Abstract
Although a beneficial effect of hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, i.e. statins, on cell-mediated immunity has been suggested in vivo and in vitro, little is known about the molecular and biochemical events by which statins inhibit T cell proliferation. To address this question, we investigated the effects of atorvastatin (AT) on intracellular cytokine production, T cell activation markers, cell cycle progression and apoptosis in human CD4+ T cells. AT did not influence intracellular cytokine production after short-term stimulation of whole blood with phorbol myristate acetate (PMA)/ionomycin or superantigen (SEB). In contrast, AT influenced CD45RA to RO switching dose-dependently, as well as CD25 expression, and caused cell cycle arrest in the G1 phase after long-term T cell stimulation. This occurred in conjunction with a reduced expression of cyclin-dependent kinases 2 and 4 and p21wav1/cip1 and was paralleled by an increased protein expression of p27kip1. In addition to G1 arrest, increased apoptosis was observed in AT-treated cells. In line with this, the expression of Bcl-xl and pBad were decreased by AT. Apoptosis was independent of caspases 3 and 9 activation. The inhibitory effect of AT on T cell proliferation could be overcome by addition of mevalonic acid or geranylgeranyl pyrophosphate, but not by farnesyl pyrophosphate or squalen, suggesting reduced protein prenylation. Activation of Rho, Rac and Ras were strongly reduced in AT-treated T cells, suggesting that impaired geranylation of these molecules might underlie the inhibitory effect of AT on T cell proliferation.
Keywords: HMG-CoA reductase, small GTPases, CD4+ T cells, apoptosis
Introduction
Statins inhibit the rate-limiting enzyme of the cholesterol pathway, i.e. hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase, thereby preventing the conversion of HMG-CoA into mevalonic acid. Clinically, these compounds are used primarily to treat hypercholesterolaemia. In addition, statins have pleiotropic effects far beyond their cholesterol-lowering characteristics [1]. Among these, modulation of inflammatory responses by statins has gained much interest, as they might provide an additive therapeutic effect, beneficial for treatment of atherosclerosis, autoimmune diseases or allograft rejection [2–5]. The anti-inflammatory response of statins is believed to be mediated through the action on both the endothelium and leucocytes [6,7]. Some statins, in particular simvastatin and lovastatin, are able to inhibit leucocyte endothelial cell adhesion directly by interfering with leucocyte function antigen/intercellular adhesion molecule (LFA-1/ICAM-1) interactions [8]. Statins also inhibit interferon (IFN)-γ-induced major histocompatibility complex (MHC) class II expression by inhibition of class II trans-activator (CIITA) gene expression [9]. Furthermore, statins have the propensity to shift the T cell cytokine profile from T helper (Th)1 to Th2, as demonstrated in animal models [10,11] and in patients suffering from acute coronary syndrome [12].
The pleiotropic effects of statins are likely to be a consequence of impaired activation of signalling molecules [6,7,13]. Because mevalonic acid also serves as an important intermediate for the synthesis of isoprenoids, i.e. geranylgeranyl pyrophosphate and farnesyl phosphate, inhibition of HMG-CoA reductase will result in inhibition of isoprenoid synthesis [14]. Hence, post-translational prenylation of signalling molecules, required for anchoring to the inner leaflet of the cell membrane, is lost as well as their signalling capacity [14,15]. In particular small guanosine triphosphatase (GTP)-binding proteins, e.g. small GTPases, require isoprenoids to retain their function [16–20]. They are involved in a number of cellular processes such as cell growth, differentiation and survival, cytoskeletal organization, endocytotic/exocytotic transport and gene expression [13,21–24].
Small GTPases are involved at various levels in the cascade of T cell activation [25,26]. They are involved in T cell receptor (TCR) signalling as well as in actin dynamics required for formation of the immunological synapse (IS) and T cell polarization. Several studies have indicated that statins impair T cell activation directly [27,28]. It remains controversial, however, whether inhibition of T cell proliferation by statins is exclusively the result of impaired T cell activation or if other mechanisms are equally involved in this. In the present study, we investigated the influence of AT on cytokine production, T cell activation markers, cell cycle progression and apoptosis in order to obtain a better understanding of the mechanisms by which AT treatment of human T cells leads to a diminished proliferation.
Materials and methods
Atorvastatin, kindly provided by Pfizer (Pfizer, Karlsruhe, Germany), was dissolved in dimethylsulphoxide (DMSO) and prepared as 10 mM stock.
