Abstract
A single infusion of pamidronate was given to patients with systemic sclerosis (scleroderma, SSc) to assess effects on cytokine production by peripheral blood mononuclear cells (PBMC) and lymphocyte subsets. Eighteen patients with SSc received a single intravenous dose of 60 mg of pamidronate and were followed for 6 months. Assessment of cytokine production [interferon (IFN)-γ, interleukin (IL)-10, transforming growth factor (TGF)-β1, tumour necrosis factor (TNF)-α and IL-4] by PBMC and lymphocyte subsets by flow cytometry was carried out before and after the pamidronate infusion. Unstimulated PBMC produced increased amounts of IFN-γ and TNF-α and reduced levels of TGF-β1 for up to 24 weeks after the infusion. γδ T cells from patients with SSc were activated in vitro and produced increased IFN-γ. The effects of pamidronate on modulation of cytokine profiles in patients with SSc may merit future study.
Keywords: cytokines, lymphocytes, pamidronate, scleroderma, systemic sclerosis
Introduction
Systemic sclerosis (SSc) is a fibrotic disorder characterized by heightened immune system activation associated with autoreactivity to a variety of tissue and cellular autoantigens. Although the exact role of cytokines in the pathogenesis of SSc remains to be elucidated, distinct patterns of T helper (Th1) and (Th2) type cytokines are present in many patients [1]. Recent reports suggest that pamidronate, an aminobisphosphonate which shares structural homology with γδ T cell ligands whose main pharmacological target is the osteoclast [2], is able to activate the Vγ9Vδ2 subsets (up-regulation of CD25 and CD69) and in some patients when administered with interleukin (IL)-2 is able to expand γδ T cells in vivo [3–6]. Of potential interest in SSc, these pamidronate-activated γδ T cells produce interferon (IFN)-γ, a Th1 cytokine, with direct anti-fibrogenic effects on fibroblasts and indirect anti-fibrogenic effects by classically activating macrophages to increase production of the anti-fibrogenic cytokine, tumour necrosis factor (TNF)-α [7–11]. In addition, pamidronate synergizes with small amounts of IFN-γ to markedly up-regulate TNF-α gene and protein expression by monocytes/macrophages and may directly modulate the function of monocytes [12].
The major purpose of the present study was to determine whether a single intravenous infusion of pamidronate in patients with SSc would affect production by peripheral blood mononuclear cells (PBMC) of cytokines that may modulate fibrogenesis and/or alter lymphocyte subsets. Safety of a single dose of pamidronate and its effects on clinical parameters including modified Rodnan skin score (MRSS), modified health assessment questionnaire (MHAQ), quality of life [short form (SF)-36] and pulmonary function tests (PFTs) and on biomarkers of bone turnover were also determined during the 24-week period of post-pamidronate infusion observation.
Materials and methods
Study design
Twenty patients with limited or diffuse SSc received a single intravenous infusion of pamidronate 60 mg in 500 cc ½ normal saline solution over 4 h. Two patients, both with limited SSc, were excluded from the analysis; one with chronic lymphocytic leukaemia (CLL) and another because her baseline production of IFN-γ in PBMC culture was greater than 5 standard deviations above mean IFN-γ production compared to the remaining patients at baseline. A total of 18 patients are included in this report.
Inclusion criteria were as follows: men or women of any race, at least 18 years of age, an American College of Rheumatology (ACR) [13] clinical diagnosis of limited or diffuse SSc, women were post-menopausal or, if premenopausal, were surgically sterile or agreed to use two forms of birth control, men agreed not to father a child during the study or for 18 months after study termination, and if a patient was on an angiotensin-converting enzyme (ACE) inhibitor, that dose was stable for at least 3 months prior to study entry. Patients were excluded from this study for any of the following reasons: inability to render an informed consent in accordance with institutional guidelines, premenopausal women with a positive pregnancy test or who were breastfeeding, use of prednisone, cyclophosphamide, d-penicillamine, cyclosporin A, methotrexate, azathioprine or other immune modulator therapies within 1 month of the study, history of an organ or stem cell transplant, use of a bisphosphonate within 6 months of study initiation or contraindication to pamidronate (including hypersensitivity, hypocalcaemia, creatinine > 5 mg/dl, white blood cell count < 3·5 mg/dl). Written consent for participation in the study was obtained from all participants in accordance with the Helsinki II declaration, and the protocol was approved by the University of Tennessee Health Science Center Institutional Review Board.
Cultures of PBMC for cytokine production
Heparinized blood was diluted 1 : 3 in RPMI-1640 medium containing 100 U/ml penicillin and 100 µg/ml streptomycin and subjected to isopycnic centrifugation using Histopaque 1077 (Sigma-Aldrich, St Louis, MO, USA) as a separation medium [14]. Isolated PBMC were washed twice in high glucose Dulbecco’s minimal essential medium containing 9% heat incubated fetal calf serum (FCS), 100 U/ml penicillin, 100 µg/ml streptomycin, non-essential amino acids and sodium pyruvate, hereafter referred to as ‘complete medium’. A single batch of FCS was used throughout the study.
The PBMC were cultured at a concentration of 2 × 106 500/µl complete medium in 48-well tissue culture plates (Nunc, Kamstrupvej, Denmark) for 6 days. At the end of culture, PBMC supernatants were harvested and frozen at −70°C until they were analysed within 2 weeks for levels of cytokines.
Cytokine quantification by enzyme-linked immunosorbent assay (ELISA)
PBMC culture supernatants were thawed at room temperature and assayed undiluted for levels of TNF-α and IL-4 at a 1 : 2 dilution for IFN-γ, a 1 : 4 dilution for IL-10 and a 1 : 7·8 dilution for transforming growth factor (TGF)-β after acid activation of latent TGF-β according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Levels of IFN, IL-4, IL-10 and TNF-α were measured at 1 : 30 dilution in stored serum samples which had been collected at each visit before and after pamidronate infusion.
