Abstract
Anti-tumour T cell response requires antigen presentation via efficient immunological synapse between antigen presenting cells, e.g. dendritic cells (DC), and specific T cells in an adapted Th1 cytokine context. Nine renal cell carcinoma (RCC) primary culture cells were used as sources of tumour antigens which were loaded on DC (DC-Tu) for autologous T cell activation assays. Cytotoxic activity of lymphocytes stimulated with DC-Tu was evaluated against autologous tumour cells. Assays were performed with 75 grays irradiated tumour cells (Tu irr) and with hydrogen peroxide ± heat shock (Tu H2O2 ± HS) treated cells. DC-Tu irr failed to enhance cytotoxic activity of autologous lymphocytes in seven of 13 assays. In all these defective assays, irradiated tumour cells displayed high interleukin (IL)-6 and vascular endothelial growth factor (VEGF) release. Conversely, when tumour cells released low IL-6 levels (n = 4), DC-Tu irr efficiently enhanced CTL activity. When assays were performed with the same RCC cells treated with H2O2 + HS, DC-Tu stimulation resulted in improved CTL activity. H2O2 + HS treatment induced post-apoptotic cell necrosis of tumour cells, totally abrogated their cytokine release [IL-6, VEGF, transforming growth factor (TGF)-β1] and induced HSP70 expression. Taken together, data show that reduction in IL-6 and VEGF release in the environment of the tumour concomitantly to tumour cell HSP expression favours induction of a stronger anti-tumour CTL response.
Keywords: cytokine, dendritic cell, heat shock protein, lymphocyte, renal cell carcinoma
Introduction
The capacity of the immune system to reject tumours is supported by both innate immune cells such as natural killer (NK), NK T, γδ T cells and by cytotoxic T lymphocytes (CTL) [1–4]. The activation of specific CTL able to kill tumour cells [5,6] involves a cross-talk between dendritic cells (DC) [7] which present tumour antigenic epitopes and naive T cells. The reproduction of T cell priming in vitro requires knowledge of the capture of antigens by DC [8] and the cytokinic environment at the time of priming, which can skew the immune response toward T helper 1 (Th1), Th2 response or tolerance [9]. The use of tumour biopsy material as source of antigens is particularly attractive for clinical applications as it contains the patient’s own specific array of tumour-associated antigens. Interest in whole tumour cells is enhanced by studies demonstrating that tumour cell lysates [10], apoptotic [11] or necrotic tumour cells [12,13] can induce specific stimulation of class I-restricted CTL. However, the putative release of immunosuppressive cytokines by tumour cells during DC loading could impede T cell activation and may be a limit for the use of whole tumour cells in immunotherapy protocols [11]. Thus, the choice of treatment to induce tumour cell death is a crucial point, as it could influence cytokine release by tumour cells.
Renal cell carcinoma (RCC) cells are known to secrete both interleukin (IL)-6 and vascular endothelial growth factor (VEGF) [14,15]. These molecules present mitogenic and immunosuppressive effects [16–18]. IL-10 and transforming growth factor (TGF)-β1 also contribute to immunosuppression in cancer [18–20]. In this study, we investigated IL-6, VEGF, IL-10 and TGF-β1 production by RCC either spontaneously and after killing treatments and evaluated the cytotoxic activity of tumour-specific CTL stimulated with DC loaded with tumour cells.
Materials and methods
Patients and biopsies
Nine RCC patients, 48 ± 20 years of age, without previous therapy, underwent curative nephrectomy. Tumours were classified according to tumour size–lymph nodes–metastases (TNM) staging (five T3, two T2, two T1) and evaluated with the Fuhrman grading system (six grade III and three grade IV) [21]. Two patients were metastatic at time of surgery. Tumour biopsies (n = 9), proximal lymph nodes (n = 4) and peripheral blood (n = 7) samples were collected.
Cell culture
DC were generated within 7 days from monocytes in X-Vivo 10 medium with 10% fetal calf serum (FCS, Invitrogen, Cergy Pontoise, France), 1000 IU/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (Schering-Plough, Huningue, France) and 400 IU/ml IL-4 (Promocell).
