Abstract
Deafness is attributable to autoimmunity in a subset of adult patients with sensorineural hearing loss (SNHL) of unknown aetiology. To determine the roles of self-antigens in the pathogenesis of idiopathic SNHL, we analysed antibody responses to the inner ear-specific proteins, cochlin and β-tectorin as well as the non-specific heat shock protein 70 (HSP70). Recombinant cochlin and β-tectorin proteins were used in a qualitative Western blot assay for the detection of antigen-specific IgG antibodies in 58 patients with idiopathic SNHL and 28 healthy blood donors. In the same study cohort, we also used a Western blot assay to assess IgG antibody responses to the recombinant human HSP70. Of the 58 patient samples analysed, 19 tested positive to the HSP70, eight to cochlin and one to β-tectorin, giving a prevalence of 33, 14 and 2%, respectively. Only one patient sample was reactive for HSP70, cochlin and β-tectorin, seven of the remaining eight cochlin IgG antibody-positive samples were monospecific. Thus, cochlin-specific antibodies were observed predominantly in HSP70 IgG-negative patients demonstrating an additive value for testing this antibody response in patients with idiopathic SNHL.
Keywords: autoantibodies, β-tectorin, Cochlin, hearing loss, HSP70
Introduction
Some cases of idiopathic sensorineural hearing loss (SNHL) of adult onset are attributable to immune recognition of inner ear proteins as foreign or non-self, a phenomenon referred to as autoimmune inner ear disease (AIED). Although the mechanism(s) in the pathogenesis of AIED is not known, autoimmunity may be induced either within the inner ear, in a primary end-organ response, or outside the inner ear and gain access to the inner ear as a secondary response. Thus, autoantibodies and/or self-reactive T cells of inner ear specificity may serve as indirect evidence of autoimmunity and therefore probable markers in defining clinical disease. The identity of self-antigens responsible for disease induction and maintenance remain to be defined clearly. At present, diagnosis of AIED is by the exclusion of Menière’s disease, retrocochlear disorders, otosclerosis, presbycusis, infectious or other autoimmune aetiologies and response to immunosuppressive therapy [1–3]. Available therapies are only partially effective, as progression in hearing loss has been documented regardless of treatment [4–6].
Initial attempts to identify antigen(s) important in the pathogenesis of AIED relied on immunohistochemical staining of mammalian inner ear tissues using patients’ serum [7–9]. These early studies showed antibody recognition of inner ear tissues in patients suffering from hearing loss. Further characterization of antibodies from patients with SNHL using inner ear tissues from different mammalian species by Western blot analysis showed immune reactivity with several antigens of varied molecular weights [4,10–17]. Several putative antigens have been identified as markers of AIED, but their role in the pathogenesis as well as relevance in disease-specific diagnosis remains undefined. A 68-kilodalton (kDa) antigen expressed in mammalian inner ear extract believed to be heat shock protein 70 (HSP70) [11] has received the most attention. HSPs are ubiquitous and expressed in a number of tissues and disease situations. Furthermore, antibodies against microbial pathogen and mammalian HSPs are present in healthy individuals as well as in various diseases, arguing against the significance of HSP antibodies in the serological diagnosis of AIED [18,19]. Other antigens of great potential include the cochlin protein, identified initially by Boulassel et al. [12] as a candidate autoantigen for SNHL, myelin protein Po [15], choline transporter-like protein 2 [17] as well as β-tectorin [20,21]. The hypothesis of a pathogenic role for either cochlin or β-tectorin is supported by experimental induction of hearing loss following adoptive transfer of T cell lines specific for these proteins [21]. Additionally, stimulation of splenic cells by cochlin or β-tectorin-specific peptides in that study demonstrated a Th1-type response, as interferon gamma was the predominant cytokine produced [21]. Although these findings are of significance in understanding immune pathogenesis of AIED, the implications of these results in the clinical setting remains untested.
To define further the roles of inner ear-specific proteins as well as the relevance of HSP70 autoantibodies in identifying patients with AIED, we evaluated antibody reactivity to the recombinant cochlin, β-tectorin and HSP70 antigens in individuals with idiopathic SNHL as well as adult healthy controls.
