Abstract
Interleukin-15 (IL-15) is a cytokine that induces proliferation and promotes cell survival of human T, B and NK cells. IL-15 and interleukin-2 (IL-2) exhibit a similar spectrum of immune effects and share the IL-2 receptor (IL-2R) subunits IL-2Rβ and IL-2Rγc for signalling in haematopoietic cells. Furthermore, each cytokine has a private α receptor, namely IL-2Rα for IL-2 and IL-15Rα for IL-15, that functions in ligand binding. Using reverse transcriptase-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA) methods, the expression and secretion of IL-15 and IL-15Rα in tumour-derived B-cell lines were studied. The results as presented in this study identify that IL-15 mRNA is predominantly expressed in EBV positive (EBV+) B-cell lines, although IL-15Rα is ubiquitously and constitutively expressed in all these B-cell lines. Although no detectable levels of IL-15 protein secretion were observed in any of these cell lines, we were able to detect membrane-bound expression of IL-15 protein by FACS analysis in some cell lines. These data imply that the IL-15/IL-15R system requires complex regulatory mechanisms for protein secretion. Taken together, we speculate that these results suggest a juxtacrine, intracrine function for IL-15/IL-15R.
Keywords: B-cell lines, ELISA, interleukin-15, juxtacrine, RT-PCR
Introduction
Interleukin-15 (IL-15) is a pleiotropic and redundant cytokine that is recognized as a major regulator of haematopoiesis, immune responses, cell survival and proliferation in many different cell types and tissues [1–5]. IL-15 exhibits high conservation among mammalian species and is coded by a > 34 kb gene that is located on human chromosome 4q31 [6,7]. The genomic IL-15 gene consists of nine exons, including an additional 119 nucleotides, named exon 4a, and eight introns [6,7]. There are two IL-15 precursor proteins bearing two distinct signal peptides, a 48-aa long signal peptide (LSP) and a 21-aa short signal peptide (SSP) [8–10]. These distinct isoforms exhibit differential patterns of intracellular distribution, trafficking, secretion and endosomal localization. Additionally, this also implies that the signal peptide is an important factor in IL-15 production, secretion, transport and signalling. While the IL-15-SSP may primarily exhibit intracrine function, only IL-15-LSP can be secreted or observed in the cell membrane [6,7,11]. The intracrine effect of IL-15-SSP refers to its function of initiating generation of IL-15-SSP without ever leaving the cell. Transcripts of IL-15 mRNA are widely observed. Despite the apparently ubiquitous expression pattern of IL-15 mRNA, detectable levels of IL-15 protein secretion in cell culture supernatants has not been observed except in pathological or chronic inflammatory conditions [12–15]. This discrepancy is explained in a recent review by a surprisingly complex system of controls that regulate IL-15 transcription, translation, post-translational processing, intracellular trafficking and translocation [16]. Interestingly, reports from several studies confirm the presence of membrane-bound IL-15 on several cell types [12–19], suggesting juxtacrine signalling function. Juxtacrine signalling is defined when cells can signal by direct contact between a membrane-associated form of the ligand and a cognate receptor of an immediately adjacent cell.
Even though there is no sequence homology between IL-2 and IL-15, they share specific cell surface receptors for signal transduction. IL-2 receptor (IL-2R) and Interleukin-15 receptor (IL-15R) are heterotrimers consisting of three different receptor subunits, alpha (α), beta (β) and gamma (γ). IL-2 and IL-15 have their own private receptor subunit, IL-2Rα and IL-15Rα, respectively. However, IL-2Rβ and the common γ (γc) chain are similar in both IL-2 and IL-15 [20]. The existence of eight human IL-15Rα isoforms has been identified [21,22].
IL-15 has been suggested as a potential immunomodulator in HIV infection, due to its role in the generation of CD8+ T and NK cells [23–25]. Considering the role of HIV and IL-15 interaction and similarities seen between IL-2 and IL-15, we propose to examine the mRNA expression of IL-15 and IL-15Rα, and IL-15 protein secretion and IL-15 membrane-bound forms in tumour-derived B-cell lines. We also investigated the role of EBV infection in modulating IL-15 and IL-15Rα expression on B-cell lines. We report here that IL-15 is predominantly expressed in EBV+ human B-cells, and IL-15Rα is constitutively and ubiquitously expressed in all the human B-cell lines studied. Membrane-bound expression of IL-15 protein was observed in some of these cell lines. However, consistent with myriad reports we were unable to detect any secretable IL-15 protein. We therefore speculate that IL-15 may exist in translationally inactive pools, as initially suggested by Bamford and co-authors [26]. Restriction fragment length polymorphism (RFLP) genotyping of an IL-15 SNP site (−10504 A/G) demonstrated homozygous A/A alleles across all cell lines. On this background, we propose that the IL-15/IL-15R system acts as a juxtacrine/intracrine cytokine with the ability to reverse signal in tumour-derived human B-cell lines.