Lymphocytes isolated either from blood [peripheral blood lymphocytes (PBL)], tumour [tissue-infiltrating lymphocytes (TIL)] or proximal lymph nodes (LNL) were cultured for 15–27 days in X-Vivo 15 medium with 10% FCS, 2% l-glutamine, 2% pyruvate, 1% non-essential amino acids and 150 IU/ml IL-2 (Chiron, Heidelberg, Germany). Tumour biopsies were minced into small pieces and cultured for a short time (< four passages) in RPMI-1640 medium (Eurobio, Suresnes, France) containing 10% FCS. Tumour cells were then recovered and treated with either 75 grays irradiation (Tu irr) [22] or with 1 mM hydrogen peroxide (H2O2, Sigma, Saint Quentin Fallavier, France) for 12 h (Tu H2O2) [13]. H2O2 treatment could be combined with heat shock for 30 min at 44°C (Tu H2O2 + HS).
Treated tumour cells were incubated, without previous washing, overnight, with DC in a 1 : 10 (DC : tumour cell) ratio. DC were then co-cultured with lymphocytes in a 1 : 100 (DC : lymphocyte) ratio during 7 days. Controls were performed with non-stimulated lymphocytes evaluated at the same time.
Flow cytometry analysis
Cells were stained by monoclonal antibodies (mAb) against CD3, CD4, CD8, CD56 (Beckman-Coulter, Marseille, France) and HSP70 (Stressgen, Vancouver, Canada). Apoptotic cells were detected by annexin V-propidium iodide assay (Immunotech). Data were acquired on a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View, USA).
Cytokine detection by enzyme-linked immunosorbent assay (ELISA)
RCC cells (5·104) were incubated for 48 h in 3 ml of medium. IL-6, IL-10, VEGF and TGF-β1 concentrations were measured in supernatants with ELISA kits purchased from Biosource, Montrouge, France; R&D Systems, Lille, France; Becton Dickinson and AbCys, Paris, France, respectively. For TGF-β1 measurements, the latent form was cleaved into the active form by acid treatment [18].
Immunohistochemical cytokine detection
A representative slice of the tumour with the highest nuclear grade was selected for immunostaining. Slices were incubated at room temperature for 1 h with the primary mAb: anti-IL-6 (titre 1/40; R&D Systems), anti-VEGF (titre 1/100; Santa Cruz Biotechnology Inc., Santa Cruz, USA) or anti-IL-10 (titre 1/100; R&D Systems). Primary mAb were revealed using a biotin–streptavidin detection system (Dako, Glostrup, Denmark). The cytokine labelling index (LI) was expressed as percentage of positive cells by counting at least 1000 tumour cells within at least 10 consecutive high-power fields.
Western immunoblotting
Cells were lysed and equal quantities of protein (50 µg) were separated by 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) before transfer to nitrocellulose membrane. Blots were incubated with anti-HSP70 mAb (Stressgen) and with control anti-glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mAb (Biochain, Hayward, CA, USA). The membrane was incubated with peroxidase-labelled anti-mouse IgG (Bio-Rad, Jury-sur-Seine, France) and bands were revealed using the enhanced chemiluminescence kit from Amersham, Aylesbury, UK.
Cytotoxicity assays
RCC cells (5·103) labelled with 51Cr sodium chromate (1 mCi/106 cells, Amersham) were co-cultured in RPMI-1640 medium in 96-well U-bottomed plates for 4 h with autologous lymphocytes at a 50 : 1 effector : target cell ratio. 51Cr release was assessed in culture supernatants using a Top-count gamma counter (Packard Instruments, Rungis, France). Specific activity was calculated using the formula: [mean experimental counts per minute (cpm)–mean spontaneous cpm]/(mean maximum cpm–mean spontaneous cpm) × 100.