Materials and method
Patients and healthy controls
Fifty-eight patients (12 males and 46 females) with idiopathic SNHL and 28 healthy blood donors were recruited for this study. The patient cohort consisted of individuals with variable disease onset and patterns with a mean age of 50·9 ±15·6. The Institutional Review Board (IRB) of the University of Utah approved the study protocol (IRB no. 13634).
Construction of plasmids expressing either cochlin or β-tectorin fusion proteins
Human wild-type COCH cDNA (GenBank Accession no. AF006740) cloned into a modified form of pcDNA3 was obtained from Dr C. C. Morton, Harvard University, Boston, MA, USA. In the case of β-tectorin, a full-length mouse wild-type TECTB cDNA (GenBank Accession no. X99806) was obtained from Dr G. P. Richardson, University of Sussex, UK. The COCH full-length coding region was polymerase chain reaction (PCR) amplified using the primer pair: COCHFor (5′-CAC CAT GTC CGC AGC CTG GAT CCC GGC TCT C-3′) and COCHRev (5′-TTG CTG GGA TTC TAA GAA ATC TCT AC-3′). The coding region of the β-tectorin protein without the signal peptide and GPI anchor sequences was amplified using the following primer pair: TectbFor (5′-CAC CTC ATG CAC TCC GAA TAA AGC AGA T-3′) and TectbRev (5′-CTA ATC TGA GAA GTC ACA GAG GCC GGA-3′). The resulting amplicons were cloned by directional TOPO cloning into the entry vector pENTR/SD/D-TOPO for the Gateway System (Invitrogen, Carlsbad, CA, USA). To generate an expression clone for either COCH or TECTB, each entry clone was sequenced to ensure proper orientation and an LR reaction performed between the entry clones and the Gateway destination vector pEXP1/DEST (Invitrogen). An LR reaction is a recombination of the open reading frame from an entry clone into expression vector. The resulting pEXP1COCH and pEXP1TectB plasmids were transformed in Escherichia coliBL21(DE3)pLysS strain for recombinant protein expression.
Expression and purification of bacterially expressed fusion proteins
Individual clones were picked following transformation with each of the plasmids and inoculated into 50 ml of Luria–Bertani (LB) medium containing ampicillin and chloramphenicol and grown overnight (O/N) at 25°C to an optical density at 600 nm (OD600) of 1·5. A 1 : 20 subculture of the O/N culture was initiated and grown to OD600 of 0·6 after which protein expression was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) (2 mM final concentration). Three hours after the addition of IPTG, the cells were harvested by centrifugation and purified as described by Ding et al. [22] with minor modifications. Briefly, the cell pellet was resuspended in buffer A (20 mM Tris, pH 8·0, 5 mM imidazole, 500 mM NaCl), sonicated and cleared by centrifugation. The resulting cell pellet was resuspended in buffer B [buffer A plus 6 M guanidium-hydrochloride (G-HCL), pH 8·0] and rocked for 1 h at room temperature, sonicated on ice and cleared by centrifugation. The recombinant protein was purified from the supernatant by Ni–NTA (nickel–nitrilotriacetic acid) chromatography following manufacturer’s recommendation (Qiagen, Valencia, CA, USA). Briefly, 600 µl supernatant was loaded onto a Ni–NTA mini column (Qiagen), washed once with buffer B and buffer C (20 mM Tris, pH 8·0, 20 mM imidazole, 500 mM NaCl, 8 M urea) and eluted with buffer D (20 mM Tris, pH 8·0, 250 mM imidazole, 500 mM NaCl, 8 M urea) under denaturing conditions. Bacterial lysate and samples from different steps of the purification were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue to assess protein expression and purity.