Materials and methods
Cell lines and culturing conditions
The 14 tumour B-cell lines included in this study were derived from patients with undifferentiated lymphomas of Burkitt’s and non-Burkitt’s origin as previously described [27–30]. Table 1 lists the origin and characteristics of the B-cell lines used in this study. The tumour B-cell lines were classified as AABCL or non-AABCL based on their EBV status. The 5 AABCL include IOC-9, 2F7, HBL-1, HBL-2 and HBL-3. IOC-9 was established from an AIDS patient with NHL. 2F7 was established from an AIDS patient with BL. They were purchased from ATCC, Rockville, MD, USA. HBL-1, HBL-2 and HBL-3 were established from patients with AIDS-associated small non-cleaved B-cell lymphomas. They were a kind gift from Dr Riccardo Dalla-Favera, Columbia University, NY, USA [31]. The HBL-1 cell line is EBV+, while the HBL-2 and HBL-3 cell lines are EBV-negative (EBV–). No HIV-1 viral sequences were detected in any of these cell lines [31]. The non-AABCL were comprised of nine cell lines of American or African Burkitt’s origin and varying EBV status. The five EBV– American BL cell lines included BJAB, EW36, CA46, ST486 and MC116. B958 was BJAB superinfected with EBV and was a kind gift from Dr Michael Steinitz, Hebrew University, Hadassah Medical School, Jerusalem, Israel. The three EBV+ African BL cell lines included Raji, Daudi and Namalva. All these cell lines were purchased from ATCC. Apart from the cell lines, CD19+ cells were isolated by incubating PBMC for 30 min at 4 °C with Dynabeads anti-CD19 mAb-coated magnetic beads (Dynal Biotech, Oslo, Norway), as previously described [30].
Table 1.
Characteristics of B-cell lines.
| Cell line | EBV status | Continent | Histology | Tissue source | ||
|---|---|---|---|---|---|---|
| American Burkitt’s Lymphoma | AABCL | IOC-9 | Positive | N. America | Burkitt’s | Peripheral blood |
| 2F7 | Positive | N. America | Burkitt’s | Peripheral blood | ||
| HBL-1 | Positive | N. America | UL | Peripheral blood | ||
| HBL-2 | Negative | N. America | UL | Pleural effusion | ||
| HBL-3 | Negative | N. America | UL | Liver | ||
| Non-AABCL | EW36 | Negative | N. America | UL | Pleural effusion | |
| CA46 | Negative | N. America | Burkitt’s | Ascitic fluid | ||
| ST486 | Negative | N. America | UL | Ascitic fluid | ||
| MC116 | Negative | N. America | UL | Pleural effusion | ||
| BJAB | Negative | N. America | Burkitt’s-like | Lymphoblastoid | ||
| B958 | Positive | N. America | Burkitt’s-like | Lymphoblastoid | ||
| African Burkitt’s lymphoma | Non-AABCL | RAJI | Positive | Africa | Burkitt’s | Jaw tumour |
| DAUDI | Positive | Africa | Burkitt’s | Orbital tumour | ||
| NAMALVA | Positive | Africa | Burkitt’s | Lymphoblastoid |
AABCL, acquired immune deficiency syndrome (AIDS)-associated B-cell line; EBV, Epstein-Barr virus; UL, undifferentiated lymphoma.
The cell lines were cultured in PRMI-1640 media with 10 mM N-2-Hydroxyethylpiperazine-N′-2-Ethanesulphonic acid (HEPES) buffer, 10% heat-inactivated foetal bovine serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Cellgro®, Mediatech Inc., Herndon, VA, USA). The cell cultures were grown in a humidified incubator at 37 °C with 5% CO2, and subcultured every 3–5 days as needed.
Exposure of cells to phorbol 12-myristate 13-acetate
Cells obtained on the fourth day after subculture were resuspended in fresh medium at a cell density of 106 cells/ml. Each cell line was divided into two T-25 flasks; one remained as a control, while the second was stimulated with the tumour promoter phorbol 12-myristate 13-acetate (PMA) (Sigma Chemical Co., St Louis, MO, USA). A 5–100 ng/ml range of PMA stimulation was studied and optimal results were achieved at a 10 ng/ml concentration, following 24 h of incubation. Total mRNA was isolated using guanidium isothiocynate-phenol chloroform, and Poly A+ RNA was also isolated by using Invitrogen Kits (Invitrogen, Carlsbad, CA, USA), as previously described [24–26]. Supernatants were collected and stored at −152 °C for protein secretion assay.