Peripheral T cells
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats of healthy donors by Ficoll Hypaque (Amersham, Freiburg, Germany) density gradient centrifugation. All procedures were performed according to the ethical committee guidelines of the University of Heidelberg, University Hospital Mannheim. Informed consent was obtained. CD4+ T cells were isolated from PBMC by negative selection (Miltenyi Biotec, Bergisch-Gladbach, Germany). Overall purity of isolated CD4+ T cells was above 95%, as confirmed by fluorescence activated cell sorter (FACS) analysis.
T cell stimulation assays
Purified CD4+ T cells were seeded in high-binding 96-well flat-bottomed plates (Greiner Bio-One, Frickenhausen, Germany) coated with anti-CD3 (clone UCHT-1) and anti-CD28 (clone 37407·11), both 1 µg/ml (R&D Systems, Wiesbaden, Germany) or 50 ng/ml phorbol myristate acetate (PMA) and 1 µg/ml ionomycin (both from Sigma-Aldrich, St Louis, MO, USA). The cells were cultured for 3 days in Iscove’s modified Dulbecco’s medium (IMDM) containing 10% fetal calf serum (FCS) (both from PAN Biotech, Aidenbach, Germany) and 1% penicillin/streptomycin (Sigma-Aldrich) in the presence or absence of various concentrations of AT. After 2 days, 50 µl of [3H]-thymidine (1 µCi, Amersham) containing culture medium was added during the final 16 h of the culturing period. [3H]-thymidine incorporation was assessed by scintillation counting in a liquid scintillation counter (LS 6500, Beckman Coulter, Krefeld, Germany).
Flow cytometry
Antigen expression on CD4+ T cells was determined by quadruple immunofluorescence staining using directly conjugated antibodies to CD3, CD4, CD19, CD25, CD45RA, CD45RO and CD69 (all from BD Biosciences, Heidelberg, Germany). To this end, CD4+ T cells were preincubated overnight with AT (1–10 µM) or left untreated and subsequently stimulated with either PMA/ionomycin or cross-linked antibodies directed against CD3 and CD28 (CD3 × CD28). On days 1–3 cells were harvested, washed twice with phosphate-buffered saline (PBS) and incubated for 30 min with specific monoclonal antibodies. The antibodies were either conjugated to fluorescein isothiocyanate (FITC), R-phycoerythrin (RPE), peridinin chlorophyll (PerCP) or allophycocyanin (APC), depending on the combination of specific antibodies used. Four-colour analysis was performed on a FACSCalibur flow cytometer (BD Biosciences) and the data were analysed using WinMDI 2·8 software.
To assess intracellular interleukin IL-2, IL-4, IL-10, IL-12 and IFN-γ upon T cell activation, intracellular FACS stainings were performed. AT pretreated (overnight, 1–10 µM) and untreated CD4+ T cells were either stimulated with 50 ng/ml PMA and 1 µg/ml ionomycin or with 2·5 µg/ml Staphylococcus aureus enterotoxin B (SEB) in heparinized whole blood in the presence of 1 µg/ml anti-CD28 and anti-CD49d (clones L293 and 9F10, BD Biosciences). Cells were incubated in polypropylene tubes at 37°C for a total of 6 h. During the last 4 h, 10 µg/ml of Brefeldin A (Sigma) was added to block extracellular secretion of cytokines. After 4 h, cells were permeabilized and stained by incubating with specific primary antibodies diluted in 10% perm-wash buffer (BD Biosciences).
Cell cycle progression as well as DNA fragmentation was assessed by propidium iodide staining; 1 × 10−6 CD4+ T cells were pretreated overnight with 1–10 µM of AT or left untreated and stimulated with PMA/ionomycin or anti-CD3/anti-CD28. On days 1–5, cells were harvested in complete IMDM and centrifuged at 300 g for 5 min. Pellets were washed twice with PBS, lysed and stained with 500 µl of propidium iodide (PI) solution (50 µg/ml in PBS, Sigma-Aldrich). Cells were incubated at 4°C overnight and analysed by flow cytometry. Apoptotic cells were defined as cells with DNA content below the G1 peak. In addition, apoptosis was analysed by annexin–FITC stainings following the manufacturer’s instructions (BD Biosciences).
