Abstract
Although it is believed widely that distinct patterns of the host immune response are associated with the outcome of chronic human T cell lymphotropic virus type 1 (HTLV-I) infection toward asymptomatic or symptomatic neurodegenerative myelopathy (HAM/TSP), the exact mechanism underlying these immunological events still remains unknown. In this study, we have evaluated the cytokine pattern [interleukin (IL)-12, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, IL-4 and IL-10] of innate and adaptive immunity cells present at the peripheral blood from non-infected (NI) and HTLV-I infected individuals [asymptomatic (AS), oligosymptomatic (OL) and HAM/TSP-HT], following in vitro short-term incubation in the absence/presence of phorbol myristate acetate (PMA) pan-leucocyte stimulation. In the absence of PMA stimulation, our data demonstrate that despite the overall immunological profile of AS mimicry that observed for NI, the high frequency of IL-12+ neutrophils and TNF-α+ monocytes are also a hallmark of this group of individuals. However, the outstanding positive correlation between the high frequency of TNF-α+ monocytes and high levels CD4+ IL-10+ and CD8+ IL-10+ T cells suggests the establishment of immunoregulatory mechanisms that guarantee their asymptomatic clinical status. On the other hand, OL and HT did not present any association between the high frequency and TNF-α+ neutrophils and monocytes and this immunoregulatory profile at their adaptive immunity cells. Upon PMA-index analysis, high levels of type 1 CD4+ T cells, as well as higher IFN-γ/IL-10 and TNF-α/IL-10 ratios, were observed in HT, and re-emphasize the role of Th1-cytokines from CD4+ cells to HTLV-I immunity and disease. Moreover, increasing frequency of CD8+ IFN-γ+ and CD8+ TNF-α+ cells were observed in the HT, which corroborates the marked inflammatory profile underlying this pathological condition and the role of CD8+ T cells in the pathogenesis of HAM/TSP.
Keywords: asymptomatic carries, HAM/TSP, HTLV-I, IL-10, TNF-α
Introduction
The human T cell lymphotropic virus type 1 (HTLV-I), a retrovirus belonging to the oncovirus subfamily, is associated with neoplasic disorders as well as degenerative inflammatory diseases, including HTLV-associated uveitis and HTLV-I-associated myelopathy or tropical spastic paraparesis (HAM/TSP) [1–3]. This neurological disorder, observed in approximately 3–5% of HTLV-I-infected individuals, is characterized by a chronic progressive demyelinating inflammatory disease sited frequently in the spinal cord and white matter of the central nervous system (CNS). HAM/TSP patients frequently present gait disturbance, weakness and stiffness of the lower limbs, lumbar pain, sexual impotence, spasticity, and bladder and bowel dysfunction [4]. Although it is believed widely that host immune responses are involved in the pathogenesis of HAM/TSP, the exact mechanism and the role of the immune system in the development/maintenance of this pathological condition still remains unknown and constitutes the target of current investigations. Several studies have suggested that the pattern of the host cellular immune response to HTLV-I-infection, associated with high proviral load, may be relevant to trigger the development of severe neurological disease [5–8]. The participation of immunological events is also attributed to a large number of other abnormal immunological features, such as hypergammaglobulinaemia with high levels of anti-HTLV-I antibodies, as well as elevated levels inflammatory cytokines [9]. Moreover, HAM/TSP patients display a higher frequency of CD4+ and CD8+ lymphocytes within inflammatory infiltrates at neuronal sites, with a predominance of the CD8+ T cell subset with the progression of the disease [8,10]. Recently, we have demonstrated that an increase of both T/B cells ratio and percentage of CD8+ human leucocyte antigen (HLA)-DR+ lymphocytes and a decrease of B lymphocytes may be important biomarkers of disease progression toward HAM/TSP [11]. Therefore, there is a consensus that CD8+ T lymphocytes represent the pivotal element in the pathogenesis of HAM/TSP favouring a proinflammatory microenvironment compatible with chronic inflammatory disease. This microenvironment has been characterized by increased levels of type 1 cytokines as interferon (IFN)-γ, tumour necrosis factor (TNF)-α and interleukin (IL)-2 [12]. Although most studies have demonstrated the contribution of T lymphocytes for the proinflammatory cytokine profile associated with tissue damage in HAM/TSP, few studies have focused on the contribution of the immune response, both innate and adaptive, to the establishment of immunoregulatory mechanism underlying the asymptomatic infection.
In this work we performed a flow cytometric analysis of intracytoplasmic cytokines, including IL-12, IFN-γ, TNF-α, IL-4 and IL-10 of innate and adaptive immunity cells present in the peripheral blood of HTLV-I-infected individuals aiming to understand more clearly the dynamic of immunological events during HTLV-I infection. Besides re-emphasizing the role of inflammatory cytokines derived from CD8+ T cells to the pathogenesis of HAM/TSP, in a pioneer investigation our findings indicated IL-10 produced by CD4+ and CD8+ T cells as a putative immunoregulatory mechanism to counterbalance monocyte-derived TNF-α and guarantee the asymptomatic clinical status during chronic HTLV-I infection.