Detection of IL-15 and IL-15Rα mRNA by reverse transcriptase-polymerase chain reaction (RT-PCR)
A 1 µg equivalent of total RNA or 0·1 µg of Poly A+ RNA of each cell type (control and PMA) was transcribed into cDNA by reverse transcription (RT) using a GenAmp® PCR system 2700 PCR Thermocycler (Applied Biosystems, Foster City, CA, USA), as described previously [27–30]. Briefly 5 µl of cDNA was amplified with 2·5 U Ex-Taq HS Polymerase (Takara, Kyoto, Japan). The reaction mixture consisted of 2 µl of 10X buffer, 1 µl of 25 mM MgCl2, 0·5 µl of 10 pmol 5′-3′ primer, and 0·5 µl of 10 pmol 3′-5′ primer. The mixture was amplified over 37 cycles. The first cycle for IL-15 was composed of two cycles of 3 min at 95 °C (denaturing), 1 min at 52 °C (annealing), and 1 min at 72 °C (primer extension), followed by 35 cycles of 40-s incubations at 94 °C, 52 °C, and 72 °C. A 3-min extension cycle at 72 °C and infinite 4 °C storage hold cycle followed. The IL-15Rα PCR cycle was composed of two cycles of 3 min at 97 °C, 1 min at 63·5 °C, and 1 min at 72 °C, followed by 35 cycles of 1-min incubations at 95 °C, 63·5 °C and 72 °C. A 3-min extension cycle at 72 °C and infinite 4 °C storage hold cycle followed. Primers specific for the IL-15 and IL-15Rα sequences were designed using MacVector™ 6.5.3 software (Accelrys Inc., San Diego, CA, USA). Primers were tested to ensure they did not dimerize, form hairpin loops, or bind to multiple sites on either cDNA strand.
The IL-15 primers employed were bases 977–994 (sense), 5′-TAA AAC AGA AGC CAA CTG-3′, and bases 1314–1333 (antisense), 5′-CAA GAA GTG TTG ATG AAC AT-3′. The PCR results in a 357 bp product. The IL-15Rα sense and antisense primers are bases 218–238 (sense), 5′-GTC AAG AGC TAC AGC TTG TAC-3′, and bases 977–995 (antisense), 5′-GGT GAG CTT GCT CCT GGA C-3′. The PCR results in two products, a 680 bp and a 778 bp, due to a natural alternative splicing pattern. Primers were custom made by Epoch Biosciences (San Diego, CA, USA). In order to ensure the success of the RT reaction and semi-quantify the amplification of the PCR product, a 358 bp region of the gene for glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was amplified along with each sample. The GAPDH primer sequences used were 5′-CTA CTG GCG CTG CCA AGG CTG-3′ (sense), and 5′-GCC ATG CGG TCC ACC ACC CTG T-3′ (antisense). Thermocycling conditions for GAPDH included two cycles of denaturation at 97 °C, annealing at 60 °C, and primer extension at 72 °C for 1 min each, followed by 17 cycles of 8 s at 94 °C, 2 s at 60 °C, and 5 s at 72 °C. After the 19 cycles a 3-min extension was performed to ensure complete target sequence extension, followed by an infinite hold cycle at 4 °C. Semi-quantification was performed as previously described using the Collage 3·0 for Macintosh intensity scanning function [27–30,32].
Cloning and sequencing of RT-PCR products
The 357 bp amplicon of IL-15 and the 778 bp amplicon of IL-15Rα from three representative cell lines, HBL-3, B958 and Raji, were subcloned into the dephosphorylated HincII site of the pBluescript II plasmid vector (Stratagene, La Jolla, CA, USA). Prior to subcloning, the T4 DNA Polymerase (Promega) was modified to remove the 3′ non-template-directed base addition, as previously described [27–30]. The blunt end amplicons were treated with T4 polynucleotide kinase, and ligated into the vector using T4 DNA ligase (Promega) at 12 °C overnight. Following transformation of competent bacteria, white colonies growing on Luria-Bertani (LB) agar containing 100 µg ampicillin/ml, 40 µg 5-bromo-4-chloro-3-indoyl-β-D-galactoside/ml were selected and grown overnight in 5 ml of LB broth containing 100 µg ampicillin/ml. Plasmid mini preparations were performed by alkali lysis of 1·5 ml of the overnight culture. Determination of plasmids containing the appropriate size inserts was performed by restriction digest with PvuII (Promega), followed by electrophoresis through 1·5% Tris acetate/EDTA agarose gels. Appropriate plasmids were sequenced using the Applied Biosystems Sequencer model 373 A (Applied Biosystems).
IL-15 enzyme-linked immunosorbent assay (ELISA)
Supernatants obtained from control and PMA-activated cells (106 cells/ml) were collected and concentrated 10-fold with 3000 membrane molecular weight cut-off filters (YM-3 Centrifugal Filter Devices, Millipore, Billerica, MA, USA) following the manufacturer’s guidelines. Cells were washed three times with PBS-Ca/Mg free solution. After the last wash, the cells were spun and suspended in 500 µl of 250 mM Tris-HCL (pH 8·0). Using liquid nitrogen, cells were freeze-thawed three times; 100 µl of the concentrated supernatant/cell extracts were assayed independently after normalizing for equal amounts of protein, using Quantikine® Human IL-15 immunoassay (R & D Systems, Minneapolis, MN, USA) following the manufacturer’s guidelines. This kit recognizes both natural and recombinant human IL-15 with no significant cross-reactivity of interference with other cytokines, as suggested by the manufacturer’s guidelines. The minimum detectable dose of IL-15 is typically 3·9 pg/ml. Results were measured by MRX II Microplate Reader (Dynatech Laboratories, Chantilly, VA, USA).