Flow cytometry
PBMC were surface-stained for 30 min at 4°C with fluorescein isothiocyanate (FITC)-, peridinin chlorophyll (PercP)- or phycoerythrin (PE)-conjugated monoclonal antibody (mAb) against CD3, CD4, CD8, γδ T cell receptor (TCR), CD16 and CD69 (all from BD Biosciences, San Jose, CA, USA). Appropriate isotype controls were used to determine background staining. After washing of unbound antibody, samples were analysed by three-colour flow cytometry on a fluorescence activated cell sorter (FACScan) flow cytometer (Becton Dickinson, San Jose, CA, USA), and the frequencies of cell subsets were calculated using WinMDI software (Joseph Trotter, Scripps Research Institute, LaJolla, CA, USA).
IFN-γ secretion assay
Production in vitro of IFN-γ by unstimulated PBMC subsets in response to culture with pamidronate was measured using the Miltenyi Cytokine Secretion Assay (Miltenyi Biotec, Auburn, CA, USA). This was performed at one time-point (24-week visit) at completion of the trial. PBMC were cultured in complete medium at 106 cells/ml/well in a 24-well tissue culture plates [Falcon (Becton Dickinson), Franklin Lakes, NJ, USA]. The cells were stimulated with 15 µM pamidronate solution (the least cytotoxic dose of pamidronate in vitro), and incubated overnight at 37°C, 5% CO2. The cells were then harvested, washed in cold buffer [phosphate-buffered saline (PBS pH 7·2, containing 0·5% bovine serum albumin (BSA) and 2 mM ethylenediamine tetraacetic acid (EDTA)] and resuspended in 90 µl of cold medium per 106 cells per tube. Ten µl of IFN-γ catch reagent was added per 106 cells, mixed and incubated for 5 min at 4°C. In order to promote IFN-γ secretion, 1 ml of warm medium was added per 106 cells, and cells were incubated for 45 min at 37°C under slow continuous rotation. Cells were then washed, resuspended, and 10 µl of IFN-γ detection antibody (PE-labelled) was added per 106 cells. Additionally, the cells were also surface-stained with anti-CD4 FITC, CD8 FITC and γδ FITC. Propidium iodide (PI) was added at a concentration of 0·5 µg/ml for exclusion of dead cells during flow cytometric analysis. The frequencies of cell subsets were calculated using WinMDI software (Joseph Trotter).
Clinical assessments
MRSS, MHAQ, short form (SF)-36 and PFTs were followed for 24 weeks after the pamidronate infusion in each patient. MRSS was quantified using a maximum value of 51 by assessing 17 body areas with a 0–3 score (i.e. 0 = normal; 1 = mild thickness; 2 = moderate thickness; and 3 = severe thickness). The same trained investigator performed all skin scores. The intra-observer variability for this examiner was < 2 [15]. A standard scleroderma MHAQ [16] and SF-36 questionnaire [17] were administered by trained personnel. PFTs, including forced vital capacity (FVC) and diffusion capacity of carbon monoxide (DLCO), were measured in SSc patients. All testing was performed under the direct supervision of a qualified pulmonologist. Quality assurance of generated data was implemented incorporating the American Thoracic Society (ATS) guidelines [18]. FVC was measured until the best two measurements of each agreed within 5%. DLCO was determined until results were within 10% + 3 ml according to ATS, 1995 guidelines [18,19].
Measurement of biomarkers
Blood was collected after an overnight fast. The aminoterminal propeptide of type I collagen (P1NP) (DiaSorin, Stillwater, MN, USA), a marker of bone formation, was measured in the serum by radioimmunoassay; pyridinoline, a marker of bone resorption, and creatinine were measured in the serum by ELISA (Metra Biosystems, Inc., Mountain Home, CA, USA). All specimens were measured in duplicate, and the mean values recorded. The intra- and interassay coefficients of variation in our laboratory were 2·68% and 2·56% for P1NP and 5·70% and 7·06% for pyridinoline.
Statistical analysis
Statistical analysis was performed using the SAS System for Windows (version 9·1; SAS Institute, Cary, NC, USA) using sas proc mixed. Model selection was based upon the value of the Akaike information criterion (AIC), and the unstructured covariance model was chosen as the best fit. Besides testing for a significant treatment effect for each of the outcomes [PBMC cytokines, γδ T cell activation (Figs 1–6) and MRSS, PFTs, MHAQ, SF-36, biomarkers (Table 2)], contrasts were developed within the overall model to compare the outcomes at each of the follow-up time-points. Four observation time-points (baseline, 1, 3 and 6 months) were entered into the model separately for each patient. All hypothesized effects were tested at an α of 0·05.
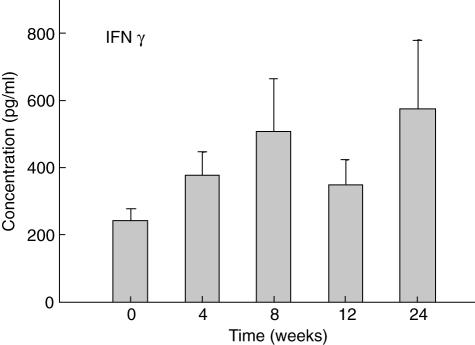
Fig. 1.
Levels of interferon (IFN)-γ in peripheral blood mononuclear cells (PBMC) supernatants. Mean levels ± s.e.m. of IFN-γ measured by enzyme-linked immunosorbent assay (ELISA) are shown in PBMC culture supernatants (0 weeks) and at the indicated times after infusion of 60 mg of pamidronate.