Results
Cytokine release by RCC cells
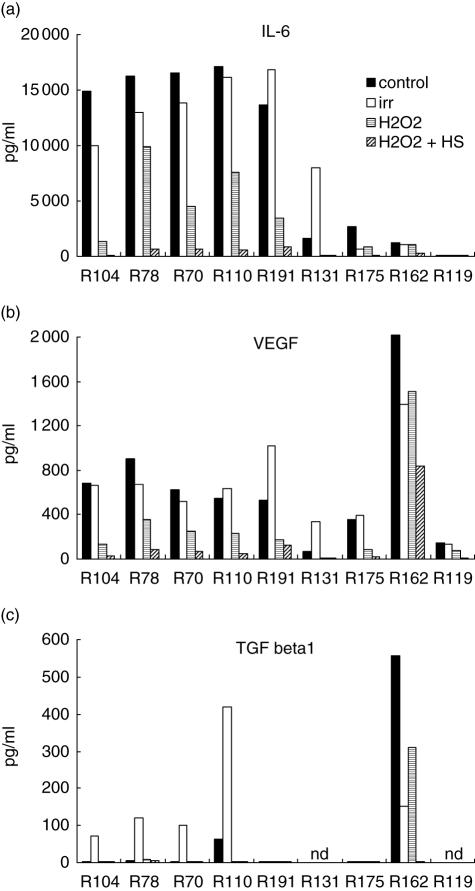
Nine RCC cultures were investigated for IL-6, VEGF, TGF-β1 and IL-10 release in culture supernatant. All but one constitutively produced IL-6 at various levels ranging from 1·3 to 16 ng/ml (Fig. 1a) and could be sorted into two groups: five of nine with high IL-6 secretion (> 10 ng/ml) and four of nine with low release. VEGF was detected in all cultures, ranging from 50 to 900 pg/ml (Fig. 1b). Tumour cell irradiation, treatment performed commonly for DC loading, did not significantly modify IL-6 and VEGF secretions except for R131 and R191, which present an increased release. Interestingly, cultures characterized by high IL-6 release also present the strongest VEGF secretion except for R162. Constitutive secretion of TGF-β1 was low or undetectable except, again, for R162 (Fig. 1c). However, irradiation induced TGF-β1 release (= 100 pg/ml) for four of seven assays. Finally, IL-10 release could never be detected (data not shown). The role of contaminating endothelial cells or leucocytes in the immunosuppressive cytokine secretion was excluded with regard to the absence of CD45+ or CD31+ cells in flow cytometry analyses (data not shown).
Fig. 1.
Interleukin (IL)-6, vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β1 production in renal cell carcinoma (RCC) cells. RCC cells (n = 9) were either untreated, irradiated (irr) or treated by hydrogen peroxide (H2O2) combined or not with heat shock (H2O2 + HS). Cells (5·104) were incubated for 48 h in 3 ml of RPMI-1640 medium. IL-6, VEGF and TGF-β1 release was measured in supernatant of cell culture by enzyme-linked immunosorbent assay; nd = not determined.
In parallel, biopsy specimens of the same nine RCC were submitted to immunochemistry analysis. IL-6, VEGF and IL-10 immunostaining was variable depending on the tumour. Labelling index was from 5 to 40% (mean 14%) for IL-6, from 0 to 100% (mean 44%) for VEGF and from 10 to 100% (mean 45%) for IL-10.
IL-6 and VEGF impede DC tumour cells (DC-Tu) stimulation of lymphocytes
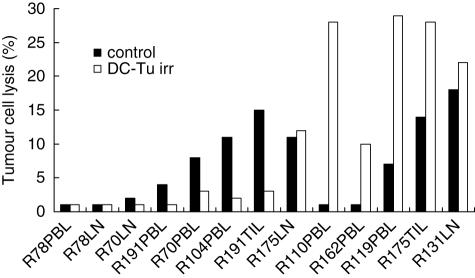
The cytotoxic activity of lymphocytes stimulated with DC loaded with irradiated tumour cells (DC-Tu irr) was evaluated against autologous tumour cells. For seven of 13 assays, stimulation did not improve the cytotoxic activity of lymphocytes (Fig. 2). For these unsuccessful assays, irradiated tumour cells were characterized by high IL-6 and VEGF release. Conversely, when tumour cells (n = 4) were characterized by low IL-6 secretion (< 10 ng/ml), stimulation was efficient and resulted in an enhancement of the cytotoxic activity. R110 was an exception because stimulation by DC was efficient despite the high IL-6 secretion.