Detection of membrane-bound IL-15 by flow cytometry
Indirect immunofluorescence assay was performed on these cell lines; 2·5 × 105 cells were washed twice in staining buffer (PBS, 2% FCS, 0·1% NaN3). Each of the cell lines was stained and analysed for control, without primary and secondary Ab, with secondary Ab alone, and with both primary and secondary Ab, following the manufacture’s guidelines. The monoclonal mouse anti-human IL-15 antibody mAb (MAB2471) was incubated for 30 min at 4 °C in the dark. After staining, cells were washed with staining buffer and incubated for 30 min at 4 °C in the dark with goat FITC-labelled anti-mouse mAb as the secondary antibody. Cells were washed for a final three times with staining buffer prior to FACS analysis, as suggested by the Manufacturer (R & D systems). Labelled cells were analysed by FACS Vantage SE with FACSDiVa software (Becton Dickinson, San Jose, CA).
IL-15 genotyping
DNA was isolated from all cell lines using the Promega® Wizard SV genomic DNA purification system (Promega) as previously described [33]. A 5 µl sample of DNA from each cell line was amplified with 2·5 U Ex-Taq HS polymerase using a GenAmp PCR system 2700 themocycler. The reaction mixture consisted of 5 µl of 10X buffer, 4 µl of 25 mM MgCl2, 4 µl of dNTP mixture (2·5 mM each), 1 µl of 10 pmol 5′-3′ primer, and 1 µl of 10 pmol 3′-5′ primer. The primers employed were 5′-ATG TGC TCG GTG AGA AAA A-3′ (sense) and 5′-CAA AAA GTC AAT CCA AAT ATT GTA-3′ (antisense) [34]. The mixture was amplified over 32 cycles. The first cycle was composed of two cycles of 1 min at 97 °C (denaturing), 1 min at 60 °C (annealing), and 1 min at 72 °C (primer extension), followed by 30 cycles of 1-min incubations at 94 °C, 60 °C, and 72 °C. A 7-min extension cycle at 72 °C and infinite 4 °C storage hold cycle followed. The PCR product was digested with restriction enzyme RsaI (Promega) for 3 h at 37 °C and resolved on a 20% (29:1) polyacrylamide gel electrophoresis. The bands were visualized using VersaDoc™ Model 4000 Imaging System (Bio-Rad, Hercules, CA, USA).
Results
The HBL-1 cell line was used as a representative B-cell line to study the kinetics of IL-15 and IL-15Rα mRNA transcripts from 1 to 96 h in culture. HBL-1 cells obtained on day 4 after subculture were resuspended at a concentration of 106 cells/ml in fresh medium containing 10% FCS and incubated at 37 °C with and without PMA for 1, 3, 5, 8, 24, 48, 72 and 96 h. At each time-point, the culture was harvested for RNA isolation and the supernatants were collected for ELISA. Constitutive IL-15 and IL-15Rα transcripts were expressed from 1 h with a peak accumulation at 24 h. Furthermore, mitogenic stimulation did not significantly change mRNA expression in any of the transcripts compared with untreated control cells, at each time-point. To ensure that the differences seen in mRNA expression at these time-points were not caused by variations in PCR efficiency or relative amounts of amplicons products, but indeed reflected changes in IL-15 and IL-15Rα kinetics, these expressions were normalized to that of GAPDH and repeated several times. Having identified the optimal time-point as 24 h in the representative HBL-1 cell line (data not shown), we performed RT-PCR analysis several times on constitutive and PMA-induced expression of IL-15 and IL-15Rα in all the other B-cell lines.