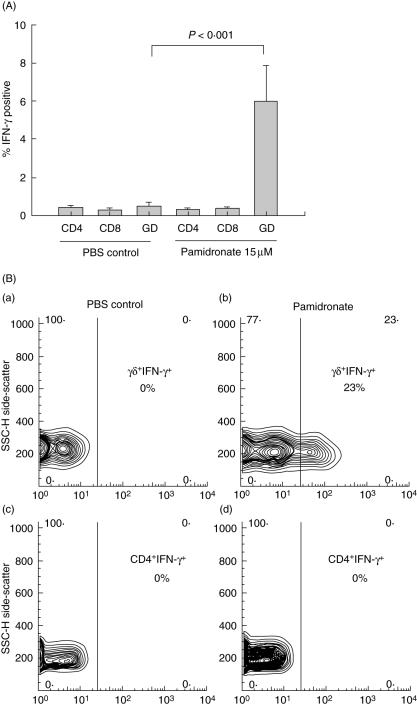
Fig. 6.
(a) In vitro culture of peripheral blood mononuclear cells (PBMC) with pamidronate; mean percentage ± s.e.m. Interferon (IFN)-γ positive lymphocyte subsets after in vitro culture of PBMC with pamidronate. Pamidronate directly induces secretion of IFN-γ from γδ T cells, but not other T cell subsets. GD = γδ T cells. (b) Contour plots from flow cytometry data demonstrating secretion of IFN-γ from γδ T cells in response to in-vitro stimulation with pamidronate. Upper panels (a and b) show gating on γδ T cells (unstimulated: a; pamidronate stimulation: b), whereas in lower panels gating is on CD4 T cells for comparison (unstimulated: c; pamidronate: d).
Table 2.
Clinical parameters (mean ± s.d.) over time.
| Clinical parameter | n | Baseline | 1 month | 3 months | 6 months |
|---|---|---|---|---|---|
| MRSS | 18 | 16·00 (9·32) | 16·67 (8·95) | 17·00 (8·56) | 16·39 (7·93) |
| FVC | 18 | 3·17 (0·98) | n.a. | 3·11 (0·97)* | 3·08 (0·98)* |
| Adjusted DLCO2 | 171 | 15·57 (4·35) | n.a. | 16·25 (5·77) | 16·08 (5·18) |
| MHAQ | 173 | 0·53 (0·51) | 0·44 (0·48) | 0·46 (0·35) | 0·40 (0·33) |
| SF-36 physical functioning scale | 173 | 48·53 (27·66) | 53·07 (25·35) | 48·82 (26·90) | 52·64 (27·90) |
| Pyrdinoline (nmol/l) | 18 | 64·80 (260·60) | n.a. | 4·12 (3·43) | 4·24 (2·74) |
| P1NP (ug/l) | 18 | 86·86 (52·66) | n.a. | 37·37 (22·72)* | 48·64 (23·03)† |
P < 0·05 adjusted for disease type compared with baseline.
One patient was unable to complete DLCO.
Adjusted for haemoglobin.
One patient did not complete the MHAQ and SF-36 physical functioning scale; n.a. = not applicable (pulmonary parameters and biomarkers were only measured at baseline, 12 and 24 weeks). DLCO: diffusion capacity for carbon monoxide; MHAQ: modified health assessment questionnaire; MRSS: modified Rodnan skin score; P1NP: aminoterminal propeptide of tyle I collagen; SF: short form.
Results
Demographics of the study population
Baseline characteristics of the SSc population are shown in Table 1. Both males and females, African Americans and Caucasians, were included in this study. More patients had limited (72%), than diffuse (28%), disease. The majority of patients reported a history of Raynaud’s phenomenon (94%), gastrointestinal (94%) or pulmonary disease (67%) or arthralgias (83%) stemming from SSc. Cardiac and muscle disease from SSc was less common in these patients, with 6% of patients reporting a history of cardiac disease or myositis from SSc. The average disease duration was 11·2 years.
Table 1.
Baseline characteristics of study population.
| Characteristic | Mean (s.d.) or n (%) |
|---|---|
| Age (years) | 56·7 (8·8) |
| Gender | |
| Male | 6 (32%) |
| Female | 12 (67%) |
| Race | |
| Caucasian | 14 (78%) |
| African American | 4 (22%) |
| Disease type | |
| Limited | 13 (72%) |
| Diffuse | 5 (28%) |
| Raynaud’s | 17 (94%) |
| Arthralgias | 15 (83%) |
| Myalgias | 1 (6%) |
| Gastrointestinal symptoms | 17 (94%) |
| Cardiac symptoms | 1 (6%) |
| Pulmonary symptoms | 12 (67%) |
| Duration of disease (years) | 11·2 (11·0) |
Pamidronate therapy was well tolerated, with no patients developing hypocalcaemia 48 h after infusion (data not shown). Four patients reported transient malaise and ‘flu-like symptoms’ 48 h after the infusion which resolved within 1 week.
Effects of pamidronate treatment on cytokine production by cultured PBMC
To assess the effects of pamidronate treatment on cytokine production, PBMC were isolated from patients at baselineand every 4 weeks for up to 24 weeks after pamidronate infusion. Cytokine expression was determined between 4 and 12 weeks based on a report that alendronate, another nitrogen-containing bisphosphonate, was found to affect cytokine profiles at weeks 4 and 12 after administration [20]. We measured PBMC cytokine expression at 24 weeks to determine whether there were any prolonged effects of pamidronate on cytokines, given the long skeletal retention of this drug [21].