RNase protection assay
Total RNA was isolated from PMA (50 ng/ml)/ionomycin (1 µg/ml)-stimulated CD4+ T cells cultured in the presence or absence of 10 µM of AT after 72 h using TRIzol reagent (Invitrogen). RNA was precipitated with isopropanol, washed with 70% ethanol and subjected to RNase protection assays using BD RiboQuant™ multi-probe sets (BD Biosciences) following the supplier’s instructions. The gels were dried and subjected to autoradiography. The RNA transcripts were identified by appropriate length of the protective fragments. In each of the template sets the housekeeping genes L32 and glyceraldehye-3-phosphate-dehydrogenase (GAPDH) were included to control for equal loading.
Western blot
Cells were resuspended in lysis buffer [150 mM NaCl, 10 mM Tris-HCl, 5 mM ethylenediamine tetraacetic acid (EDTA), 1% Triton X-100, 0·5% Na-deoxycholate, 1 µM dithiothreitol (DTT), 1 µg/ml aprotinin, 1 mM phenylmethylsulphonyl fluoride (PMSF)]. After 5 min incubation on ice, lysates were centrifuged for 15 min at 16 000 g. Protein concentrations in the supernatants were measured using Coomassie reagent (Pierce, Rockford, IL, USA). The samples were separated on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted semidry onto a polyvinyl difluoride (PVDF) membrane (Roche Diagnostics, Mannheim, Germany). The membrane was incubated overnight in 5% milk powder in Tris-buffered saline (TBS) (10 mM Tris-HCl, pH 8·0, 150 mM NaCl) solution. Thereafter, the blot was incubated for 1 h with specific primary antibodies, followed by incubation with appropriate horseradish peroxidase (HRP) secondary antibodies. Proteins were visualized using enhanced chemoluminescence technology according to the manufacturer’s instructions (Pierce). To confirm equal protein loading, membranes were stripped with 62·5 mM Tris-HCl, 2% SDS and 100 mM β-mercaptoethanol and incubated with antibodies against GAPDH and β-actin.
Pull-down assays
Activation of small GTP-binding proteins Rho, Rac and Ras were assessed by pull-down assays (Pierce) according to the manufacturer’s manuals. Guanosine triphosphate (GTPγS) (1 mM) and guanosine diphosphate (GDP) (1 mM) served as controls. Pull-down extracts were separated using 12% SDS-PAGE and blotted onto PVDF membranes (Roche). Following 3 h of blocking [3% bovine serum albumin (BSA) in TBS] at room temperature, membranes were incubated overnight at 4°C with specific primary antibodies to Rho, Rac and Ras. The membranes were washed extensively with TBS and incubated with appropriate HRP-labelled secondary antibodies [45 min at room temperature (RT)]. Proteins were visualized using enhanced chemoluminescence technology according to the manufacturer’s instructions (Pierce).
Statistical analysis
For statistical analysis Student’s t-tests and analysis of variance (anova) with Tukey–Kramer’s adjustment for multiple comparisons were applied. A P-value P < 0·05 was considered to be significant.
Results
AT inhibits proliferation of CD4+ T cells
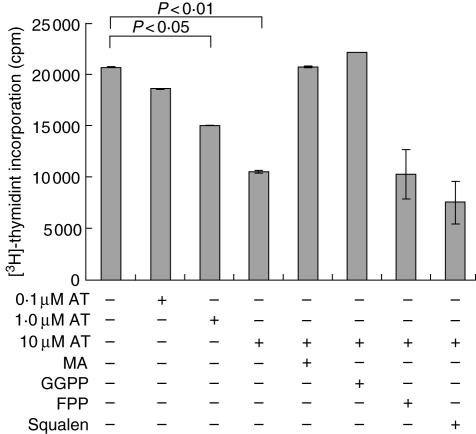
AT dose-dependently inhibited CD3/CD28 mediated proliferation (Fig. 1) as well as proliferation induced by PMA/ionomycin (data not shown) as assessed by [3H]-thymidine incorporation. Inhibition occurred 1 day after stimulation and became maximal after 3–5 days (data not shown). T cell proliferation could be restored by addition of mevalonic acid or geranylgeranyl pyrophosphate, but farnesyl phosphate or squalen could not overcome the inhibitory effect of AT (Fig. 1). This suggests that protein prenylation might be responsible for the observed inhibition of proliferation, rather than cholesterol synthesis, and thus the integrity of lipid rafts.
Fig. 1.