Materials and methods
Study population
The study population comprised 47 HTLV-I-infected individuals, classified as asymptomatic (AS): 10 males and 12 females, age median 34 years; oligosymptomatic (OL): five males and eight females, age median 41 years; and HAM/TSP (HT): five males and seven females, age median 49 years. Upon confirmatory diagnosis of HTLV-I infection, throughout positive serology by enzyme-linked immunosorbent assay (ELISA) and Western blot, the patient's clinical status was defined by two specialist attending physicians from our research staff (B. C. S. and J. G. R.) following the clinical score and impairment scale recommended by the American Spinal Injury Association (ASIA).
HTLV-I-infected individuals enrolled into AS and OL groups were contacted at the Hemominas Foundation in Belo Horizonte, Minas Gerais, Brazil. The AS group had no clinical complaints and presented normal motor and sensory functions. The OL patients (considered symptomatic not-HAM/TSP patients) displayed impairment of the tendon reflexes (hyper- or hyporeflexis) and vesical impairment, including urinary dysfunctions, paraesthesia, lumbar pain and sexual dysfunction. However, it is important to mention that patients enrolled into the OL group did not present sufficient clinical signs, according to ASIA, to be classified as HT. The HT group received medical assistance at the Sarah Kubitschek Hospital in Belo Horizonte, Minas Gerais, Brazil. These individuals presented the classical set of symptoms of HAM/TSP according to the standard neurological ASIA impairment scale classification.
All HTLV-I-infected patients included in this study presented negative serology for other relevant blood-borne pathogens, including HIV, hepatitis C virus (HCV), hepatitis B virus (HBV), syphilis and Chagas' disease. The use of corticosteroids or other immunosuppressive chemotherapy was considered as an exclusion criterion prior to the blood collection procedure.
Healthy, non-infected volunteers, with negative serology for HTLV-I, as well as the above-mentioned blood-borne pathogens considered apt for blood donation, were included in the control group of non-infected individuals (NI): seven males and nine females, age median 32 years.
Informed written consent was obtained from all participants. This work complied with the resolution number 196/1996 from the National Health Council for research evolving humans and was approved by the Ethical Committee at the Hemominas Foundation (27 October 1997) and Centro de Pesquisas René Rachou, Oswaldo Cruz Foundation (protocol no. 04/2001), Belo Horizonte, Minas Gerais, Brazil.
Specific monoclonal antibodies used on flow cytometric analysis
The specific monoclonal antibodies (MoAbs) used were purchased from Pharmingen (San Diego, CA, USA), including anti-human IgG1-fluorescein isothiocyanate (FITC) clone 679·1Mc7, anti-human IgG2a-phycoerythrin (PE) clone U7·27, anti-human CD4-FITC clone 13B8·2, anti-human CD8-FITC clone B9·11, anti-human CD16-PE 3G8 and anti-CD14-FITC (clone M0P9). The anti-human cytokines PE-labelled (PE) MoAbs included anti-TNF-α (clone MOAB11), anti-IL-12p40/p70 (clone C11-5·14), anti-IFN-γ (clone B27), anti-IL-4 (clone MP4–25D2) and anti-IL-10 (clone JES3–9D7).
Short-term whole blood culture in vitro
Peripheral blood samples were collected into Vacutainer tubes containing sodium heparin (Vacutainer; BectonDickinson, San Jose, CA, USA), and 500 µl aliquots were dispensed into individual 17 × 100 mm polypropylene tubes (Falcon 2059, Becton Dickinson, San Jose, CA, USA). In this study, analysis of the cytokine profile was first evaluated after short-term incubation in vitro in the absence of exogenous stimuli. This condition was chosen considering that the detection of cytokines, particularly in the absence of exogenous stimuli, may reflect the dynamic events of cytokine production in blood leucocyte subsets in vivo. For this purpose, whole blood samples were incubated for 4 h at 37°C in a 5% CO2 humidified atmosphere in the presence of 500 µl of RPMI-1640 (Gibco, Grand Island, NY, USA) plus Brefeldin A (BFA) (Sigma Chemical Co., St Louis, MO, USA), that inhibit cytokine secretion, at a final concentration of 10 µg/ml. The non-specific phorbol myristate acetate (PMA) pan-leucocyte stimulation was performed by incubation of 500 µl of aliquots of whole blood in the presence of RPMI-1640 plus PMA (Sigma) at a final concentration of 25 ng/ml, ionomycin (Sigma) at 1 µg/ml and BFA. Blood samples were incubated for 4 h at 37°C in a 5% CO2 humidified incubator. After this incubation time, all cultures were treated with 2 mM ethylenediamine tetraacetic acid (EDTA) (Sigma) and incubated at room temperature for 15 min.