Levels of IFN-γ were increased relative to baseline in supernatants from PMBC at all times assessed after pamidronate infusion (Fig. 1).
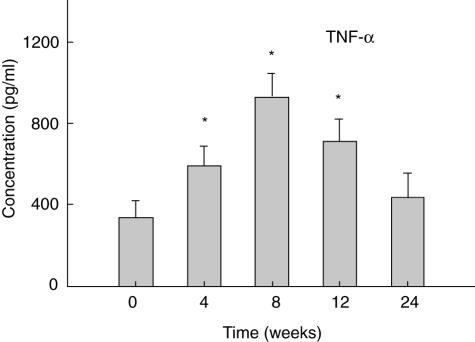
The levels of TNF-α in supernatants from PBMCs were also higher at all times after pamidronate infusion. TNF-α was significantly elevated (P < 0·05) from baseline levels at 4, 8 and 12 weeks post-pamidronate infusion (PBS) (Fig. 2).
Fig. 2.
Mean levels ± s.e.m. of tumour necrosis factor (TNF)-α in peripheral blood mononuclear cells (PBMC) culture supernatants over time; Levels are shown of TNF-α measured by enzyme-linked immunosorbent assay (ELISA) in PBMC before (0 weeks) and at the indicated times after infusion of 60 mg of pamidronate. *Indicates P-value < 0·05 adjusted for disease type compared with baseline.
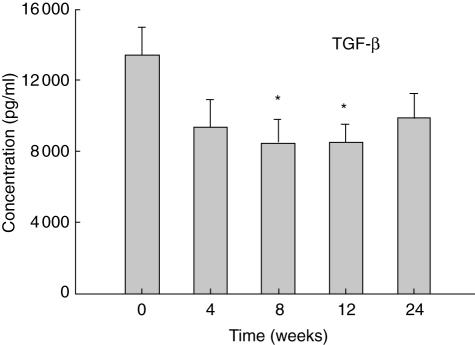
In contrast to the elevated levels of IFN-γ and TNF-α in culture supernatants of PBMC after the pamidronate infusion, the levels of TGF-β1 in the same culture supernatants decreased from baseline levels. Levels of TGF-β1 reached significantly reduced levels (P < 0·05) at 8 and 12 weeks after pamidronate infusion (Fig. 3). Levels of IL-4 and IL-10 did not change significantly from baseline values in supernatants from culture of PBMC (data not shown).
Fig. 3.
Mean levels ± of TGF-β1 in peripheral blood mononuclear cells (PBMC) culture supernatants Levels are shown of transforming growth factor (TGF)-β1 measured by enzyme-linked immunosorbent assay (ELISA) in PBMC culture supernatants before (0 weeks) and at the indicated times after infusion of 60 mg of pamidronate. *Indicates P-value < 0·05 adjusted for disease type compared with baseline.
Serum levels of cytokines
Serum levels of IFN-γ, TNF-α, IL-10 and IL-4 did not change significantly at 4, 8, 12 or 24 weeks after the pamidronate infusion (data not shown).
Effects of pamidronate on lymphocyte subsets
We assessed lymphocyte subsets at similar time-points to the cytokine measurements; in addition, we also measured lymphocyte subsets at 48 h, because of a report that pamidronate can up-regulate the expression of CD86 and MHC class I molecules at 48 h [22]. Finally, lymphocyte subsets were analysed at 48 weeks to determine whether there were any prolonged effects of pamidronate on lymphocyte subsets, given this drug’s long skeletal retention [21].
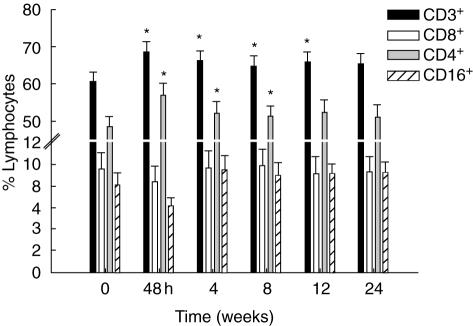
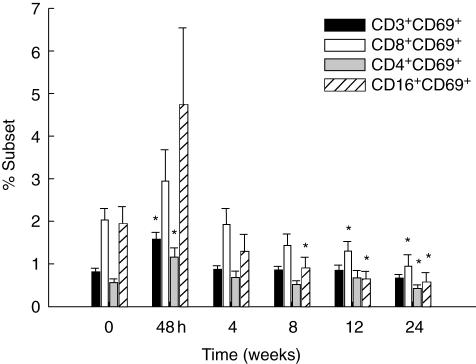
There was an increase that normalized by the end of the study in the proportion of total T cells (CD3+) and in the CD4+, but not the CD8+ T cell subset after infusion. A fall in the percentage of natural killer (NK) cells (CD16+) was seen 48 h after pamidronate infusion; this had resolved by the time of the 4-week visit (Fig. 4). Increased lymphocyte activation, measured by CD69 expression on CD3+, CD4+ and CD8+ T cells as well as NK cells was detected at the 48-h visit (P < 0·05 for CD3 and CD4 subsets) (Fig. 5).
Fig. 4.
Flow cytometry analyses over time; lymphocyte subsets were measured by flow cytometry. Mean percentage ± s.e.m. CD3, CD4, CD8 and CD16 lymphocytes are shown at each time-point. *Indicates P-value < 0·05 adjusted for disease type compared with baseline.
Fig. 5.
Up-regulation of CD69 by flow cytometry; in vivo activation of lymphocyte subsets (mean ± s.e.m. expression of CD69) is shown, measured by flow cytometry. *Indicates P-value < 0·05 adjusted for disease type compared with baseline.