Fig. 2.
Cytotoxic activity of lymphocytes after stimulation by dendritic cells (DC) loaded with irradiated tumour cells.
The distribution of T cells was not modified after the DC-Tu irr stimulation either for CD8 T lymphocytes (33 ± 10 versus 33 ± 20%, n = 10) or for CD3– CD56+ NK cells (9 ± 6 versus 9 ± 3%, n = 10).
Hydrogen peroxide and heat shock treatment of tumour cells abrogates IL-6 and VEGF production and favours stimulation of lymphocytes
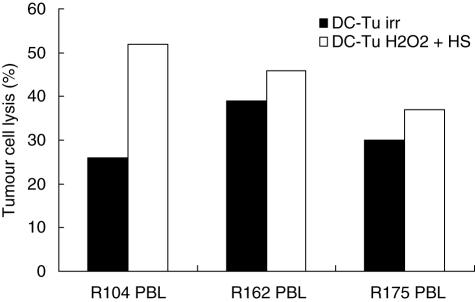
H2O2 treatment significantly reduced IL-6 and VEGF release in all cultures (Fig. 1a,b). When cells were submitted to an additional heat shock (HS), IL-6 and VEGF production was completely abrogated in eight of nine assays (except R162). TGF-β1 release was also reduced after H2O2 or H2O2 + HS treatments in comparison with the constitutive (R110, R162) or irradiation-induced (R70, R78, R104) secretions (Fig. 1c). Interestingly, when tumour cells treated with H2O2 + HS were used for DC pulsing, cytotoxic activity of lymphocytes was enhanced in comparison with irradiated tumour cells (Fig. 3).
Fig. 3.
Cytotoxic activity of lymphocytes after stimulation by dendritic cells (DC) loaded with hydrogen peroxide + heat shock treated tumour cells.
Hydrogen peroxide and heat shock treatment of tumour cells induces cell necrosis and HSP70 expression
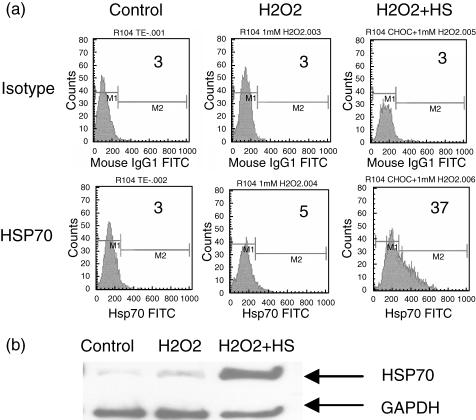
Unlike irradiation, H2O2 and H2O2 + HS treatments increase the percentage of cell necrosis in RCC cell lines in comparison to control cells (Table 1). Interestingly, HSP70 expression was evidenced only after H2O2 + HS combined treatment either in flow cytometry analysis (Fig. 4a) or in Western immunoblotting assays (Fig. 4b).
Table 1.
Effects of hydrogen peroxide (H2O2) associated or not with heat shock (HS) on necrosis of RCC cells.
| Control | Irradiation | H2O2 | H2O2 + HS | |
|---|---|---|---|---|
| % Necrotic cells | 15 ± 17 | 15 ± 18 | 32 ± 12 | 34 ± 9 |
| % Viable cells | 68 ± 24 | 67 ± 24 | 60 ± 12 | 59 ± 10 |
Renal carcinoma cells (RCC) were gamma-radiated or treated with 1 mM H2O2 for 12 h combined or not with HS (44°C during 30 min). Necrotic cells were detected by annexin V–propidium iodide assay. Control was performed with untreated cells. n = 6 for control and irradiation and n = 3 for H2O2 and H2O2 + HS. Necrotic cells: A +, PI + viable cells: A-PI-.
Fig. 4.