Antibody reactivity to recombinant HSP70, cochlin and β-tectorin proteins
To perform the Western blot assay, rhHSP70 (a kind gift from Dr Morrata Anthony, Stressgen, Victoria, BC, Canada) cochlin or β-tectorin were heated at 70°C for 10 min in loading buffer and separated on a 4–12% NuPage™ Bis Tris gel (Invitrogen) using MOPS buffer (Invitrogen). Following electrophoresis, proteins were transferred onto nitrocellulose membrane (Invitrogen) using the Invitrogen Trans-Blot SD semidry transfer apparatus. Membranes were blocked in blocking buffer [5% non-fat dry milk in Tris-buffered saline (TBS) containing 0·05% Tween 20, TBS-T] for 1 h at room temperature to prevent non-specific binding. Following blocking, patient’s serum diluted at 1 : 100 in blocking buffer was added to individual strips and incubated for 1 h. As a positive control, we used the anti-human HSP70 antibody (Extra EKS 750-P2 kit component, Stressgen Bioreagents Corp., Victoria, BC, Canada) diluted at 1 : 10 in blocking buffer. In the case of cochlin and β-tectorin, the mouse anti-Xpress monoclonal antibody (Invitrogen) reacting with a fusion epitope was employed as a positive control. Membranes were washed thrice for 10 min in TBS-T buffer followed by incubation with a 1 : 5000 dilution of horseradish peroxidase conjugated goat anti-human IgG (KPL, Gaithersburg, MD, USA) or 1 : 10 000 dilution of horseradish peroxidase conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h. After three washes of 10 min in TBS-T buffer, the substrate 3,3′,5,5′-tetramethylbenzidine (TMB) was added for colour development for 10–15 min and antibody reactivity assessed.
Results
Protein expression and purification of recombinant fusion proteins
In order to assess human antibody response to the candidate inner ear-specific proteins in AIED, we subcloned both human COCH and mouse TECTB cDNAs in the plasmid pEXP1/DEST for recombinant protein expression in E. coli (see Materials and methods). The mouse β-tectorin protein exhibits 94% identity to the human counterpart (mouse and human TECTB, Accession nos CAA68139 and AAI13500, respectively) and therefore has comparable antigenic determinants.
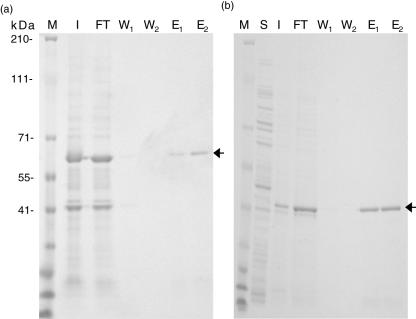
Following transformation and IPTG induction, we first attempted to purify induced proteins under native conditions; however, proteins were found to accumulate in bacterial inclusion bodies (data not shown). We therefore solubilized proteins under denaturing conditions using G-HCL treatment and evaluated expression in soluble (S) and insoluble (I) fractions of either protein by SDS-PAGE analysis (Fig. 1a,b). A major band is visible at ∼60 kDa (Fig. 1a) and ∼39 kDa (Fig. 1b) in the insoluble (I) fraction and flow through (FT) from the Ni–NTA column. Both bands correspond to the expected molecular size of cochlin and β-tectorin, respectively, fused to the 27-amino acid histidine tag of the pEXP1/DEST vector. The insoluble fraction containing the recombinant protein was cleared following G-HCL treatment and the resulting supernatant purified on a Ni–NTA column. The single distinct band in lanes labelled E1 and E2 (Fig. 1a,b) are eluates 1 and 2 of cochlin and β-tectorin, respectively, obtained following purification on the Ni–NTA column. We also confirmed the expression of purified recombinant proteins in eluates by Western blot analysis using the mouse anti-Xpress monoclonal antibody specific for the fusion tag and an unrelated mouse antibody (Fig. 2: lanes 1 and 2 for cochlin and β-tectorin, respectively).
Fig. 1.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of cochlin and β-tectorin expression in BL21(DE3)pLysS and following nickel–nitrilotriacetic acid (Ni–NTA) purification. Bacterial lysate and Ni–NTA purified products of cochlin (a) and β-tectorin (b) were electropheresed in precast 4–16% NuPage gels and stained with Coomassie blue. Arrows indicate bands corresponding to the respective antigens. M = marker; I = lysate of insoluble fraction; S = soluble faction which does not contain protein; FT = flow through; W = wash, E = eluate.
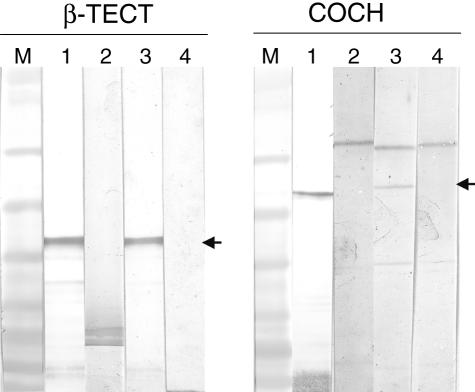
Fig. 2.