Flow cytometric immunostaining for intracellular cytokine
Cultured whole blood samples were washed with 6 ml of fluorescence activated cell sorter (FACS) buffer containing 0·015 M phosphate buffered saline (PBS), 0·5% bovine serum albumin (BSA) and 0·1% sodium azide (Sigma). All centrifugations were performed at 600 g at room temperature for 7 min. After resuspension in 1 ml of FACS buffer, 200 µl aliquots were dispensed into two 12 × 75 mm polystyrene tubes (Falcon no. 2052, Becton Dickinson, San Jose, CA, USA) and stained individually with the manufacturer's recommended amounts of MoAbs anti-CD4-FITC, anti-CD8-FITC, anti-CD14-FITC and anti-CD16-FITC in the dark for 30 min at room temperature. Stained samples were treated by vortexing gently with 2 ml of FACS lysing solution (Becton Dickinson, San Jose, CA, USA) and reincubated for an additional 10 min. After erythrocyte lysis was completed, the samples were centrifuged, the supernatant discarded and the cell pellet incubated with 2 ml of FACS permeabilizing solution containing FACS buffer and 0·5% of saponin (Sigma) in the dark for 10 min at room temperature. Following incubation the samples were centrifuged, the supernatant decanted gently and 3 ml of FACS buffer added to each tube. After centrifugation, the cells were stained with 20 µl of PE-labelled anti-cytokine MoAb (anti-TNF-α, anti-IL-12, anti-IFN-γ, anti-IL-4 and anti-IL-10) previously diluted 1 : 25 in sterile FACS permeabilizing solution and distributed in a 96-well U-bottomed microplate (Thomas 9383–A90). Cells were incubated in the dark for 30 min at room temperature. After incubation, the cells were washed with 150 µl of FACS permeabilizing solution followed by 200 µl of FACS buffer. After washing, the cell preparation were fixed in 200 µl of FACS fixing solution containing 10 g/l paraformaldehyde, 10·2 g/l sodium cacodylate and 6·63 g/l sodium chloride (Sigma), pH 7·2. The samples were stored at 4°C in the dark and analysed by flow cytometry within 24 h.
Flow cytometric acquisition and analysis
A total of 30 000 events/tube were acquired using a FACScan flow cytometer (Becton Dickinson) correctly set up to measure forward (FSC) and side (SSC) light scatters, FITC (FL-1) and PE (FL-2) fluorescence. CellQuest™ software provided by the manufacture was used for data acquisition and analysis.
Selective analysis of neutrophils was performed by establishing a specific scatter gate using the combination of anti-cell surface antigens and laser side scatter (SSC) to discriminate the neutrophils as SSChigh CD16high+. Following the selection of neutrophil population, the prevalence of cytokine-expressing cells was determined using quadrant statistics over FL2/anti-cytokine-PE dot-plots. Analysis of cytokine-positive monocytes was performed by double-staining immunophenotyping using anti-CD14-FITC and anti-cytokine-PE-labelled MoAbs. Monocytes were first selected as SSClow CD14high+ cells, using FL1/anti-CD14-FITC versus SSC dot-plots. Cytokine expression was then measured in terms of percentage of positive events within CD14high+ cells. Identification of the lymphocytes subsets was performed by the dual-colour immunophenotyping method using specific anti-surface-FITC and anti-cytokine-PE-labelled MoAbs. Initially, a lymphocyte scatter gate was set up, using FSC versus SSC dot-plots followed by the identification of cytokine-positive cells using FL1/anti-cell marker-FITC versus FL2/anti-cytokine-PE dot-plots.
All results were expressed as percentage of cytokine-positive cells for different gated leucocyte subpopulations analysed in this study, selected as described above. The numbers of subjects displayed in the figures may differ due to methodological restrictions, including insufficient numbers of cells as well as the autofluorescence interference on the flow cytometric data.
Statistical analysis
Statistical analysis was performed by non-parametric methods using analysis of variance (anova) Kruskal–Wallis test followed by Dunn's multiple comparison test. Spearman's (rS) rank correlations were computed to assess correlations between intracytoplasmic parameters. Statistical analysis was provided by the software GraphPad Prism 3·0.3(San Diego, CA, USA). The association analysis was performed by χ2 test. Statistically significant differences were considered when P < 0·05.
Results
High frequency of monocytes expressing proinflammatory cytokine TNF-α is observed in HTLV-I-infected individuals regardless of their clinical status
In order to evaluate the engagement of innate immunity on different clinical status of HTLV-I infection, we have characterized a detailed investigation of intracytoplasmic cytokine profile of circulating neutrophils and monocytes after short-term in vitro incubation, particularly in the absence of exogenous stimuli. For this purpose we have analysed type 1 (IL-12, IFN-γ and TNF-α) and type 2 (IL-4 and IL-10) cytokines at single-cell level, using the combination of anti-cell surface antigens and laser SSC to discriminate and gated the neutrophils as SSChigh CD16high+ and monocytes as SSClow CD14high+ cells. These blood cellular populations are not usually examined in studies regarding HAM/TSP pathogenesis.
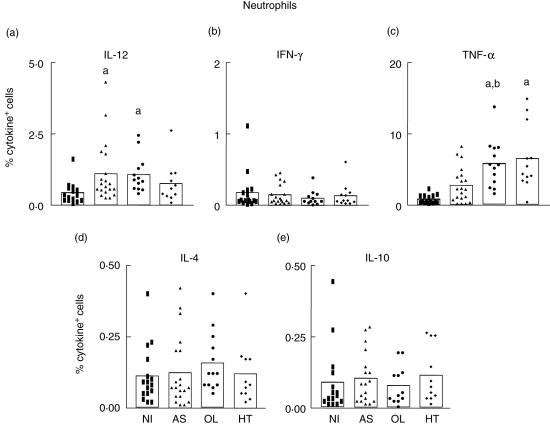
Our data demonstrate a high frequency of IL-12-expressing neutrophils in AS and OL compared to the NI group (Fig. 1a). Analysis of TNF-α demonstrated an elevated frequency of TNF-α+ neutrophils in OL compared to NI and AS groups and a high frequency of TNF-α+ neutrophils in HT individuals compared to the NI group (Fig. 1c). No differences were observed in the frequency of IFN-γ+, IL-4+ and IL-10+ neutrophils among the analysed groups (Fig. 1b,d,e, respectively).