In order to identify whether pamidronate stimulates lymphocyte subsets directly or indirectly via cytokine release, we performed in vitro studies on SSc PBMC obtained at 24 weeks post-pamidronate infusion. Using a cytokine secretion assay and flow cytometric analysis, we demonstrated that in vitro, only γδ T cells are directly activated by pamidronate. Pamidronate-activated γδ T cells, but not CD4 or CD8 cells, produced IFN-γ (Fig. 6a,b).
Effect of pamidronate on clinical parameters
Clinical parameters including MRSS, DLCO, MHAQ and SF-36 (physical functioning scale) showed no significant changes during the 24-week period of observation after the pamidronate infusion (Table 1). There was a 2·24% decrease in FVC (from 3·12 to 3·05, or 0·07 l) which, although statistically significant, was within the variability of the test [20] and not clinically significant; DLCO increased by 2·16%, which was also not clinically significant as it falls within the coefficient of variation (5–6%) of repeated measurements in normal subjects [21]. P1NP, but not pyridinoline, decreased significantly after pamidronate infusion (Table 1).
Discussion
In patients with limited and diffuse SSc, administration of a single intravenous dose of pamidronate was well tolerated and had prolonged effects on production of cytokines by unstimulated PBMC, increasing IFN-γ and TNF-α but decreasing production of TGF-β1. To our knowledge, this is the first study in any patient population (normal or diseased) to assess prolonged effects of pamidronate on PBMC subsets and cytokine production. The infusion of pamidronate induced T cell activation in vivo and expanded circulating CD3+, CD4+ T cells and decreased NK cells. In vitro, pamidronate stimulated γδ T cells directly to secrete IFN-γ.
Our results, as well as results from other studies, show low percentages of circulating γδ T cells in patients with SSc [23–25]. This may be the result of a preferential homing of γδ T cells to the skin and lungs of patients with SSc. We did not see an expansion of circulating γδ T cells in any of the patients in our study population. A single infusion of pamidronate has been reported to increase the percentages of γδ T cells in patients with increased bone resorption who develop the acute-phase reaction, but not in patients who do not develop the acute-phase reaction [3]. It is possible that we may have missed a rise in γδ T cells between 48 h and 4 weeks post-pamidronate infusion. It is also possible that this was not detected because IL-2 was not added. The addition of IL-2 may be particularly important, as expanded γδ T cells from pamidronate co stimulated with IL-2 are potent Th1 effectors capable of releasing large amounts of IFN-γ [26].
γδ T cells are involved in a wide range of immunological activities including defence against infections, regulation of inflammation, tumour surveillance, graft rejection, autoimmunity and tumour killing [6]. Both animal [27,28] and human [29,30] studies suggest that γδ T cells produce IFN-γ when activated. Of potential importance in SSc is the observation that IFN-γ has an anti-fibrogenic effect due to its ability in fibroblasts to decrease synthesis of CI and reduce transcription of Col I A2 mRNA [8,9,31]. Moreover, reports suggest that patients with clinically active SSc have lower serum levels of IFN-γ than those in clinically stable patients [32]. Bronchoalveolar lavage (BAL) cells from patients with SSc that express IFN-γ mRNA are significantly less likely to have a decline in FVC over time compared to patients whose BAL cells do not express increased IFN-γ mRNA [33]. However, for unknown reasons, exogenous administration of IFN-γ has provided only marginal benefits in the treatment of SSc [34].
Synthetic aminobisphosphonates, including pamidronate, are potent activators of γδ T cells [5] and have been shown to induce IFN-γ production in lymphoma and myeloma cells in vitro [6]. To our knowledge, this is the first report to suggest that an aminobisphosphonate can stimulate in vitro SSc γδ T cell IFN-γ production and effect up-regulation of IFN-γ production by PBMC from patients with SSc. The up-regulation of IFN-γ production by PBMCs seen in patients with SSc was not a transient phenomenon, as has been noted in patients with other diseases [6]. We postulate that in vivo, pamidronate induces IFN-γ production by γδ T cells and this cytokine in turn exerts pleiotropic effects on immune function, including synergizing of IFN-γ with pamidronate to induce TNF-α production by monocytes [12].
The Th2 cytokine, TGF-β, is up-regulated in patients with SSc [35,36], and some studies suggest that there is a positive correlation between Th2 cytokines and the severity of SSc [33]. There was a significant, small (approximately 30%) decline in production of TGF-β by cultured PBMC after pamidronate infusion, suggesting that a profibrogenic cytokine is being decreased by pamidronate treatment in SSc patients. Even small changes in TGF-β in patients with SSc may be clinically significant in the pathogenesis of this disease, as one study has suggested that PBMC TGF-β concentrations are elevated in patients with SSc compared with normal controls [37]. TGF-β1 is a potent chemoattractant for fibroblasts, increases CI protein and gene expression in fibroblasts, inhibits synthesis of matrix melloproteinase-1 (MMP-1) production, up-regulates products of tissue inhibitor of metalloproteinases and up-regulates synthesis of glycosaminoglycans [38–41]. TGF-β1 has been implicated as a major profibrogenic cytokine in the pathogenesis of SSc [42,43]. TGF-β1 is synthesized by IL-4-induced alternatively activated macrophages but not by lipopolysaccharide or IFN-γ-induced classically activated macrophages [7]. TGF-β1 production is increased in cultured PBMC from patients with SSc compared to normal control subjects [37]. The present study showed that TGF-β1 production was chronically down-regulated by cultured PMBC from patients with SSc infused with pamidronate, perhaps by changing the macrophage activation from the alternative to the classical pathway [44].
