in type 2 diabetes mellitus: higher vulnerability of polymorphonuclear leukocytes. Mutat Res. 2003;529:129–33. doi: 10.1016/s0027-5107(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 23.Zykova SN, Svartberg J, Seljelid R, et al. Release of TNF-alpha from in vitro-stimulated monocytes is negatively associated with serum levels of apolipoprotein B in patients with type 2 diabetes. Scand J Immunol. 2004;60:535–42. doi: 10.1111/j.0300-9475.2004.01509.x. [DOI] [PubMed] [Google Scholar]
- 24.Giulietti A, Stoffels K, Decallonne B, Overbergh L, Mathieu C. Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann NY Acad Sci. 2004;1037:74–8. doi: 10.1196/annals.1337.011. [DOI] [PubMed] [Google Scholar]
- 25.Glowacka E, Banasik M, Lewkowicz P, Tchorzewski H. The effect of LPS on neutrophils from patients with high risk of type 1 diabetes mellitus in relation to IL-8, IL-10 and IL-12 production and apoptosis in vitro. Scand J Immunol. 2002;55:210–7. doi: 10.1046/j.1365-3083.2002.01046.x. [DOI] [PubMed] [Google Scholar]
- 26.Collison KS, Parhar RS, Saleh SS, et al. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs) J Leuk Biol. 2002;71:433–44. [PubMed] [Google Scholar]
- 27.Demerdash TM, Seyrek N, Smogorzewski M, Marcinkowski W, Nasser-Moadelli S, Massry SG. Pathways through which glucose induces a rise in [Ca2+]i of polymorphonuclear leukocytes of rats. Kidney Int. 1996;50:2032–40. doi: 10.1038/ki.1996.526. [DOI] [PubMed] [Google Scholar]
- 28.Kuhns DB, Young HA, Gallin EK, Gallin JI. Ca2+-dependent production and release of IL-8 in human neutrophils. J Immunol. 1998;161:4332–9. [PubMed] [Google Scholar]
- 29.Salamone G, Trevani A, Martinez D, et al. Analysis of the mechanisms involved in the stimulation of neutrophil apoptosis by tumour necrosis factor-alpha. Immunology. 2004;113:355–62. doi: 10.1111/j.1365-2567.2004.01973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miesel R, Hartung R, Kroeger H. Priming of NADPH oxidase by tumor necrosis factor alpha in patients with inflammatory and autoimmune rheumatic diseases. Inflammation. 1996;20:427–38. doi: 10.1007/BF01486744. [DOI] [PubMed] [Google Scholar]
- 31.Brechard S, Bueb JL, Tschirhart EJ. Interleukin-8 primes oxidative burst in neutrophil-like HL-60 through changes in cytosolic calcium. Cell Calcium. 2005;37:531–40. doi: 10.1016/j.ceca.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 33.Komesu MC, Tanga MB, Buttros KR, Nakao C. Effects of acute diabetes on rat cutaneous wound healing. Pathophysiology. 2004;11:63–7. doi: 10.1016/j.pathophys.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–86. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 35.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]