Expression of IL-15 in human B-cell lines
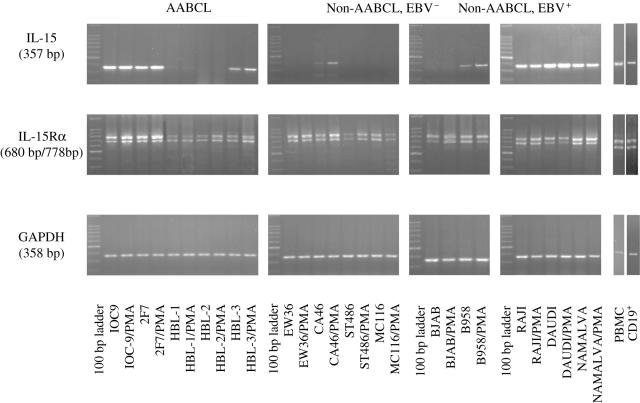
Expression of IL-15 transcript in AABCL was observed in IOC-9, 2F7, HBL-1 and HBL-3, with the exception of HBL-2, an EBV– cell line. Stimulation with PMA did not result in any significant modulations after normalizing to the amplicons of the housekeeping gene, GAPDH (Fig. 1). With the exception of CA46, no other EBV– non-AABCL expressed IL-15 mRNA transcript. PMA stimulation of CA46 resulted in a 2·3-fold increase in the transcript. Interestingly, the BJAB cell line did not express IL-15 mRNA either constituently or upon PMA stimulation. However, EBV super-infected BJAB cell line, B958, expressed IL-15 mRNA transcript constitutively that was further enhanced by PMA stimulation to 1·3-fold increase. All of the EBV+ non-AABCL (Raji, Daudi and Namalva) constitutively expressed IL-15 transcript. PMA stimulation resulted in a 1·3-fold increase in Daudi with no significant changes in Raji and Namalva (Fig. 1). These results clearly suggest a positive correlation between EBV infection and expression of IL-15 mRNA transcript.
Fig. 1.
Interleukin-15 (IL-15) expression as determined by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. (a) Acquired immune deficiency syndrome (AIDS)-associated B-cell lines (AABCL); (b) non-AABCL, EBV; and (c) non-AABCL, EBV+. The 357-bp amplicon of IL-15 was generated from RNA isolated from control and Phorbol 12-myristate 13-acetate (PMA)-stimulated cells, as well as the 680-bp and 778-bp of IL-15Rα RT-PCR product. The 358-bp amplicon represents glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA transcript.
Expression of IL-15Rα in human B-cell lines
The IL-15Rα was constitutively and ubiquitously expressed in all the cell lines included in our study and PMA stimulation did not make any significant modulations in their mRNA transcripts, as shown in Fig. 1. The RT-PCR yielded two products, a 680 bp and a 778 bp, due to a natural alternative splicing pattern, consistent with other reports [21,22].
IL-15 ELISA to detect secreted and cell-bound forms in human B-cell lines
IL-15 protein secretion and intracellular expression was analysed using the Quantikine® human IL-15 immunoassay following the manufacture’s guidelines, as described under ‘Materials and methods’. The result of the ELISA shows that B-cells do not secrete the IL-15 mature protein either constitutively or upon PMA stimulation. The presence of EBV or AIDS association did not induce detectable levels of secreted or intracellular IL-15 protein as assayed by ELISA.
Membrane-bound expression of IL-15 protein by FACS analysis
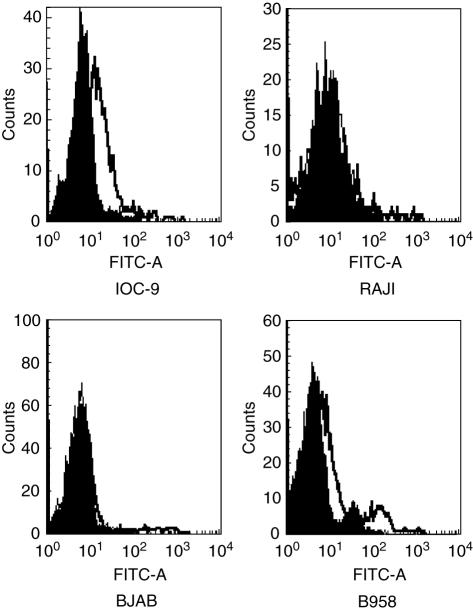
Analysis of these cell lines for membrane-bound IL-15 was done by FACS as described under ‘Materials and methods’. Mean fluorescence intensity is expressed as the mean channel number of staining with anti-human IL-15 mAb (MAB2471) minus that obtained with an isotype-matched control mAb. Low intensity specific binding was observed in the AABCL cell lines, IOC-9 and 2F7, but not in other cell lines. Although we could not detect membrane-bound IL-15 expression in the EBV– BJAB cell line, its EBV-infected counterpart, B958, showed marginal intensity specific binding, as shown in Fig. 2, consistent with the mRNA expression patterns. We could not detect in Raji cells membrane-bound forms of IL-15 protein consistent with other reports in the literature [13]. The most possible explanation as to why Raji cells did not show membrane-bound form compared with IOC-9 and 2F7, could be that these cell lines have been ‘frozen’ in different stages of development where IL-15 protein expression was absent.
Fig. 2.
FACS analysis with mouse anti-human IL-15 MAB2471 monoclonal antibody. Represantive B-cell lines: IOC-9, RAJI, BAJB and B958 were stained with MAB2471 followed by goat anti-mouse FITC-conjugated antibody. Fluorescence intensity is represented by white histograms; black histograms refer to the background staining of isotype-matched control mAB.