Influence of atorvastatin (AT) on CD4+ T cell proliferation. CD4+ T cell proliferation was determined by [3H]-thymidine incorporation after 3 days. CD4+ T cells were stimulated with anti-CD3 and anti-CD28 (CD3 × CD28). Statistical analysis was performed by analysis of variance (anova) with Tukey–Kramer’s adjustment for multiple comparisons. MA: mevalonic acid; GGPP: geranylgeranyl pyrophosphate; FPP: farnesyl pyrophosphate (n = 3).
Activation of small GTPases is reduced strongly in AT-treated CD4+ T cells
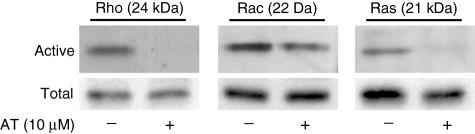
Because protein prenylation is required for small GTPases to become functionally active, we tested the influence of AT on Rho, Rac and Ras activation. CD4+ T cells were cultured overnight in the presence or absence of 10 µM AT and stimulated subsequently for 16 h. As could be demonstrated by pull-down assays, activation of Rho and Ras were inhibited strongly by AT, while this was found to a lesser extent for Rac (Fig. 2).
Fig. 2.
Inhibition of small small guanosine triphosphatases (GTPases) Rho, Ras and Rac. CD4+ T cells were stimulated overnight with phorbol myristate acetate (PMA)/ionomycin in the presence or absence of 10 µM atorvastatin (AT). Activation of small GTP-binding proteins Rho, Rac and Ras was assessed by pull-down assays and subsequent Western blot analysis (n = 3). Densitometry was performed using three independent experiments. This analysis revealed significant differences in Rho, Ras and Rac activation between atorvastatin (AT)-treated and -untreated T cells (P < 0·01).
AT inhibits long-term CD25 expression but not short-term cytokine production
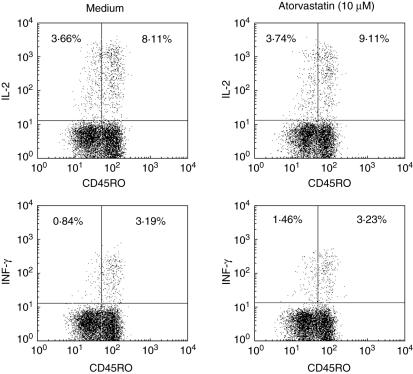
To test if T cell activation was generally impaired by AT, we investigated whether intracellular cytokine expression and surface expression of the early T cell activation marker CD69 were also influenced by AT after short-term T cell stimulation. No influence of AT on IL-2 or IFN-γ (Fig. 3) or on CD69 expression (data not shown) was found 6 h after superantigen (sAG) or PMA/ionomycin stimulation. This was also confirmed by enzyme-linked immunosorbent assay (ELISA) technique assessing the supernatants of T cells 6 h and 24 h after stimulation with PMA/ionomycin. Again, AT did not show any effect on cytokine profiles (data not shown).
Fig. 3.
Influence of atorvastatin on T cell activation. CD4+ T cells were incubated with or without 10 µM atorvastatin (AT) overnight and stimulated with superantigen (SEB) in the presence of anti-CD28/anti-CD49d. After 6 h, cells were permeabilized and stained by incubating with specific primary antibodies. Intracellular cytokine production was measured by fluorescence activated cell sorter (FACS) analysis (n = 3).
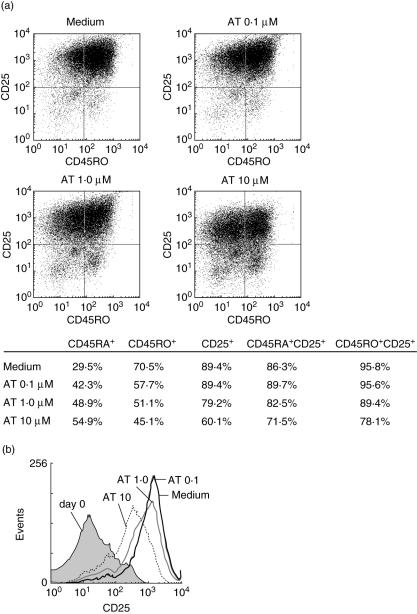
In contrast, prolonged stimulation revealed a clear influence of AT on T cell activation. Three days after PMA/ionomycin stimulation 70·5% of untreated T cells were positive for CD45RO compared to 45·1% of AT (10 µM)-treated cells. Similar results were obtained for CD25. In the naive T cell population (CD45RA+) 86·3% of untreated cells were positive for CD25 compared to 71·5% in AT-treated cells (10 µM). In the memory population (CD45RO+) 95·8% were CD25+ compared to 78·1% in AT-treated cells (10 µM) (Fig. 4a). Not only the percentage of CD25+ cells, but also the mean fluorescence intensity of CD25 was influenced by AT (Fig. 4b). The effects of AT on CD45RO and CD25 were dose-dependent and could be prevented by addition of mevalonic acid and geranylgeranyl pyrophosphate (data not shown).