HSP70 expression in renal cell carcinoma (RCC) cells after hydrogen peroxide (H2O2) + heat shock (HS) treatment. Tumour cells were treated during 12 h with 1 mM hydrogen peroxide (H2O2) or H2O2 plus heat shock for 30 min at 44°C (H2O2 + HS). Untreated cells were used as control. (a) HSP70 expression was measured by flow cytometry. Percentages of positive cells are indicated on the histograms. Representative data of seven experiments are shown. (b) HSP70 expression was measured by Western immunoblotting using an anti-HSP70 monoclonal antibody. Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) expression was used as standard protein. Representative data of three experiments are shown.
Discussion
Several studies have demonstrated, in patients with cancer, a blockade in the maturation process of DC related to the release of cytokines and other soluble factors [23,24]. In RCC, IL-6 and VEGF are known as the main immunosuppressive factors [17,25].
In this study, we investigated IL-6, VEGF, TGF-β1 and IL-10 release in RCC cultures and the influence of killing treatments on this secretion. A constitutive expression of large amounts of IL-6 (> 10 ng/ml) associated with VEGF secretion (> 500 pg/ml) was found in five of nine RCC cultures [26]. With regard to TGF-β1 secretion, only two of seven primary RCC cultures released some detectable amounts while high TGF-β1 production was observed in culture from one metastatic patient. This resultcorroborates a study indicating that TGF-β1 production is enhanced in advanced RCC tumour stage [27]. The presence of immunosuppressive cytokines during the DC loading could impede the induction of a strong immune response and this question is worth studying in the vaccination protocols.
To date, irradiation is used commonly to induce tumour cell death. However, our assays showed that viability of gamma-radiated RCC cells was similar to untreated cells. This result could be related to the clinically known resistance of human RCC against radiation [28]. Moreover, irradiation did not change IL-6 and VEGF release. It induced, in half the assays, a TGF-β1 secretion which could be the consequence of the up-regulation of TGF-β1 gene expression following irradiation [29]. In seven of 13 assays, stimulation with DC loaded by irradiated tumour cells was insufficient to enhance cytotoxic activity of autologous lymphocytes. In all these defective assays, irradiated RCC cells were characterized by a high IL-6 and VEGF release. Inversely, when irradiated RCC cells presented low IL-6 release (n = 4), stimulation efficiently enhanced CTL activity. These results suggest a negative impact of IL-6 and VEGF on T cell activation. H2O2 and heat shock are alternative treatments to induce tumour cell death. Hydrogen peroxide induces apoptosis via the caspase activation or necrosis by decreasing intracellular adenosine triphosphate (ATP) [30], while heat shock is known to favour HSP70 expression, a molecule involved in DC maturation and cross-presentation [10,31–34]. Our results show that H2O2 plus heat shock succeeded in inducing necrosis of RCC cells. It allowed the abrogation of IL-6 and VEGF secretion as well as strong HSP70 expression and led to improved CTL activity.
In conclusion, our data obtained with human tumours collected from patients with RCC show that treatment of tumour cells by H2O2 and heat shock enhances their immunogenicity. The underlying mechanisms could be the abrogation of immunosuppressive cytokine release into the tumour environment as well as the induction of danger signal such as the chaperone protein HSP70. These results could be helpful in optimizing strategies aimed at inducing a strong CTL response against tumour cells and in favouring the development of adoptive cell therapy protocols.