Representative Western blot analysis of purified inner ear-specific proteins with controls and patients’ sera. Cochlin and β-tectorin proteins were separated on a 4–12% NuPage™ Bis Tris gel (Invitrogen) and transferred onto nitrocellulose membranes. Membranes were subsequently probed with anti-Xpress monoclonal antibody, patients’ and control sera. Arrows indicate bands corresponding to the respective antigens. M = marker; 1 = positive control [anti-Xpress monoclonal antibody (MoAb) for detection of expression of cochlin and β-tectorin]; 2 = negative control (unrelated antibody); 3 = positive patient; 4 = healthy control.
Detection and scoring of HSP70 IgG antibody in patients and healthy controls
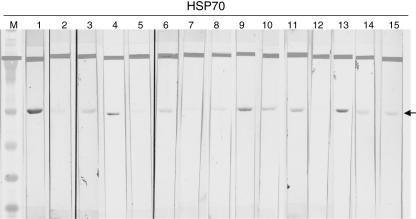
Using a qualitative Western blot assay, we assessed serum samples from all patients and healthy controls for the presence of anti-HSP70 IgG antibody response. We established the identity of the HSP70 band by using a strongly reactive HSP70 IgG control strip as a reference (Fig. 3: lane 1). A negative control (Fig. 3: lane 2) was also included for each assay. The reactivity of each test sample was visually evaluated by comparison with a reference band on a weakly reactive HSP70 IgG control (Fig. 3: lane 3). Thus, a serum sample was scored positive when a band was present with intensity equal to or stronger than that of the weakly positive control and negative by the absence of any specific band or one of a lesser intensity than our cut-off control. Using this method of scoring, the degree of antibody reactivity varied within both positive (lanes 4, 9–11 and 13) and negative (lanes 5–8, 12, 14 and 15) samples as indicated in Fig. 3. At least two investigators scored all samples blindly. Our scoring method as well as the design of the HSP70 Western blot assay ensured that the presence or absence of HSP70 antibody reactivity was consistent within and between assays. We believe these rigorous conditions may account for the low HSP70 IgG-positive rate observed in our study.
Fig. 3.
Anti-heat shock protein 70 (HSP70) IgG profile in idiopathic sensorineural hearing loss (SNHL) patients and healthy volunteers. Human recombinant HSP70 antigen was separated on a 4–12% NuPage™ Bis Tris gel (Invitrogen) and transferred onto nitrocellulose membranes. Membranes were subsequently probed with positive human anti-HSP70 antibody, weakly reactive and negative control sera as well as patients’ and volunteers’ sera. Arrow indicate band corresponding to the 70 kilodalton protein. M = marker; 1 = positive control; 2 = negative control; 3 = weakly positive control; 4, 9–11, 13 = positive patients; 5–8, 12, 14–15 = healthy controls.
Prevalence of cochlin and β-tectorin antibodies in patients with idiopathic SNHL
To determine the prevalence of IgG antibodies directed against cochlin or β-tectorin in our patient cohort, we performed Western blot analysis using the respective recombinant proteins. As controls, we used sera from healthy blood donors employing the same experimental conditions. Fifty-eight sera from patients with idiopathic SNHL and 28 from healthy controls were tested by Western blot analysis using recombinant cochlin or β-tectorin. Figure 2, lanes 3 and 4 shows representative antibody reactivity with positive patient samples and healthy controls, respectively. We found no antibody reactivity between serum samples from healthy controls with either recombinant cochlin or β-tectorin. However, 2 of the 28 healthy donor samples were found to react with HSP70. This finding is consistent with a previous study, which demonstrated that some apparently healthy individuals possess antibodies directed against HSP70 [18]. Of the 58 patients in the study cohort, 19 tested positive to the HSP70 antigen, eight to cochlin and one to β-tectorin, giving a prevalence of 33, 14 and 2%, respectively (Table 1). Overall, only one HSP70 IgG-positive sample was reactive with all inner ear-specific proteins and none was double-positive for any of the antigenic specificities tested. No patient sample reacted exclusively with β-tectorin and seven of the eight cochlin antibody-positive samples were monospecific.
Table 1.