IL-15 polymorphisms
In this study, RFLP genotyping of an IL-15 SNP site (−10504 A/G) demonstrated homozygous A/A alleles across all cell lines. This site is located 98 basepairs upstream from exon 5 within the 4th intron. As none of the cell lines secreted functional IL-15 and all expressed the same SNP it is impossible to conclude whether there is a correlation between gene expression and protein secretion.
Discussion
IL-15 is a pleiotropic cytokine effective in promoting survival, proliferation and activation of NK and CD8+ T cells. IL-15 is also important to B-cells as it enhances proliferation and immunoglobulin secretion [35–37], induces proliferation of malignant B-cells [38,39], and inhibits apoptosis of activated B-cells [40]. Although IL-15 mRNA is constitutively expressed in a variety of cell types and tissues, IL-15 protein secretion has been scarcely detected, except in certain pathological conditions [2,12,15]. Herein, we demonstrate that IL-15 mRNA expression is predominantly expressed in all EBV+ B-cell lines. Furthermore, IL-15Rα mRNA is ubiquitously expressed in these B-cell lines. In this study we investigated whether tumour-derived human B-cells either constitutively or upon PMA induction secreted IL-15 protein. The only stimulus tested in this report is using PMA, although a more appropriate trigger could be achieved by BCR or CD40. We did not detect secretable IL-15 protein consistent with the body of literature exploring for cell lines that secrete IL-15 protein. As no secreted IL-15 protein was observed, we set forth to explore for membrane-bound IL-15 protein expression. Our results using FACS analysis confirm that IL-15 protein can be detected as membrane bound in some of the tumour-derived B-cell lines, suggesting a juxtacrine role. IL-15 protein expression as a biologically active and non-soluble membrane-bound form has been found in several cell types [13,17–19,41].
Several studies of IL-15 suggest that regulation of IL-15 protein secretion is much more complex, occurring at the level of transcription, translation and intracellular trafficking. Although IL-15 expression is controlled at the transcriptional level, it appears that the predominant regulation of IL-15 expression is controlled at the post-transcriptional level. This is notable, as significant discrepancies between considerable amounts of widely distributed IL-15 mRNA expression and little or no IL-15 protein secretion in the cytoplasm or cell culture supernatant were observed. There are several proposed mechanisms to explain this discordance. One such important mechanism is due to the presence of Kozak sequences [42]. Another proposed mechanism for inefficient IL-15 production is the mammalian translation systems. Although IL-15 transcripts were poorly translated in rabbit reticulocyte or in COS cells in vitro translation systems, they were readily translated in a wheat-germ in vitro translation system. This provides evidence that the mammalian translation system, somehow, had negative regulatory effects, along with the 5′ UTR Kozak sequences [1].
Experimentally, IL-15 protein secretion has been increased by replacing its signal peptide with that of a foreign one, suggesting that the native signal sequence is involved in negative control of IL-15 protein secretion [10]. This experiment also demonstrated a non-correlation between the expressions of wild-type IL-15 mRNA and efficient IL-15 protein secretion, supporting the view that IL-15 expression is controlled mainly at the post-transcriptional level of translation and secretion [1]. Another regulatory translation mechanism may exist in the C-terminus of the IL-15 mature protein coding sequence or protein [26,43]. IL-15 exhibits a very complex mechanism of intracellular trafficking into diverse compartments such as the endoplasmic reticulum (ER), cytoplasm and nucleus. Two IL-15 isoforms, SSP-IL-15 and LSP-IL-15, have a profound impact on the intracellular trafficking of IL-15 [8,10,26,34,43]. Although the SSP-IL-15 isoform was translated [8–10], it was not secreted; but was stored intracellularly in cytoplasmic components and the nucleus [8,43]. Furthermore, the SSP-IL-15 isoform was not detected either in the Golgi or in the extracellular fluid [43], suggesting that the SSP-IL-15 signal sequence was unable to mediate translocation into the secretary pathway. On the other hand, LSP-IL-15 precursor protein was expressed in placenta, skeletal muscle, heart, lung, liver and kidney [20], and was observed in the ER secretion through the ER/Golgi pathway [8,20]. The isoforms explain in part the IL-15 expression mechanisms by sorting of the same protein to different cellular compartments by modification of the regulatory sequences, SSP and LSP [8].
Reports from numerous studies confirm the presence of membrane-bound IL-15 on several cell types [13,17–20]. These cells induce a reverse-signalling pathway that leads signal transduction to enhance the biological activity of membrane-bound IL-15. Neely et al. [19] found intracellularly stored IL-15 protein in CD14+ monocytes. Interestingly, stored IL-15 protein shifted to the plasma membrane under stimulation with either LPS/IFN-γ or GM-CSF, confirming that post-translational regulatory stages limit IL-15 secretion. Furthermore, Neely et al. [19] found that cell surface IL-15 can function as a receptor and can initiate the activation of signal pathways that enhance adhesion ability to target cells. This demonstrates that the cell surface IL-15 is more than just a ligand, also functioning as a receptor and participating in reverse signalling that leads to biological activities [19,41].