Pamidronate treatment of SSc patients in our study up-regulated production of IFN-γ from PBMC. Although we did not have a control population of SSc patients who did not receive pamidronate, the increase in IFN-γ that we noted was probably secondary to the therapy itself, as data from our institution suggest that PBMC production of IFN-γ in patients with SSc not given pamidronate does not change significantly over time [45]. TNF-α by PBMC was also up-regulated by pamidronate treatment. In vitro, in healthy patients with increased bone turnover, TNF-α increases with a single intravenous administration of pamidronate [46]. IFN-γ, both soluble and bound to T cell membranes, inhibits collagen synthesis in fibroblasts stimulated by TGF-β [47]. TNF-α inhibits collagen synthesis by fibroblasts induced by TGF-β1 by down-regulating TGF-β Type II receptors and by inhibiting induction of connective tissue growth factor expression [10,48]. TNF-α is also a potent stimulant of MMP-1 synthesis of fibroblasts [49]. However, of concern, SSc cells appear to be resistant to the anti-fibrotic effects of TNF-α, as TNF-α is unable to suppress the basal level of connective tissue growth factor in SSc fibroblasts [11], and TNF-α significantly reduces collagen production by normal fibroblasts [50]. The mechanisms by which pamidronate induces up-regulation of TNF-α by human monocytes and mouse macrophages has been studied recently [12]. Pamidronate, in the presence of non-stimulating levels of IFN-γ (1–10 pg/ml) by inhibiting protein tyrosine phosphatase activity, augments TNF-α protein and gene expression by sustained phosphorylation of tyrosine residue at position 701 of STAT1 [12].
In our study, there were transient changes in the T cell population following pamidronate infusion, with total T cells (CD3 and CD4) increasing, NK cells (CD16) decreasing, and CD8 T cells remaining stable. In contrast, following pamidronate infusion in patients with solid tumours, Pecherstorfer et al. observed that there were transient decreases in CD3, CD4 and CD8 T cells [51]. However, activation of lymphocyte subsets measured by CD69, similar to what occurred in our SSc patients, was also reported following pamidronate infusions in these patients with solid tumours [51]. The reason for the different effects of pamidronate on T cell numbers in patients with SSc versus patients with solid tumours is not apparent, but may be related to the unique influences these disease states exert on T cells.
Our study has several limitations. We did not include a control population in our study; therefore, we could not determine the effects of the same infusion of pamidronate on cytokine and lymphocyte profiles in patients without SSc. None the less, this study demonstrates that pamidronate modulates important cytokines that regulate fibrogenesis in patients with SSc which in itself is a significant observation, regardless of whether or not it has similar effects on cytokine profiles in normal controls. In addition, the number of patients with diffuse disease (five) was too small to detect any meaningful differences in response to pamidronate in patients with limited versus diffuse SSc; however, adjustment for disease type (diffuse versus limited) in the statistical analysis did not significantly change any of the results. The study was not powered to detect changes in MRSS or PFTs and the period of observation (6 months) was probably too short to detect changes in this population; there was, however, a significant reduction in P1NP and decreases in P1NP could potentially translate into reductions in fracture rates over time [52,53]. The majority of TGF-β1 in PBMC cultures is produced by monocytes, and less by T cells [54]. Platelets may also be a source of TGF-β1, and we cannot completely exclude the possibility that the changes in TGF-β1 levels in PBMC culture supernatants may be the result of platelets being present in the culture. However, given that the same careful procedures were used throughout the study to isolate and stimulate the PBMCs, we feel that this is less likely. Finally, it is possible that the observed changes in cytokines with pamidronate may have effects on immune dysregulation and endothelial activation and dysfunction that were not measured in this study. Recent reports suggest that activation of the immune system and endothelial activation and dysfunction are of paramount importance in the pathogenesis of SSc [55] and another nitrogen-containing bisphosphonate, zolendronic acid, has been shown, in vitro, to directly inhibit proliferation of human endothelial cells [56].
Conclusions
In conclusion, a single 60-mg intravenous dose of pamidronate administered to patients with limited or diffuse SSc induced T cell activation and up-regulated production of the cytokines, IFN-γ and TNF-α, and down-regulated TGF-β1 production by PBMC. In vitro, pamidronate directly stimulated γδ T cells from patients with SSc to secrete IFN-γ. Clinical parameters including MRSS, PFTs and MHAQ over the 24-week period of observation did not change significantly with single-dose pamidronate therapy in these patients. The risk/benefit ratio of pamidronate in patients with SSc may merit future study.
Acknowledgments
This work was supported by grants from the Scleroderma Foundation (L. Carbone and M. Watsky), the General Clinical Research Center (MO1 RR 00211) of The University of Tennessee, a Scleroderma SCOR grant from NIAMS (AR44890, A. Postlethwaite), a NIAMS Rheumatic Diseases Research Core Center grant (AR47379) and research funds from the Department of Veterans Affairs (A. Postlethwaite and L. Carbone).
Declaration of interest
Laura Carbone, MD has consulted for Novartis.