Fig. 1.
Intracellular cytokine profile of circulating neutrophils in peripheral blood from non-infected (NI) (▪ = 16), asymptomatic (AS) (▴ = 20), oligosymptomatic (OL) (• = 13) and HAM/TSP (HT) ♦ = 11) individuals, after short-term in vitro incubation, particularly in the absence of exogenous stimuli. The frequency of cytokine-expressing neutrophils was determined within gated SSChigh CD16high+ cells. The results are expressed as scattering of individual values and mean percentage of interleukin (IL)-12+ (a), interferon (IFN)-γ+ (b), tumour necrosis factor (TNF)-α+ (c), IL-4+ (d) and IL-10+ (e) neutrophils. Letters ‘a’ and ‘b’ represent statistically significant differences (P < 0·05) compared to non-infected (NI) and asymptomatic (AS) groups, respectively.
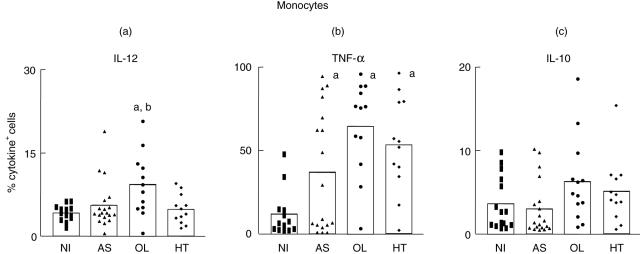
As shown in Fig. 2a, an elevated frequency of IL-12-expressing monocytes was observed in OL compared to NI and AS groups. Our data also demonstrate an elevated frequency of TNF-α+ monocytes in AS, OL and HT compared to the NI group (Fig. 2b). No difference was observed in the frequency of IL-10+ monocytes among the analysed groups (Fig. 1c).
Fig. 2.
Intracellular cytokine profile of circulating monocytes in peripheral blood from non-infected (NI) (▪ = 13), asymptomatic (AS) ▴ = 19), oligosymptomatic (OL) (• = 12) and HAM/TSP (HT) (♦ = 12) individuals after short-term in vitro incubation, particularly in the absence of exogenous stimuli. A double-staining immunophenotyping platform was used to identify cytokine-positive cells within monocytes selected as SSClow CD14high+ leucocytes. The results are expressed as scattering of individual values and mean percentage of interleukin (IL)-12+ (a), tumour necrosis factor (TNF)-α+ (b) and IL-10+ (c) monocytes. Letters ‘a’ and ‘b’ represent statistically significant differences (P < 0·05) compared to NI and AS groups, respectively.
IL-10 produced by CD4+ and CD8+ T cells emerge as a putative immunoregulatory mechanism to counterbalance the monocyte-derived TNF-α in AS patients
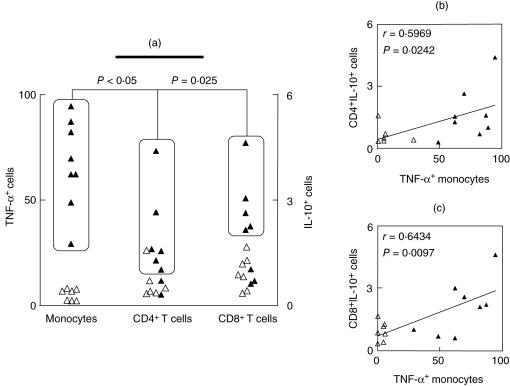
Despite the importance of the proinflammatory cytokine profile in the disease outcome associated with OL, and especially HT clinical forms, our data also demonstrated that AS presented an elevated frequency of TNF-α+ monocytes (Fig. 2b). This unexpected finding raises an intriguing question: which immunological feature could compensate the high levels of monocyte-derived proinflammatory cytokine in AS, in order to guarantee their asymptomatic clinical status? Interestingly, our data indicated that AS individuals who presented a high frequency of TNF-α+ monocytes also displayed high levels of IL-10+ cells within their CD4+ and CD8+ T lymphocytes (Fig. 3a). Analysis of individual data confirmed this association between the low frequency of TNF-α+ monocytes with a low frequency of CD4+ IL-10+ and CD8+ IL-10+ T cells in AS (Fig. 3a, rectangles). Correlation analysis validated these findings further, showing a positive correlation between TNF-α+ monocytes/CD4+ IL-10+ T cells and TNF-α+ monocytes/CD8+ IL-10+ T cells (Fig. 3b,c, respectively).
Fig. 3.
Analysis of major discriminatory immunophenotypes of asymptomatic (AS) individuals: (a) Analysis of individual data demonstrates an association between high frequency of tumour necrosis factor (TNF)-α+ monocytes with high frequency of CD4+ IL-10+ and CD8+ IL-10+ T cells in AS (▴). The boxed regions represent subgroups of patients, highlighting the association between the individuals displaying high levels of TNF-α+ monocytes as well as IL-10+ CD4+ and IL-10+ CD8+ lymphocytes. Analysis of individual data demonstrates an association (dotted rectangles and lines) between the low frequency of TNF-α+ monocytes with low frequency of CD4+ IL-10+ and CD8+ IL-10+ T cells in AS (▵). χ2 analysis demonstrated that the associations are significant and the P-values are shown in the figure. (b, c) Confirmatory correlation analysis validates the association between TNF-α+ monocytes with the frequency of CD4+ IL-10+ and CD8+ IL-10+ T cells. Correlation indexes (r and P-values) are shown in the figure.