In summary, results as presented here demonstrate that several tumour-derived B-cells express IL-15 mRNA transcript and ubiquitously express IL-15Rα transcript. Although none of these cell lines secreted detectable IL-15 protein, some expressed low to marginal levels of membrane-bound IL-15 protein, as observed by FACS analysis. Perhaps, as suggested by the experimental evidence of Bamford et al. [26], IL-15 may exist in translationaly inactive pools. This could also indicate that excessive IL-15 activity might have potentially harmful effects upon a cell or whole organism and therefore is designed by several of these complex mechanisms to be a selective regulatory cytokine. Interestingly, the clinical importance of targeting the IL-15/IL-15R system could prove to become an effective therapeutic tool in the treatment of several autoimmune diseases, where increased levels of IL-15 have been reported. These results further confirm that modulation of viral expression influences several regulatory cytokines, and vice versa, as outlined in the review of Sharma [44]. Therefore, it is tempting to speculate that the IL-15/IL-15R system might function as a juxtacrine/intracrine cytokine with reverse signalling, in these tumour-derived B-cell lines.
Acknowledgments
This research was partially supported by Grants Merck-AAAS Undergraduate Research and the Office of Research, University of West Florida, Pensacola (VS). We thank John Van Steenbergen for assistance and Dr Ray Hester of the Laboratory of Flow Cytometry at the University of South Alabama, Mobile, for FACS analysis.
References
- 1.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Fehniger T, Caligiuri M. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–27. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Waldman TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–10. [PubMed] [Google Scholar]
- 4.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002;13:429–39. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 5.Schluns KS, Stoklasek T, Lefrancois L. The role of interleukin-15 receptor α: trans-presentation, receptor component or both? Int J Biochem Cell Biol. 2005;37:1567–71. doi: 10.1016/j.biocel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Krause H, Jandrig B, Wenicke C, Bulfone-Paus S, Pohl T, Diamantstein T. Genome structure and chromosomal localization of the human interleukin-15 gene (IL-15) Cytokine. 1996;8:667–74. doi: 10.1006/cyto.1996.0089. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DM, Johnson L, Glaccum MB, et al. Chromosomal assignment and genomic structure of IL-15. Genomics. 1995;25:701–6. doi: 10.1016/0888-7543(95)80013-c. [DOI] [PubMed] [Google Scholar]
- 8.Tagaya Y, Kurys G, Thies TA, et al. Generation of secretable and nonsecretable interleukin-15 isoforms through alternate usage of signal peptides. Pro Natl Acad Sci USA. 1997;94:14444–9. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meazza R, Verdiani S, Biassoni R, et al. Identification of a novel interleukin-15 (IL-15) transcript isoform generated by alternative splicing in human small cell lung cancer cell lines. Oncogene. 1996;12:2187–92. [PubMed] [Google Scholar]
- 10.Onu A, Pohl T, Krause H, Bulfone-Paus S. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J Immunol. 1997;158:255–62. [PubMed] [Google Scholar]
- 11.Pereno R, Giron-Michel J, Gaggero A, et al. IL-15/IL-15Rα intracellular trafficking in human melanoma cells and signal transduction through the IL-15Rα. Oncogene. 2000;19:5153–62. doi: 10.1038/sj.onc.1203873. [DOI] [PubMed] [Google Scholar]
- 12.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 13.Musso T, Calosso L, Zucca M, et al. Human monocytes constitutively express membrane-bound, biologically active, and Interferon-γ-regulated Interleukin-15. Blood. 1999;93:3531–9. [PubMed] [Google Scholar]
- 14.Fehniger T, Caligiuri M. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–27. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 15.Waldmann TA. The IL-2/IL-15 receptor systems: target for immunotherapy. J Clin Immunol. 2002;22:51–6. doi: 10.1023/a:1014416616687. [DOI] [PubMed] [Google Scholar]
- 16.Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays. 2006;28:362–77. doi: 10.1002/bies.20380. [DOI] [PubMed] [Google Scholar]
- 17.Buldagian V, Bulanova E, Orinska Z, et al. Reverse signaling through membrane-bound interleukin-15. J Biol Chem. 2004;279:42192–201. doi: 10.1074/jbc.M403182200. [DOI] [PubMed] [Google Scholar]
- 18.Giron-Michel J, Giuliani M, Fogli M, et al. Membrane-bound and soluble IL-15/Il−15Rα complexes display differential signaling and functions on human haematopoietic progenitors. Blood. 2005;106:2302–10. doi: 10.1182/blood-2005-01-0064. [DOI] [PubMed] [Google Scholar]
- 19.Neely GG, Robbins SM, Amankwah EK, et al. Lipopolysaccharide-stimulated or granulocyte-macrophage colony-stimulating factor-stimulated monocytes rapidly express biological active IL-15 on their cell surface independent of new protein synthesis. J Immunol. 2001;167:5011–7. doi: 10.4049/jimmunol.167.9.5011. [DOI] [PubMed] [Google Scholar]