Authors’ contributions
L. D. C. participated in the design of the study, performed physical examinations and skin scores on participants and drafted the manuscript. K. D. B. participated in the design of the study, performed the statistical analysis and helped to draft the manuscript. K. J. W. performed the flow cytometry and helped to draft the manuscript. M. P. performed quality control of pulmonary function tests and helped to draft the manuscript. M. A. W. performed the biomarker of bone metabolism analysis and helped to draft the manuscript. G. W. S. performed the statistical analysis and helped to draft the manuscript. J. I. participated in the design of the study, performed the cytokine analysis and helped to draft the manuscript. A. E. P. generated preliminary data in vitro that formed the basis for the study and participated in the design of the study, performed the cytokine analyses and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Majumdar S, Li D, Ansari T, et al. Different cytokine profiles in cryptogenic fibrosing alveolitis and fibrosing alveolitis associated with systemic sclerosis: a quantitative study of open lung biopsies. Eur Respir J. 1999;14:251–7. doi: 10.1034/j.1399-3003.1999.14b03.x. [DOI] [PubMed] [Google Scholar]
- 2.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9:2643–58. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]
- 3.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 4.Schilbach K, Geiselhart A, Handgretinger R. Induction of proliferation and augmented cytotoxicity of gammadelta T lymphocytes by bisphosphonate clodronate. Blood. 2001;97:2917–18. doi: 10.1182/blood.v97.9.2917. [DOI] [PubMed] [Google Scholar]
- 5.Das H, Wang L, Kamath A, Bukowski JF. Vgamma2Vdelta2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood. 2001;98:1616–18. doi: 10.1182/blood.v98.5.1616. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 7.Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh AK, Yuan W, Mori Y, Chen S, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J Biol Chem. 2001;276:11041–8. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- 9.Varga J, Olsen A, Herhal J, Constantine G, Rosenbloom J, Jimenez SA. Interferon-gamma reverses the stimulation of collagen but not fibronectin gene expression by transforming growth factor-beta in normal human fibroblasts. Eur J Clin Invest. 1990;20:487–93. doi: 10.1111/j.1365-2362.1990.tb01890.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamane K, Ihn H, Asano Y, Jinnin M, Tamaki K. Antagonistic effects of TNF-alpha on TGF-beta signaling through down-regulation of TGF-beta receptor type II in human dermal fibroblasts. J Immunol. 2003;171:3855–62. doi: 10.4049/jimmunol.171.7.3855. [DOI] [PubMed] [Google Scholar]
- 11.Abraham DJ, Shiwen X, Black CM, Sa Xu S, Leask Y. A tumor necrosis factor alpha suppresses the induction of connective tissue growth factor by transforming growth factor-beta in normal and scleroderma fibroblasts. J Biol Chem. 2000;275:15220–5. doi: 10.1074/jbc.275.20.15220. [DOI] [PubMed] [Google Scholar]
- 12.Takagi K, Takagi M, Kanangat S, Warrington KJ, Shigemitsu H, Postlethwaite AE. Modulation of TNF-alpha gene expression by IFN-gamma and pamidronate in murine macrophages: regulation by STAT1-dependent pathways. J Immunol. 2005;174:1801–10. doi: 10.4049/jimmunol.174.4.1801. [DOI] [PubMed] [Google Scholar]
- 13.American Rheumatism Association. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. [DOI] [PubMed] [Google Scholar]
- 14.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 15.Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 16.Steen VD, Medsger TA., Jr The value of the Health Assessment Questionnaire and special patient-generated scales to demonstrate change in systemic sclerosis patients over time. Arthritis Rheum. 1997;40:1984–91. doi: 10.1002/art.1780401110. [DOI] [PubMed] [Google Scholar]
- 17.Del Rosso A, Boldrini M, D’Agostino D, et al. Health-related quality of life in systemic sclerosis as measured by the Short Form 36: relationship with clinical and biologic markers. Arthritis Rheum. 2004;51:475–81. doi: 10.1002/art.20389. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique − 1995 update. Am J Respir Crit Care Med. 1995;152:2185–98. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 20.Papadaki HA, Tsatsanis C, Christoforidou A, et al. Alendronate reduces serum TNFalpha and IL-1beta, increases neutrophil counts, and improves bone mineral density and bone metabolism indices in patients with chronic idiopathic neutropenia (CIN)-associated osteopenia/osteoporosis. J Bone Miner Metab. 2004;22:577–87. doi: 10.1007/s00774-004-0526-y. [DOI] [PubMed] [Google Scholar]
- 21.Cremers SC, Papapoulos SE, Gelderblom H, et al. Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res. 2005;20:1543–7. doi: 10.1359/JBMR.050522. [DOI] [PubMed] [Google Scholar]
- 22.Conti L, Casetti R, Cardone M, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–60. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 23.Ercole LP, Malvezzi M, Boaretti AC, Utiyama SR, Rachid A. Analysis of lymphocyte subpopulations in systemic sclerosis. J Invest Allergol Clin Immunol. 2003;13:87–93. [PubMed] [Google Scholar]
- 24.Holcombe RF, Baethge BA, Wolf RE, Betzing KW, Stewart RM. Natural killer cells and gamma delta T cells in scleroderma. relationship to disease duration and anti-Scl-70 antibodies. Ann Rheum Dis. 1995;54:69–72. doi: 10.1136/ard.54.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riccieri V, Parisi G, Spadaro A, et al. Reduced circulating natural killer T cells and gamma/delta T cells in patients with systemic sclerosis. J Rheumatol. 2005;32:283–6. [PubMed] [Google Scholar]
- 26.Casetti R, Perretta G, Taglioni A, et al. Drug-induced expansion and differentiation of V gamma 9V delta 2 T cells in vivo: the role of exogenous IL-2. J Immunol. 2005;175:1593–8. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]