Similar analyses were carried out with data generated from OL and HT individuals. However, we did not observe any association or correlation between the high levels of monocyte-derived proinflammatory cytokines and the levels of IL-10 produced by the adaptive immunity cells (data is not shown).
High levels of type 1 CD4+ T cells leading to higher IFN-γ/IL-10 and TNF-α/IL-10 ratios in HT re-emphasize the role of inflammatory Th1-cytokine phenotype in CD4+ cells to HTLV-I immunity and disease.
Levels of both pro- and anti-inflammatory cytokine-expressing cells within the circulating lymphocyte population were investigated in NI, AS, OL and HT individuals. The identification of the lymphocytes subsets was performed initially using FSC versus SSC dot-plots followed by the identification of cytokine-positive cells using FL1/anti-cell marker-FITC versus FL2/anti-cytokine-PE dot-plots.
Results were calculated as the denominator between the intracellular cytokine profiles observed after short-term in vitro incubation with pan-leucocyte stimuli and the percentage of cytokine-positive cells detected in the control cultures (PMA index). As demonstrated in Table 1, significant higher PMA indexes for CD4+ T cells expressing type 1 cytokines, including INF-γ and TNF-α, were observed in HT.
Table 1.
Intracellular cytokine profile of T lymphocytes subpopulations CD4+ and CD8+ in peripheral blood from non-infected (NI), asymptomatic (AS), oligosymptomatic (OL) and HAM/TSP (HT) individuals after in vitro short-term stimulation.*
| Pan-leucocyte short-term stimulation in vitro (PMA index) | ||||
|---|---|---|---|---|
| Cell phenotype | NI | AS | OL | HT |
| CD4+ T cells | ||||
| INF-γ+ | 9·3(1·7–25·9) | 10·0(0·6–34·7) | 8·8(1·2–101·8) | 23·2(5·9–115·0)b |
| TNF-α+ | 14·4(1·7–53·7) | 14·9(0·6–95·1) | 28·9(2·1–74·9) | 37·0(18·3–115·8)a,b |
| IL-10+ | 1·5(0·2–3·0) | 1·0(0·2–4·8) | 1·5(0·2–7·1) | 1·0(0·2–7·5) |
| IFN-γ+/IL-10+ | 6·0(0·6–1·5.5) | 8·1(0·9–25·1) | 8·8(0·9–81·4) | 12·2(4·8–88·0)a,b |
| TNF-α+/IL-10+ | 13·6(0·7–36·7) | 7·4(0·5–36·9) | 23·6(1·6–153·4) | 35·6(8·0–135·9)a,b |
| CD8+ T cells | ||||
| INF-γ+ | 14·4(3·1–31·7) | 16·2(0·9–79·5) | 20·4(1·4–130·7) | 38·8(17·5–175·9)a,b |
| TNF-α+ | 6·8(0·5–45·5) | 11·8(0·4–45·6) | 17·8(0·7–56·6) | 23·3(5·5–60·2)a |
| IL-10+ | 0·4(0·1–3·9) | 0·6(0·1–4·9) | 1·1(0·2–5·1) | 0·8(0·2–1·1) |
| IFN-γ+/IL-10+ | 26·2(3·3–85·3) | 13·4(1·7–113·9) | 22·7(0·6–148·2) | 46·0(17·5–314·6)a,b |
| TNF-α+/IL-10+ | 17·6(1·6–23·3) | 14·8(1·1–98·2) | 21·0(0·3–66·2) | 26·4(5·1–201·3)a |
Data are expressed as phorbol myristate acetate (PMA) index, calculated as the ratio between the frequency of cytokine positive cells observed in the PMA-stimulated culture by the one detected in the control culture. Results are presented as mean PMA index of cytokine-expressing cells ± standard deviation. NI: n = 16; AS: carriers: n = 22; OL: n = 13; and HT: n = 12.
Statistically significant differences (P < 0·05) observed in the HT groups compared to the NI and AS groups, respectively.
Additional analysis also revealed increased IFN-γ/IL-10 and TNF-α/IL-10 ratios in HT, re-emphasizing the role of inflammatory Th1 cytokines derived from CD4+ T cells to the pathogenesis of HAM/TSP (Table 1).
Increasing levels of CD8+ IFN-γ+ and CD8+ TNF-α+ T cells highlighted the higher frequency of CTL precursor of HT patients, re-emphasizing the role of CD8+ T cells in the pathogenesis of HAM/TSP
Analysis of the type 1 cytokine profile in CD8+ T cells, which probably reflects CTL precursor frequency, has also been expressed as the PMA index among the groups analysed (Table 1). Data analysis revealed a predominant type 1 immune response in CD8+ T cells from HT individuals, which may reflect the increasing frequency of CTL precursor already well documented in the literature of HAM/TSP patients. Our data also demonstrated an increased ratio of CD8+ IFN-γ+/CD8+ IL-10+ and CD8+ TNF-α+/CD8+ IL-10+ T cells in HT (Table 1).