Distribution of IgG antibody responses to recombinant HSP70, cochlin and β-tectorin proteins in patients and healthy controls.
| Subjects | HSP70 (%) | Cochlin (%) | β-tectorin (%) |
|---|---|---|---|
| All patients | 19/58 (33) | 8/58 (14) | 1/58 (2) |
| Controls | 2/28 (7) | 0/28 (0) | 0/28 (0) |
HSP: heat shock protein.
Discussion
This study addresses the hypothesis that antibody response directed against specific inner ear proteins is responsible for the induction of autoimmune hearing loss and therefore may serve as markers for disease diagnosis. Using antigen-specific Western blot analysis of sera from patients and healthy controls, we demonstrate that cochlin rather than β-tectorin-specific IgG antibodies are more prevalent in patients with idiopathic SNHL, 14% and 2%, respectively. In the same study cohort, we found HSP70 IgG responses to be significantly higher in prevalence than either cochlin or β-tectorin-specific antibodies. To our knowledge, this study is the first to compare the prevalence of IgG antibodies to recombinant proteins that are highly expressed in the inner ear in association with HSP70 antibody responses. Since McCabe first proposed the concept of autoimmunity in some cases of idiopathic SNHL [1], there has been interest and speculation about the antigenic specificity that may be responsible for disease pathogenesis. Antibody recognition of inner tissues by immunofluorescence staining using serum from patients with SNHL [7–9] as well as the induction of hearing loss via adoptive transfer of inner ear-specific T cells in experimental studies [21] strengthens the notion that autoimmunity may indeed be involved in some hearing disorders of adult onset of unknown origin.
Harris and Sharp [10] first reported that a significant proportion of sera from patients affected by idiopathic SNHL showed antibody recognition of a 68-kDa protein in bovine inner ear extract which correlated with disease activity. Subsequent studies by other investigators reported that patients who were antibody-positive for the 68 kDa antigen responded to steroid treatment more frequently than non-responders [3,4,11]. The identity of this antigen was shown by Billing et al. [11] to be HSP70, which is a non-specific response associated with several inflammatory responses. By direct sequencing analysis of protein bands recognized by sera from patients with idiopathic SNHL, Boulassel et al. [16] identified cochlin as a target antigen.
The cochlin protein is of considerable significance as the COCH gene is expressed preferentially in the inner ear and constitutes 70% of the inner ear proteins [23]. In the mutated form, COCH results in non-syndromic autosomal dominant deafness (DFNA9) [24]. Thus, cochlin appears to play an important function in hearing and due to its relative abundance may be an ideal target of the host’s immune system by virtue of constitutive expression by the resident antigen-presenting cells (APCs) in the inner ear. In addition to cochlin in the inner ear, β-tectorin together with alpha-tectorin, consist of the major non-collagenous proteins of the tectorial membrane of the cochlea and their expression is associated with hair cells in the organ of Corti [20,25]. A pathogenic role of cochlin- and β-tectorin-specific T cells was shown by experimental induction of hearing loss following adoptive transfer of these specific T cell lines [21].
The differential prevalence of HSP70, cochlin and β-tectorin antibodies observed in our study cohort may reflect either one or more of the following: (i) variability in disease onset in the patients; (ii) a differential role of these molecules in the induction and/or maintenance of pathogenesis; and (iii) the type of hearing disorder − that is, Menière’s disease or AIED. HSP70 antibodies have been reported in many inflammatory diseases [18] and may mark the early onset of hearing loss. Indeed, several studies have shown that HSP70 antibodies are less prevalent in individuals with inactive or stable disease [4,10,11]. Therefore, the presence of cochlin antibodies in HSP70-negative subjects may represent a subset of patients with chronic AIED for whom immunosuppressive therapy may be beneficial. With respect to β-tectorin, to which only one patient had a detectable antibody response, there is an alternative possibility that cell-mediated rather than humoral immunity may be implicated in the induction of this response, as reported in a single study of experimental hearing loss [21]. On the other hand, the kinetics of the β-tectorin-specific antibody response may be different from that of cochlin and may appear later in disease. The only patient for whom anti-β-tectorin IgG antibody was detected was a 79-year-old woman. Interestingly, she also tested positive for both cochlin and HSP70 antibodies.