Fig. 4.
Atorvastatin (AT) inhibits T cell maturation. CD4+ T cells were stimulated with phorbol myristate acetate (PMA)/ionomycin in the presence or absence of various concentrations of AT (0·1–10 µM). After 72 h T cells were stained for CD25 and CD45RO and fluorescence activated cell sorter (FACS) analysis was performed. (a) Dot plot diagrams for CD45RO and CD25. (b) Histogram for CD25. Representative experiments (n = 3) are depicted.
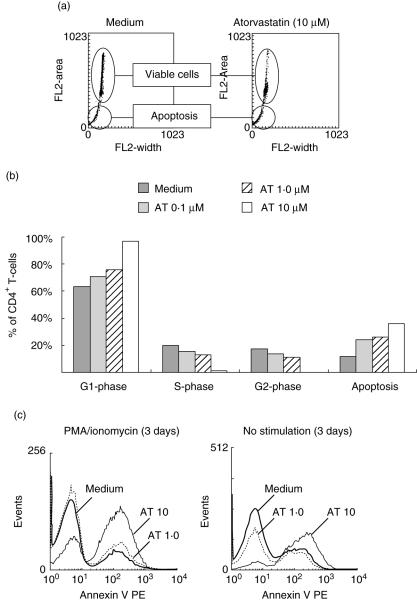
AT treatment leads to cell cycle arrest and apoptosis
PMA/ionomycin stimulation of untreated CD4+ T cells resulted in an increased proportion of T cells in the S- and G2-phase (Fig. 5a,b). In contrast, stimulation in the presence of AT (10 µM) revealed an arrest in cell cycle progression, as evidenced by an increased proportion of CD4+ T cells in the G1-phase. Concomitant with a G1 arrest, there was an increase in DNA fragmentation over time in AT-treated T cells, suggesting the occurrence of apoptosis. In line with this, an increased annexin V expression was found in AT-treated cells. This was not dependent on T cell activation, as apoptosis also occurred in unstimulated T cells (Fig. 5c), and was present in both the naive CD45RA+ and the memory CD45RO+ subpopulation (data not shown). Again, the addition of mevalonic acid or geranylgeranyl pyrophosphate, but not farnesyl phosphate or squalen, could overcome the inhibitory effect of AT on cell cycle proliferation and T cell apoptosis (data not shown).
Fig. 5.
Influence of atorvastatin (AT) on cell cycle progression and induction of apoptosis. CD4+ T cells were stimulated with phorbol myristate acetate (PMA)/ionomycin for 72 h in the presence of various concentrations of AT (0–10 µM). (a) Representative dot plots of treated and untreated cells. (b) Percentage of apoptotic cells was calculated on the basis of all events. Cell cycle progression was measured only in viable cells. Dot plots were transformed into histograms after gating to determine the percentage of each of the cell cycle phases. (c) Staining for annexin V and subsequent fluorescence activated cell sorter (FACS) measurements were performed to assess the amount of apoptotic cells after 72 h (n = 3).
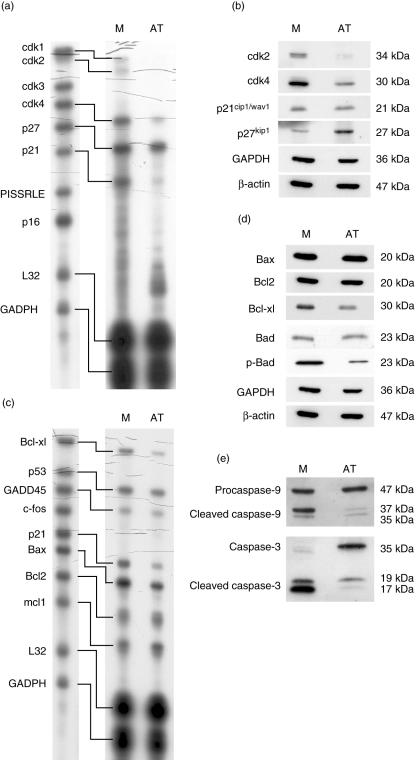
To substantiate further the finding of G1 arrest and increased apoptosis, multi-probe RNase protection assays and Western blot analysis were performed. In AT (10 µM)-treated cells mRNA expression of cdk1, 2 and 4 and p21 was strongly inhibited. On protein levels, we could confirm a reduced expression of cdk2 and 4 in the AT-treated cells, while the expression of p21wav1/cip1 was unchanged. Although no influence of AT on mRNA expression of p27 was observed, protein expression of p27kip1 was clearly increased (Fig. 6a,b).