Discussion
Several studies have suggested that the host cellular immune response to HTLV-I infection plays a pivotal role in the development of the severe neurological disease known as HAM/TSP syndrome [5–8]. Because cytokines control both the intensity and the quality of the immune response, changes in the pattern of proinflammatory and regulatory cytokines may be related to immunological events underlying the risk factors that are involved in the HTLV-I-related diseases. A possibility is that leucocytes from HAM/TSP patients expressing higher levels of type 1 cytokines are prone to trigger the inflammatory events in the CNS. The resulting cellular recruitment to affected sites would promote/maintain the microenvironment that contributes to HTLV-I associate neurological damage. It has been proposed that inflammation and activated immune response may induce T cell proliferation, including HTLV-I-infected T cells, which leads to an increased amount of HTLV-I provirus load as well as tissue damage [13]. However, the benefits of the immune system coupled to lower risks of HTLV-I-associated diseases, resulting in a situation whereby the cytokines milieu confers protection and predisposes lower disease morbidity, are also well recognized [14]. Despite the relevance of the immunological status driving distinct patterns of HTLV-I chronic infection, the precise mechanisms underlying the immunological events associated with asymptomatic chronic HTLV-I infection is still poorly investigated. Regardless of the concept of compartmentalized immune response at privileged immune sites, the assessment of phenotypic features in peripheral blood has been applied successfully by our group to investigate the immunological mechanism underlying the establishment/maintenance of HAM/TSP, as well as the asymptomatic infection [11,15].
In order to evaluate the immunological parameter further as a marker of HTLV-I asymptomatic infection and HAM/TSP progression, in this study we have enrolled HTLV-I-infected individuals presenting distinct clinical status into a cross-sectional investigation, and performed a detailed analysis of the intracellular cytokine profile of peripheral blood cells following short-term stimulation in vitro in the absence/presence of PMA pan-leucocyte stimuli. We have chosen to evaluate the cytokine pattern after in vitro short-term culture, in particular in the absence of exogenous stimuli, in order to mimic and reflect the dynamic of the immunological events that take place in vivo during HTLV-I infection. Additionally, we have also characterized the cytokine profile following non-specific PMA pan-leucocyte stimulation, aiming to verify whether, upon mitogenic stimuli, dissimilar functional activities of peripheral blood cell subsets would be associated with the clinical status of HTLV-I-infected patients.
While adaptive immunity has been studied extensively, little is known about the host's innate immunity to HTLV-I infection. It has been demonstrated that, despite their clinical status, the spontaneous neutrophils activation is significantly higher in HTLV-1-infected patients compared to healthy individuals [16]. These authors have proposed that spontaneous neutrophil activation would be another general immunological marker resulting from HTLV-1 infection. Herein, focusing on the cytokine pattern of innate immunity cells, we have observed that both AS and OL display high levels of IL-12+ neutrophils, whereas OL and HT also display a high frequency of TNF-α+ neutrophils (Fig. 1). Moreover, despite their clinical status, all HTLV-I-infected individuals showed a high percentage of TNF-α+ monocytes (Fig. 2). Previous studies have reported the relevance of the immunological balance between type 1 and type 2 towards type 1 in the periphery and CNS for the pathogenesis of HAM/TSP [17]. Therefore, the high level of proinflammatory neutrophils and monocytes observed in AS patients would apparently seem controversial. This predominant type 1 immune response, observed for the innate immunity cells from both AS individuals and HAM/TSP patients, raised an intriguing question: which immunoregulatory event would take place during asymptomatic HTLV-I infection in order to guarantee the establishment of protective mechanism and avoid the tissue damage leading to a symptomless clinical condition?
Once the cytokine pattern of both innate and adaptive immunity cells were determined, we observed that although AS patients display a typical proinflammatory type 1 cytokine profile on their innate immunity cells (high frequency of IL-12+ neutrophils and TNF-α+ monocytes) they also present, at their adaptive immunity compartment, an outstanding putative immunomodulatory event that would be the key to controlling morbidity during chronic HTLV-I infection (Fig. 3). We have observed that those AS patients displaying a high frequency of proinflammatory TNF-α+ monocytes also present high levels of anti-inflammatory CD4+ IL-10+ and CD8+ IL-10+ T cells (Fig. 3a). Moreover, additional statistical analysis further confirms the presence of positive correlation between the frequency of TNF-α+ monocytes and both CD4+ IL-10+ and CD8+ IL-10+ T cells (Fig. 3b,c, respectively). Interestingly, it is relevant to consider that similar analyses were carried out in OL and HT individuals and no association between the levels of TNF-α+ monocytes and the frequency of IL-10+ T cells was observed (data not shown). These findings suggest that the establishment of immunoregulatory mechanisms is relevant to guarantee the asymptomatic clinical status during HTLV-I chronic infection. Indeed, it has been reported that IL-10 is able to modulate the IFN-γ production in HTLV-I-infected individuals [14]. Moreover, a relationship between the clinical efficacy of pentoxifilline treatment and the high seric levels of type 2 cytokines in HAM/TSP patients has also been reported [18]. These authors suggested that upon therapeutic intervention, the correction of immunological imbalance from type 1 to type 2 cytokines, with up-regulation of IL-10, might account for the clinical improvement in HAM/TSP patients.