- 27.Vesosky B, Turner OC, Turner J, Orme IM. Gamma interferon production by bovine gamma delta T cells following stimulation with mycobacterial mycolylarabinogalactan peptidoglycan. Infect Immun. 2004;72:4612–8. doi: 10.1128/IAI.72.8.4612-4618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponomarev ED, Novikova M, Yassai M, Szczepanik M, Gorski J, Dittel BN. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J Immunol. 2004;173:1587–95. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- 29.Wisnewski AV, Herrick CA, Liu Q, Chen L, Bottomly K, Redlich CA. Human gamma/delta T-cell proliferation and IFN-gamma production induced by hexamethylene diisocyanate. J Allergy Clin Immunol. 2003;112:538–46. doi: 10.1016/s0091-6749(03)01865-7. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Das H, Kamath A, Bukowski JF. Human V gamma 2V delta 2 T cells produce IFN-gamma and TNF-alpha with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 31.Serpier H, Gillery P, Salmon-Ehr V, et al. Antagonistic effects of interferon-gamma and interleukin-4 on fibroblast cultures. J Invest Dermatol. 1997;109:158–62. doi: 10.1111/1523-1747.ep12319207. [DOI] [PubMed] [Google Scholar]
- 32.Molteni M, Della Bella S, Mascagni B, et al. Increased interferon-gamma (IFN-gamma) levels produced in vitro by alloactivated T lymphocytes in systemic sclerosis and Raynaud’s phenomenon. Clin Exp Immunol. 1999;116:164–8. doi: 10.1046/j.1365-2249.1999.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atamas SP, Yurovsky VV, Wise R, et al. Production of type 2 cytokines by CD8+ lung cells is associated with greater decline in pulmonary function in patients with systemic sclerosis. Arthritis Rheum. 1999;42:1168–78. doi: 10.1002/1529-0131(199906)42:6<1168::AID-ANR13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Polisson RP, Gilkeson GS, Pyun EH, Pisetsky DS, Smith EA, Simon LS. A multicenter trial of recombinant human interferon gamma in patients with systemic sclerosis. effects on cutaneous fibrosis and interleukin 2 receptor levels. J Rheumatol. 1996;23:654–8. [PubMed] [Google Scholar]
- 35.Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35:67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328–32. [PubMed] [Google Scholar]
- 37.Hasegawa M, Sato S, Takehara K. Augmented production of transforming growth factor-beta by cultured peripheral blood mononuclear cells from patients with systemic sclerosis. Arch Dermatol Res. 2004;296:89–93. doi: 10.1007/s00403-004-0472-5. [DOI] [PubMed] [Google Scholar]
- 38.Raghow R, Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987;79:1285–8. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165:251–6. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards DR, Leco KJ, Beaudry PP, Atadja PW, Veillette C, Riabowol KT. Differential effects of transforming growth factor-beta 1 on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in young and old human fibroblasts. Exp Gerontol. 1996;31:207–23. doi: 10.1016/0531-5565(95)02010-1. [DOI] [PubMed] [Google Scholar]
- 41.Philips N, Keller T, Gonzalez S. TGF beta-like regulation of matrix metalloproteinases by anti-transforming growth factor-beta, and anti-transforming growth factor-beta 1 antibodies in dermal fibroblasts: implications for wound healing. Wound Repair Regen. 2004;12:53–9. doi: 10.1111/j.1067-1927.2004.012111.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith EA, LeRoy EC. A possible role for transforming growth factor-beta in systemic sclerosis. J Invest Dermatol. 1990;95(Suppl. 6):S125–7. doi: 10.1111/1523-1747.ep12874998. [DOI] [PubMed] [Google Scholar]
- 43.Denton CP, Abraham DJ. Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol. 2001;13:505–11. doi: 10.1097/00002281-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Atamas SP, White B. Cytokine regulation of pulmonary fibrosis in scleroderma. Cytokine Growth Factor Rev. 2003;14:537–50. doi: 10.1016/s1359-6101(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 45.Postlethwaite AE, Furst DE, Wong WK, Clements P CSCT Investigators. American College of Rheumatology Annual Meeting. San Diego, CA: 2005. Oral tolerance (OT) induction to type I collagen (CI) significantly reduces the skin score in patients with diffuse systemic sclerosis (SSc) with late-phase disease. Results of a NIAMS/NIAID Multicenter Phase II placebo-controlled double blind clinical trial. [Google Scholar]
- 46.Thiebaud D, Sauty A, Burckhardt P, et al. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997;61:386–92. doi: 10.1007/s002239900353. [DOI] [PubMed] [Google Scholar]
- 47.Chizzolini C, Rezzonico R, Ribbens C, Burger D, Wollheim FA, Dayer JM. Inhibition of type I collagen production by dermal fibroblasts upon contact with activated T cells: different sensitivity to inhibition between systemic sclerosis and control fibroblasts. Arthritis Rheum. 1998;41:2039–47. doi: 10.1002/1529-0131(199811)41:11<2039::AID-ART20>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell Signal. 2004;16:873–80. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Rekdal O, Osterud B, Svendsen JS, Winberg JO. Evidence for exclusive role of the p55 tumor necrosis factor (TNF) receptor in mediating the TNF-induced collagenase expression by human dermal fibroblasts. J Invest Dermatol. 1996;107:565–8. doi: 10.1111/1523-1747.ep12582818. [DOI] [PubMed] [Google Scholar]
- 50.Chizzolini C, Parel Y, De Luca C, et al. Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Arthritis Rheum. 2003;48:2593–604. doi: 10.1002/art.11129. [DOI] [PubMed] [Google Scholar]
- 51.Pecherstorfer M, Jilch R, Sauty A, et al. Effect of first treatment with aminobisphosphonates pamidronate and ibandronate on circulating lymphocyte subpopulations. J Bone Miner Res. 2000;15:147–54. doi: 10.1359/jbmr.2000.15.1.147. [DOI] [PubMed] [Google Scholar]
- 52.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051–6. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 53.Reid IR, Davidson JS, Wattie D, et al. Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone. 2004;35:224–30. doi: 10.1016/j.bone.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakkas LI. New developments in the pathogenesis of systemic sclerosis. Autoimmunity. 2005;38:113–16. doi: 10.1080/16066350500095415. [DOI] [PubMed] [Google Scholar]
- 56.Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–61. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]