Fig. 6.
RNase protection assays and Western blot analysis. CD4+ T cells were stimulated with phorbol myristate acetate (PMA)/ionomycin in the presence or absence of 10 µM atorvastatin (AT) for 72 h. RNase protection assays (a + c) and Western blot analysis (b + d) for cell cycle proteins and proteins involved in programmed cell death were performed. Densitometry was performed using three independent experiments. This analysis revealed significant differences between AT-treated and -untreated T cells (P < 0·01).
Inhibition of apoptosis-related mRNA expression was found for Bcl-xl and Bax in AT-treated cells. In addition the expression of Bcl-xl, but not Bax, was decreased in Western blot analysis (Fig. 6c,d). Furthermore, Western blot analysis revealed a strongly reduced phosphorylation of Bad in the presence of AT, although the overall amount of Bad was unchanged. Interestingly, activation of caspase 9 and caspase 3 was inhibited by AT, despite increased apoptosis in AT-treated T cells (Fig. 6e).
Discussion
How statins influence T cell activation is of utmost importance to understand the beneficial effect of these compounds on inflammatory conditions. In the present paper, we have analysed how AT inhibits T cell proliferation. Our data suggest that AT does not inhibit T cell activation in general, as intracellular cytokine and surface CD69 expression were not influenced after short-term T cell stimulation. In contrast, markers for long-term T cell activation, i.e. CD25 expression and skewing of CD45RA to CD45RO, were diminished by AT. Moreover, cell-cycle arrest in G1 occurred together with an increase in apoptosis. The latter was independent of T cell activation and not related mechanistically to caspase activation. These effects occurred using concentrations of 0·1–10 µM of AT, and are well within the therapeutic range measured in human plasma [29,30].
Studies on T cell activation have shown that TCR signalling depends critically on functional compartmentalization of membrane lipids into ordered microdomains, or lipid rafts. The significance of lipid rafts lies, on one hand, in their ability to recruit signalling partners that assemble into a mature IS, and on the other hand in their ability to regulate actin dynamics to achieve polarization and stabilization of the IS. Rho, Ras and Rac GTPases are key players in orchestrating TCR signalling. Activation of these molecules can be influenced in various ways, such as by inhibition of GDP/GTP exchange factors (GEF), by GDP dissociation inhibitors or by inhibiting membrane targeting [31]. Statins inhibit the mevalonate pathway and consequently protein prenylation, and therefore might prevent anchorage of small GTPases to cell membranes. Moreover, statins might influence the composition of lipid rafts by decreasing the cellular cholesterol content [32]. Ghittoni et al., however, observed no effects of simvastatin on intracellular cholesterol levels, raft integrity or on initial tyrosine phosphorylation following T cell activation [28]. In addition, Aktas et al. showed an unaffected calcium influx upon T cell activation in AT-treated cells [33]. It therefore seems that the IS as a signalling device is not completely impaired by statins. In line with Ghittoni et al. and Aktas et al., our own data demonstrate that early expression of IL-2 or CD69 is not influenced by AT.
Previously published observations in animal models revealed that treatment with statins modulates T cell differentiation towards a Th2 phenotype with an increased production of IL-10 and a decreased secretion of IFN-γ [10,11,27]. In our study AT did not influence cytokine production in general, measured early after superantigen (SEB) or PMA/ionomycin (data not shown) stimulation. Our results are supported by a study in experimental autoimmune uveitis using human-derived T cells, where no differences in cytokine production after AT treatment were observed [34]. It still remains to be elucidated why AT influences cytokine production of T cells from rodents but has no effect on cytokine profiles obtained from human T cells.
Although early T cell activation markers seem not to be affected by AT, a strong inhibitory effect of AT on T cell proliferation was observed after 72 h of stimulation, suggesting that full T cell activation was clearly influenced. In line with this, the expression of CD25 and skewing of naive CD45RA to memory CD45RO was reduced by AT.