Acknowledgments
We thank Matthieu Le Gallo* and Frederic Epstein (Inserm U601) for Western immunoblotting assays. Pascaline Charlot and Selim Zerrouki (Laboratoire de Génétique moléculaire et hormonologie, CHU de Rennes) for technical assistance. This work was supported by grants from the Comité Grand Ouest de la Ligue Contre le Cancer, the Faculté de Médecine de Rennes and the Groupement d’Intérêt Scientifique de Thérapie Cellulaire de Rennes, France.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 4.Seino K, Fujii S, Harada M, et al. Valpha14 NKT cell-mediated anti-tumor responses and their clinical application. Springer Semin Immunopathol. 2005;27:65–74. doi: 10.1007/s00281-004-0194-y. [DOI] [PubMed] [Google Scholar]
- 5.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–45. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- 8.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 9.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation. new players and new insights. J Clin Immunol. 2003;23:147–61. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 10.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–9. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 11.Albert ML. Death-defying immunity. do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–31. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 12.Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001;61:8105–9. [PubMed] [Google Scholar]
- 13.Gervais A, Bouet-Toussaint F, Toutirais O, De La Pintiere CT, Genetet N, Catros-Quemener V. Ex vivo expansion of antitumor cytotoxic lymphocytes with tumor-associated antigen-loaded dendritic cells. Anticancer Res. 2005;25:2177–85. [PubMed] [Google Scholar]
- 14.Alberti L, Thomachot MC, Bachelot T, Menetrier-Caux C, Puisieux I, Blay JY. IL-6 as an intracrine growth factor for renal carcinoma cell lines. Int J Cancer. 2004;111:653–61. doi: 10.1002/ijc.20287. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen J, Grankvist K, Rasmuson T, Bergh A, Landberg G, Ljungberg B. Expression of vascular endothelial growth factor protein in human renal cell carcinoma. BJU Int. 2004;93:297–302. doi: 10.1111/j.1464-410x.2004.04605.x. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 17.Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- 18.Toutirais O, Chartier P, Dubois D, et al. Constitutive expression of TGF-beta1, interleukin-6 and interleukin-8 by tumor cells as a major component of immune escape in human ovarian carcinoma. Eur Cytokine Netw. 2003;14:246–55. [PubMed] [Google Scholar]
- 19.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 20.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Bouet-Toussaint F, Patard JJ, Gervais A, et al. Cytotoxic effector cells with antitumor activity can be amplified ex vivo from biopsies or blood of patients with renal cell carcinoma for cell therapy use. Cancer Immunol Immunother. 2003;52:699–707. doi: 10.1007/s00262-003-0412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–8. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gervais A, Leveque J, Bouet-Toussaint F, et al. Dendritic cells are defective in breast cancer patients: a potential role for polyamine in this immunodeficiency. Breast Cancer Res. 2005;7:R326–35. doi: 10.1186/bcr1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikov MM, Ohm JE, Ray N, et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174:215–22. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 26.Knoefel B, Nuske K, Steiner T, et al. Renal cell carcinomas produce IL-6, IL-10, IL-11, and TGF-beta 1 in primary cultures and modulate T lymphocyte blast transformation. J Interferon Cytokine Res. 1997;17:95–102. doi: 10.1089/jir.1997.17.95. [DOI] [PubMed] [Google Scholar]
- 27.Mitropoulos D, Kiroudi A, Christelli E, et al. Expression of transforming growth factor beta in renal cell carcinoma and matched non-involved renal tissue. Urol Res. 2004;32:317–22. doi: 10.1007/s00240-003-0360-z. [DOI] [PubMed] [Google Scholar]
- 28.Ramp U, Caliskan E, Mahotka C, et al. Apoptosis induction in renal cell carcinoma by TRAIL and gamma-radiation is impaired by deficient caspase-9 cleavage. Br J Cancer. 2003;88:1800–7. doi: 10.1038/sj.bjc.6600984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chorna I, Bilyy R, Datsyuk L, Stoika R. Comparative study of human breast carcinoma MCF-7 cells differing in their resistance to doxorubicin. effect of ionizing radiation on apoptosis and TGF-beta production. Exp Oncol. 2004;26:111–7. [PubMed] [Google Scholar]
- 30.Saito Y, Nishio K, Ogawa Y, et al. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic Res. 2006;40:619–30. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 31.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 32.Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood. 2003;101:4485–91. doi: 10.1182/blood-2002-10-3108. [DOI] [PubMed] [Google Scholar]
- 33.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101:245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 34.Masse D, Ebstein F, Bougras G, Harb J, Meflah K, Gregoire M. Increased expression of inducible HSP70 in apoptotic cells is correlated with their efficacy for antitumor vaccine therapy. Int J Cancer. 2004;111:575–83. doi: 10.1002/ijc.20249. [DOI] [PubMed] [Google Scholar]