Inner ear-specific autoantibodies as well as HSP70 antibodies have been reported in patients with Menière’s disease, sudden deafness and other forms of SNHL. Because the clinical presentation of AIED can be variable [11,26,27], it is unknown if AIED is a completely different clinical entity from the above-mentioned hearing disorders. Several investigators have suggested that Menière’s disease may be an earlier manifestation or a continuum of AIED [11,26,27]. Although we have not addressed directly the difference in prevalence of antibodies in the scenario of clinical presentation, it is worth speculating that the extent of inner ear antigen-specific responses in patients with Menière’s disease may be different from patients with AIED. As there is no consensus for diagnosing these hearing disorders, interpretations of results based on the patient selection criteria used as well as the experimental procedures employed are difficult to assess. However, based on experimental evidence, there appears to be some concurrence in the literature that there is an ongoing immune response directed against proteins expressed in the inner ear [7–11,21,26,27]. What remains to be defined are the early events leading to the recognition of these molecules in these patients, the mechanism(s) of immune-mediated tissue destruction and the nature of antigenic spread, if any, in the different clinical manifestations. Furthermore, it is important to note here that other inner ear antigens such as the myelin protein, Po [15] and choline transporter-like protein 2 [17], as well as other unidentified proteins not investigated in this study, may well be involved in disease pathogenesis.
We are currently examining the relative prevalence of cochlin- and β-tectorin-specific antibody and T cell responses in patients with early, chronic AIED as well as in subjects with Menière’s disease to define more clearly the role of these antigens in disease pathogenesis. There is a possibility that an immune response directed against cochlin and/or β-tectorin may be either antibody- or cell-mediated or both. Dissecting the relative contribution of each arm of the antigen-specific immune response to these and other inner ear antigens may have implications for patient care and management in the future.
Acknowledgments
This work was supported by the ARUP Institute for Clinical and Experimental Pathology. We would like to thank Dr C. C. Morton (Harvard University, Boston, MA, USA) and Dr G. P. Richardson (University of Sussex, UK) for the COCH and TECTB plasmids, respectively. We are also grateful to Dr Anthony Marotta and the technical staff of Stressgen Bioreagents Corp, Victoria BC, Canada for support with reagents.
References
- 1.McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88:585–9. doi: 10.1177/000348947908800501. [DOI] [PubMed] [Google Scholar]
- 2.McCabe BF, McCormick KJ. Tests for autoimmune disease in otology. Am J Otol. 1984;5:447–9. [PubMed] [Google Scholar]
- 3.Hirose K, Weener MH, Ducker LG. Utility of laboratory testing in autoimmune inner ear disease. Laryngoscope. 1999;109:1749–54. doi: 10.1097/00005537-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Moscicki RA, San Martin JE, Quintero CH, Rauch SD, Nadol JB, Jr, Bloch KJ. Serum antibody to inner ear proteins in patients with progressive hearing loss. Correlation with disease activity and response to corticosteroid treatment. JAMA. 1994;272:611–16. [PubMed] [Google Scholar]
- 5.Harris JP, Weisman MH, Derebery JM, et al. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA. 2003;290:1875–83. doi: 10.1001/jama.290.14.1875. [DOI] [PubMed] [Google Scholar]
- 6.Loveman DM, de Comarmond C, Cepero R, Baldwin DM. Autoimmune sensorineural hearing loss: clinical course and treatment outcome. Semin Arthritis Rheum. 2004;34:538–43. doi: 10.1016/j.semarthrit.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Anrnold W, Pfaltz R, Altermatt HJ. Evidence of serum antibodies against inner ear tissues in the blood of patients with certain sensorineural hearing disorders. Acta Otolaryngol. 1985;99:437–44. doi: 10.3109/00016488509108935. [DOI] [PubMed] [Google Scholar]
- 8.Anrnold W, Pfaltz R. Critical evaluation of the immunofluorescence microscopic test for identification of serum antibodies against human inner ear tissue. Acta Otolaryngol. 1987;103:373–8. [PubMed] [Google Scholar]