Inhibition of T cell proliferation was due most probably to both cell cycle arrest and increased apoptosis. Cell cycle arrest occurred in the G1-phase and was reflected by an increased protein expression of p27kip1, which is known to govern cell cycle progression in the late G1 phase. We also show that the induction of p21 and various cyclin-dependent kinases on transcriptional level are abolished in AT-treated T cells. Similar effects of statins have also been described in other cell types [33,35].
Increased apoptosis was not dependent on T cell activation and occurred in both CD45RA+ and CD45RO+ T cells. In stimulated T cells we observed that cycle arrest occurred before apoptosis was evident, suggesting that apoptosis and cell cycle arrest were two independent events.
Blocking HMG-CoA reductase and mevalonate synthesis prevents post-translational protein prenylation of certain cell-signalling proteins [20]. Among the small GTP-binding proteins, Rho, Rac and Ras are involved in cell cycle regulation [36–39]. AT inhibited activation of Rho and Ras and, to a smaller extent, of Rac. Because Rho and Ras require geranylation to become functionally active [31], this might explain why the addition of mevalonic acid or geranylgeranyl pyrophosphate, but not farnesyl pyrophosphate or squalen, was able to overcome the inhibitory effects of AT on T cells. In contrast, Ras needs to be farnesylated [31]. Although AT inhibits Ras activation, addition of FPP did not overcome the inhibitory effects of AT on T cell proliferation or apoptosis.
Bcl-xl acts as an anti-apoptotic factor by governing mitochondrial permeability and catalysing mitochondrial outer membrane polarization (MOMP) [40–43]. Expression of Bcl-xl is under the control of nuclear factor kappa B (NFκB) activation [44]. Its counterpart, Bad, the physiological function of which is determined by phosphorylation, is localized in the cytosol as phosphorylated protein. Upon dephosphorylation Bad wanders into mitochondria and acts as a pro-apoptotic factor by interfering with Bcl-xl. Although the expression of Bad was not influenced by AT treatment, a decreased phosphorylation was observed. This might influence the balance between Bcl2 family members in favour of cell death, thus triggering pro-apoptotic mitochondrial pathways. Release of cytochrome c triggers the cleavage of pro-caspase 9, finally resulting in the activation of effector caspases, i.e. caspases 3, 6 and 7. Cleavage of caspases was observed mainly in stimulated T cells not treated with AT, indicative of activation-induced cell death [36]. Therefore, AT-mediated apoptosis was not caspase-dependent. This is in contrast to earlier reports by Cafferio et al., who claimed that AT induces activation of caspases 3 and 9 [45]. Their experiments, however, were performed with Jurkat lymphoma T cells and not with primary human T cells from healthy volunteers, as used in this study. Evidence for caspase-independent apoptosis has been shown previously in T cells [43], although the importance of this finding in terms of T cell homeostasis still remains to be defined.
In conclusion, AT treatment is not influencing activation of human T cells a priori. However, AT seems to interfere with sustained T cell activation by inhibiting small GTPase activation, causing an arrest in the late G1 phase and, finally, inducing apoptosis.