As well as the cytokine profile from the innate immunity cells, the main focus of this study, we have had also the opportunity to highlight the role of the type 1 cytokine pattern from CD4+ T cells, as well as the relevance of increasing levels of CTL precursors in the peripheral blood as a biomarker of HTLV-I immunity towards disease progression, re-emphasizing the role of the Th1 cytokine phenotype in CD4+ cells as well as CTL precursor CD8+ T cells in the pathogenesis of HAM/TSP (Table 1). Our results demonstrate that upon PMA index analysis, a higher frequency of CD4+ T cells expressing IFN-γ and TNF-α was observed in the HT group, which supports the marked inflammatory profile underlying this pathological condition. Flow cytometric studies have demonstrated previously a high frequency of inflammatory cytokines (IFN-γ and TNF-α) producing lymphocytes in HAM/TSP patients compared to asymptomatic carriers [19]. Supporting our findings of increasing CTL precursor frequency in HT, the data reported by Santos et al. [19] were also accounted for mainly by an increased frequency of CD8+ T cells producing cytokines. The importance of the CD8+ T cell subset in the immunopathogenesis of HAM/TSP has been documented in a large number of reports [4,10,12,20]. It has been suggested that CD8+ T cells could self-react with homologous antigens shared between the virus and host cell structures and that this action would facilitate key immunological events in HAM/TSP. We have also reported previously that HT patients presented high levels of CD18 expression on their circulating CD8+ T cells, as well as a higher frequency of CD8+ HLA-DR+ and lower levels of CD8+ CD28+ in peripheral blood [11,15]. A higher frequency of CD8+ CD28– cells has been also reported by Santos et al. [19]. Recently, methods have been developed to quantify lymphocyte turnover rates in vivo and the efficiency of anti-HTLV-1 CTLs ex vivo. Data from these new techniques substantiate that variation between individuals, regarding host population genetics, viral genetics and host immunological functions, at which CTL anti-HTLV-1 activity plays the most relevant and perhaps the decisive determinant function, controls the proviral load and the risk of HAM/TSP [21].
Taken together, these findings are relevant to understanding of the dynamics of immunological events associated with the differing clinical status of HTLV-I infections. We have demonstrated here that an outstanding inflammatory profile, especially driven by TNF-α+ neutrophils and monocytes, as well as type 1 CD4+ T cells and an increased frequency of CTL precursors CD8+ T cells, are important for the pathophysiological changes observed in HT patients. The cytokine profiles of leucocytes from OL individuals also display a typical type 1 cytokine profile in the innate cell-mediated immune response, similar to the HT patients. The similarity in cytokine profiles between the OL and HT groups is expected, as OL represents a stage close to HT in disease development. Interestingly, in HT the type 1 immunological profiles were observed in both innate and adaptive contexts, suggesting the important contribution of innate immunity in disease outcome and the CD8+ T cells' role in the maintainance of chronic alterations described in HT patients [22,23]. On the other hand, the AS-infected individuals differ significantly from the HT group. These individuals present a mixed cytokine profile with a typical type 1 profile in their innate compartment, with IL-10 derived from adaptive immunity as an important immunomodulator, attenuating host defence mechanisms and favouring control of the exacerbate immune response.
IL-10 is a potent anti-inflammatory cytokine and inhibitor of TNF-α production. It has been proposed that IL-10 suppresses such responses via deactivation of monocytes/macrophages and repression of inflammatory cytokine expression. The mechanisms of IL-10's suppressive action are, however, characterized incompletely. Although the molecular pathways via which IL-10 inhibits TNF-α production are still obscure, some mechanisms have been proposed. It has been demonstrated by Denys et al. [24] that dual mechanisms for IL-10 function could be considered. Using an adenovirus to deliver TNF-α promoter-based luciferase reporter genes to primary human monocytes, these authors have demonstrated that IL-10 required the 3′ untranslated region of the TNF-α gene to inhibit luciferase mRNA and protein expression, indicating a post-transcriptional mechanism. However, when macrophages were incubated with IL-10 before activation, another mechanism displaying the inhibition of gene expression mediated by the 5′ promoter was also triggered, suggesting a transcriptional mechanism. Recently, it has been proposed by Rajasingh et al. [25] that the mechanisms underlying the inhibition of TNF-α production by IL-10 requires the IL-10-induced TNF-α mRNA destabilization via the ability of IL-10 to inhibit the expression of mRNA-stabilizing protein HuR and mediate the repression of p38 mitogen-activated protein (MAP) kinase activation. These authors have documented, at the molecular level, using a human monocytic cell line (U937) that IL-10 suppressed a number of inflammatory cytokines, mainly via post-transcriptional mRNA destabilization. Detailed studies on IL-10 regulation of TNF-α mRNA expression identified AU-rich elements (ARE) in the 3′ untranslated region as a necessary determinant of IL-10-mediated TNF-α mRNA destabilization [25].
Moreover, it has been demonstrated that polymorphisms in the IL-10 promoter can also influence the outcome of HTLV-I infection [26]. These authors have analysed a single-nucleotide region in HAM/TSP patients as well as asymptomatic carriers and demonstrated that the IL-10-592 A allele was associated with a more than twofold reduction in the odds of developing HAM/TSP. This odds ratio and the observed frequency of IL-10-592 A allele demonstrate that this allele prevents approximately 44·7% of potential cases of HAM/TSP, which indicates that it defines one component of the genetic susceptibility to HAM/TSP [26].