- 20.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 21.Anderson DM, Kumaki S, Ahdiesh M, et al. Functional characterization of the human interleukin-15 receptor α chain linkage of IL-15 RA and IL-2RA genes. J Biol Chem. 1995;270:29862–9. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 22.Dubois S, Magrangeas F, Lehours P, et al. Natural splicing of Exon 2 of human interleukin-15 receptor α-chain mRNA results in a shortened form with a distinct pattern of expression. J Biol Chem. 1999;274:26978–84. doi: 10.1074/jbc.274.38.26978. [DOI] [PubMed] [Google Scholar]
- 23.Forcina G, d’Ettorre G, Mastroianni CM, et al. Interleukin-15 modulates interferon-γ and β-chemokine production in patients with HIV infection: implications for immune-based therapy. Cytokine. 2004;25:283–90. doi: 10.1016/j.cyto.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Lum JJ, Schnepple DJ, Nie Z, et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J Virol. 2004;78:6033–42. doi: 10.1128/JVI.78.11.6033-6042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller YM, Bojczuk PM, Halstead ES, et al. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–9. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 26.Bamford R, DeFilippis A, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;106:4418–26. [PubMed] [Google Scholar]
- 27.Sharma V, Walper D, Deckert R. Modulation of macrophage inflammatory protein-1alpha and its receptors in human B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt’s lymphoma. Biochem Biophys Res Commun. 1997;235:576–81. doi: 10.1006/bbrc.1997.6828. [DOI] [PubMed] [Google Scholar]
- 28.Sharma V, Sparks JL, Vail JD. Human B-cell lines constitutively express and secrete interleukin-16. Immunol. 2000;99:266–71. doi: 10.1046/j.1365-2567.2000.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma V, Zhang L. Interleukin-8 expression in AIDS-associated lymphoma B-cell lines. Biochem Biophys Res Commun. 2001;282:369–75. doi: 10.1006/bbrc.2001.4579. [DOI] [PubMed] [Google Scholar]
- 30.Lorey SL, Huang YC, Sharma V. Constitutive expression of interleukin-18 and interleukin-18 receptor mRNA in tumour derived human B-cell lines. Clin Exp Immunol. 2004;136:456–62. doi: 10.1111/j.1365-2249.2004.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaidano G, Parsa NZ, Tassi V, et al. In vitro establishment of AIDS-related lymphoma cell lines: phenotypic characterization, oncogene and tumor suppressor gene lesions, and heterogeneity in Epstein-Barr viral infection. Leukemia. 1993;7:1621–9. [PubMed] [Google Scholar]
- 32.Sharma V, Xu M, Vail JD, Campbell R. Comparative analysis of multiple techniques for semi-quantitation of RT-PCR amplicons. Biotechnol Techniques. 1998;12:521–4. [Google Scholar]
- 33.Huang YC, Tsukamoto K, Sharma V. Interleukin-10 promoter gene polymorphisms have no clear influence on interleukin-10 protein secretion in AIDS-associated B-cell lines. Biochem Biophys Res Commun. 2005;335:529–35. doi: 10.1016/j.bbrc.2005.07.107. [DOI] [PubMed] [Google Scholar]
- 34.Kurz T, Strauch K, Dietrich H, et al. Multilocus haplotype analyses reveal association between 5 novel IL-15 polymorphisms and asthma. J Allergy Clin Immunol. 2004;113:896–901. doi: 10.1016/j.jaci.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–90. [PubMed] [Google Scholar]
- 36.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 37.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P. DCs induce CD40-independent immunoglobulin class switching through BlyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tinhofer I, Marschiz I, Henn T, Egle A, Greil R. Expression of function interleukin-15 receptor and autocrine production of interleukin-15 as mechanism of tumor propargation in multiple myeloma. Blood. 2002;15:610–8. [PubMed] [Google Scholar]
- 39.Trentin L, Zambello R, Facco M, Sancetta R, Agostini C, Semenzato G. Interleukin-15: a novel cytokine with regulatory properties on normal and neoplastic B lymophocytes. Leuk Lymphoma. 1997;27:35–42. doi: 10.3109/10428199709068269. [DOI] [PubMed] [Google Scholar]
- 40.Bulfone-Paus S, Ungureanu D, Pohl T, et al. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–8. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 41.Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J Immunol. 2004;173:6676–83. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 42.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–41. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaggero A, Azzarone B, Andrei C, et al. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Euro J Immunol. 1999;29:1265–74. doi: 10.1002/(SICI)1521-4141(199904)29:04<1265::AID-IMMU1265>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Sharma V. Current perspectives on cytokines for anti-retroviral therapy in AIDS related B-cell lymphomas. Curr Drug Targets Infect Disord. 2003;3:137–49. doi: 10.2174/1568005033481178. [DOI] [PubMed] [Google Scholar]