References
- 1.Bellosta S, Ferri N, Arnaboldi L, Bernini F, Paoletti R, Corsini A. Pleiotropic effects of statins in atherosclerosis and diabetes. Diabetes Care. 2000;23(Suppl. 2):B72–8. [PubMed] [Google Scholar]
- 2.Foody JM, Nissen SE. Effectiveness of statins in acute coronary syndromes. Am J Cardiol. 2001;88:31F–5F. doi: 10.1016/s0002-9149(01)01875-6. [DOI] [PubMed] [Google Scholar]
- 3.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 5.Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–8. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 6.Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- 7.Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci. 2002;23:482–6. doi: 10.1016/s0165-6147(02)02077-1. [DOI] [PubMed] [Google Scholar]
- 8.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 9.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 10.Leung BP, Sattar N, Crilly A, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. doi: 10.4049/jimmunol.170.3.1524. [DOI] [PubMed] [Google Scholar]
- 11.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Miyauchi K, Daida H. Early intervention with atorvastatin modulates TH1/TH2 imbalance in patients with acute coronary syndrome: from bedside to bench. Circulation. 2004;109:e213–4. doi: 10.1161/01.CIR.0000127616.70152.5D. author reply e–4. [DOI] [PubMed] [Google Scholar]
- 13.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–19. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–30. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–69. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 16.Davignon J, Mabile L. [Mechanisms of action of statins and their pleiotropic effects] Ann Endocrinol (Paris) 2001;62:101–12. [PubMed] [Google Scholar]
- 17.Hausding M, Witteck A, Rodriguez-Pascual F, von Eichel-Streiber C, Forstermann U, Kleinert H. Inhibition of small G proteins of the rho family by statins or clostridium difficile toxin B enhances cytokine-mediated induction of NO synthase II. Br J Pharmacol. 2000;131:553–61. doi: 10.1038/sj.bjp.0703607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortego M, Bustos C, Hernandez-Presa MA, et al. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis. 1999;147:253–61. doi: 10.1016/s0021-9150(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 19.Essig M, Nguyen G, Prie D, Escoubet B, Sraer JD, Friedlander G. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells. Role of geranylgeranylation and Rho proteins. Circ Res. 1998;83:683–90. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]
- 20.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–8. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–77. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 22.Mackay DJ, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–8. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 23.Macara IG, Lounsbury KM, Richards SA, McKiernan C, Bar-Sagi D. The Ras superfamily of GTPases. Faseb J. 1996;10:625–30. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 24.Somsel Rodman J, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–92. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]
- 25.Andre P, Boretto J, Hueber AO, et al. A dominant-negative mutant of the Rab5 GTPase enhances T cell signaling by interfering with TCR down-modulation in transgenic mice. J Immunol. 1997;159:5253–63. [PubMed] [Google Scholar]
- 26.Cantrell DA. GTPases and T cell activation. Immunol Rev. 2003;192:122–30. doi: 10.1034/j.1600-065x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 27.Waiczies S, Prozorovski T, Infante-Duarte C, et al. Atorvastatin induces T cell anergy via phosphorylation of ERK1. J Immunol. 2005;174:5630–5. doi: 10.4049/jimmunol.174.9.5630. [DOI] [PubMed] [Google Scholar]
- 28.Ghittoni R, Patrussi L, Pirozzi K, et al. Simvastatin inhibits T cell activation by selectively impairing the function of Ras superfamily GTPases. Faseb J. 2005;19:605–7. doi: 10.1096/fj.04-2702fje. [DOI] [PubMed] [Google Scholar]
- 29.Lea AP, McTavish D. Atorvastatin: a review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997;53:828–47. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- 30.Stern RH, Yang BB, Hounslow NJ, MacMahon M, Abel RB, Olson SC. Pharmacodynamics and pharmacokinetic-pharmacodynamic relationships of atorvastatin, an HMG-CoA reductase inhibitor. J Clin Pharmacol. 2000;40:616–23. [PubMed] [Google Scholar]
- 31.Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–12. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 32.Ehrenstein MR, Jury EC, Mauri C. Statins for atherosclerosis − as good as it gets? N Engl J Med. 2005;352:73–5. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 33.Aktas O, Waiczies S, Smorodchenko A, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–33. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas PB, Albini T, Giri RK, See RF, Evans M, Rao NA. The effects of atorvastatin in experimental autoimmune uveitis. Br J Ophthalmol. 2005;89:275–9. doi: 10.1136/bjo.2004.050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danesh FR, Sadeghi MM, Amro N, et al. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/p21 signaling pathway: implications for diabetic nephropathy. Proc Natl Acad Sci USA. 2002;99:8301–5. doi: 10.1073/pnas.122228799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–3. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 37.Hughes DA. Control of signal transduction and morphogenesis by Ras. Semin Cell Biol. 1995;6:89–94. doi: 10.1016/1043-4682(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 38.Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–4. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 39.Albanese C, Johnson J, Watanabe G, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–97. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- 40.Kim R, Emi M, Tanabe K. Caspase-dependent and -independent cell death pathways after DNA damage [Review] Oncol Rep. 2005;14:595–9. [PubMed] [Google Scholar]
- 41.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–9. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 42.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–30. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 43.Jaattela M, Tschopp J. Caspase-independent cell death in T lymphocytes. Nat Immunol. 2003;4:416–23. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 44.Tsukahara T, Kannagi M, Ohashi T, et al. Induction of Bcl-x(L) expression by human T cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T cell transfectants with Tax. J Virol. 1999;73:7981–7. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cafforio P, Dammacco F, Gernone A, Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005;26:883–91. doi: 10.1093/carcin/bgi036. [DOI] [PubMed] [Google Scholar]