- 9.Salomon P, Charachon R, Lejeune JM. Indirect immunofluorescence in the investigation of rapidly progressive sensorineural hearing loss and Menière’s disease. Acta Otolaryngol. 1993;113:318–20. doi: 10.3109/00016489309135816. [DOI] [PubMed] [Google Scholar]
- 10.Harris JP, Sharp PA. Inner ear autoantibodies in patients with rapidly progressive sensorineural hearing loss. Laryngoscope. 1990;100:516–24. doi: 10.1288/00005537-199005000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Billings PB, Keithley EM, Harris JP. Evidence linking the 68-kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann Otol Rhinol Laryngol. 1995;104:181–8. doi: 10.1177/000348949510400302. [DOI] [PubMed] [Google Scholar]
- 12.Boulassel MR, Tomasi JP, Deggouj N, Gersdorff M. COCH5B2 is a target antigen of anti-inner ear antibodies in autoimmune inner ear diseases. Otol Neurotol. 2001;22:614–18. doi: 10.1097/00129492-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka K, Yamanobe S, Tomiyama S, Yagi T. Inner ear autoantibodies in patients with sensorineural hearing loss. Acta Otolaryngol Suppl. 1995;519:176–7. doi: 10.3109/00016489509121897. [DOI] [PubMed] [Google Scholar]
- 14.Cao MY, Deggouj N, Gersdorff M, Tomasi JP. Guinea pig inner ear antigens: extraction and application to the study of human autoimmune inner ear disease. Laryngoscope. 1996;106:207–12. doi: 10.1097/00005537-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Cao MY, Dupriez VJ, Rider MH, et al. Myelin protein Po as a potential autoantigen in autoimmune inner ear disease. FASEB J. 1996;10:1635–40. doi: 10.1096/fasebj.10.14.9002556. [DOI] [PubMed] [Google Scholar]
- 16.Boulassel MR, Deggouj N, Tomasi JP, Gersdorff M. Inner ear autoantibodies and their targets in patients with autoimmune inner ear diseases. Acta Otolaryngol. 2001;121:28–34. doi: 10.1080/000164801300006236. [DOI] [PubMed] [Google Scholar]
- 17.Nair TS, Kozma KE, Hoefling NL, et al. Identification and characterization of choline transporter-like protein 2, an inner ear glycoprotein of 68 and 72 KDa that is the target of antibody-induced hearing loss. Neuroscience. 2004;24:1772–9. doi: 10.1523/JNEUROSCI.5063-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zügel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious disease. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia Berrocal JR, Ramirez-Camacho R, Arellano B, Vargas JA. Validity of the Western blot immunoassay for heat shock protein-70 in associated and isolated immunorelated inner ear disease. Laryngoscope. 2002;112:304–9. doi: 10.1097/00005537-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm–egg adhesion system. J Biol Chem. 1997;272:8791–801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- 21.Solares CA, Edling AE, Johnson JM, et al. Murine autoimmune hearing loss mediated by CD4+ T cells specific for inner ear peptides. J Clin Invest. 2004;113:1210–7. doi: 10.1172/JCI18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding YS, Owen SM, Lal BB, Ikeda RA. Efficient expression and rapid purification of human T-cell leukemia virus type 1 protease. J Virol. 1998;72:3383–6. doi: 10.1128/jvi.72.4.3383-3386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikezono T, Omori A, Ichinose S, Pawankar R, Watanabe A, Yagi T. Identification of the protein product of the COCH gene (hereditary deafness gene) as the major component of bovine inner ear protein. Biochim Biophys Acta. 2001;1535:258–65. doi: 10.1016/s0925-4439(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 24.Robertson NG, Lu L, Heller S, et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat Genet. 1998;20:299–303. doi: 10.1038/3118. [DOI] [PubMed] [Google Scholar]
- 25.Heller S, Sheane CA, Javed Z, Hudspeth AJ. Molecular markers for cell types of the inner ear and candidate genes for hearing disorders. Proc Natl Acad Sci USA. 1998;95:11400–5. doi: 10.1073/pnas.95.19.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes GB, Kinney SE, Barna BP, Calabrese LH. Autoimmune reactivity in Menière’s disease: a preliminary report. Laryngoscope. 1983;93:410–7. doi: 10.1002/lary.1983.93.4.410. [DOI] [PubMed] [Google Scholar]
- 27.Riente L, Bongiorni F, Nacci A, et al. Antibodies to inner ear antigens in Menière’s disease. Clin Exp Immunol. 2004;135:159–63. doi: 10.1111/j.1365-2249.2004.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]