Together, these studies demonstrate the diversity and complexity of the mechanisms underlying the IL-10 anti-inflammatory activity that controls TNF-α synthesis by human monocyte/macrophages.
The observation that high levels of IL-10+ T cells are associated with the presence of proinflammatory monocytes in HTLV-1 asymptomatic carriers suggests that to some extent this cytokine may be one tool to modulate the immune response in subjects chronically infected with HTLV-1. However, it is important to mention that as an intrinsic characteristic of a cross-sectional study, the results presented in this investigation should be interpreted with caution. Only prospective investigations, albeit difficult, will provide definitive evidence of the role of the various aspects of the innate immune response in disease development.
Acknowledgments
We would like to thank Jaqueline Gontijo de Souza for her technical assistance during sample processing and flow cytometry data collection. This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico − CNPq, Fundação Oswaldo Cruz − FIOCRUZ and Fundação Hemominas, Brazil.
References
- 1.Popovic M, Reitz MS, Jr, Sarngadharan MG, et al. The virus of Japanese adult T cell leukaemia is a member of the T cell leukaemia virus group. Nature. 1982;300:63–6. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- 2.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type I in patients with tropical pararesis. Lancet. 1985;2:407–10. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 4.Moore GRW, Traugott U, Sheunberg LC, Raine CS. Tropical spastic paraparesis: a model of virus-induced cytotoxic T cell mediated demyelination? Ann Neurol. 1989;26:523–30. doi: 10.1002/ana.410260405. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson S, Zaninovic V, Mora C, et al. Immunological finding in neurological diseases: activated lymphocytes in tropical spastic paraparesis. Ann Neurol. 1988;23:S196–200. doi: 10.1002/ana.410230744. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida S, Osame M, Kawai H, et al. Increased replication of HTLV-I in HTLV-I associated myelopathy. Ann Neurol. 1989;26:331–5. doi: 10.1002/ana.410260304. [DOI] [PubMed] [Google Scholar]
- 7.Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. HTLV-I specific IFN-gamma+ CD8+ lymphocytes correlate with the proviral load in peripheral blood of infected individuals. J Neuroimmunol. 2000;102:208–15. doi: 10.1016/s0165-5728(99)00175-7. [DOI] [PubMed] [Google Scholar]
- 8.Nagai M, Jacobson S. Immunopathogenesis of human T cell lymphotropic virus type I-associated myelopathy. Curr Opin Neurol. 2001;14:381–6. doi: 10.1097/00019052-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda Y, Matsui M. Cerebrospinal fluid interferon-gamma is increased in HTLV-I associated myelopathy. J Neuroimmunol. 1993;42:223–6. doi: 10.1016/0165-5728(93)90014-p. [DOI] [PubMed] [Google Scholar]
- 10.Greten TF, Slasky JE, Kubota R, et al. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–73. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brito-Melo GE, Martins-Filho OA, Carneiro-Proietti AB, et al. Grupo Interdisciplinar de Pesquisas Em HTLV. Phenotypic study of peripheral blood leucocytes in HTLV-I-infected individuals from Minas Gerais, Brazil. Scand J Immunol. 2002;55:621–8. doi: 10.1046/j.1365-3083.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 12.Kubota R, Kawanishi T, Matsubara H, Manns a, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J Immunol. 1998;161:482–8. [PubMed] [Google Scholar]
- 13.Nishimura M, Maeda M, Yasunaga J, et al. Influence of cytokine and mannose biding protein gene polymorphisms on human T cell leukemia virus type I (HTLV-I) provirus load in HTLV-I asymptomatic carriers. Hum Immunol. 2003;64:453–7. doi: 10.1016/s0198-8859(02)00829-7. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho EM, Bacellar O, Porto AF, et al. Cytokine profile and immunomodulation in asymptomatic human T-lymphotropic vírus type 1-infected blood donors. J Acquir Immune Defic Syndr. 2001;27:1–6. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Brito-Melo GE, Souza JG, Barbosa-Stancioli EF, et al. Interdisciplinar de Pesquisas em HTLV. Grupo Establishing phenotypic features associated with morbidity in human T cell lymphotropic virus type 1 infection. Clin Diagn Lab Immunol. 2004;11:1105–10. doi: 10.1128/CDLI.11.6.1105-1110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerreiro JB, Porto MAF, Santos SB, Lacerda L, Ho JL, Carvalho EM. Spontaneous neutrophils activation in HTLV-I infected patients. Braz J Infect Dis. 2005;9:510–4. doi: 10.1590/s1413-86702005000600010. [DOI] [PubMed] [Google Scholar]
- 17.Furuya T, Nakamura T, Fujimoto T, et al. Elevated levels of interleukin-12 and interferon-gamma in patients with human T lymphotropic virus type I-associated myelopathy. J Neuroimmunol. 1999;95:185–9. doi: 10.1016/s0165-5728(98)00263-x. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto T, Nakamura T, Furuya T, et al. Relationship between the clinical efficacy of pentoxifylline treatment and elevation of serum T helper type 2 cytokine levels in patients with human T-lymphotropic virus type I-associated myelopathy. Intern Med. 1999;38:717–21. doi: 10.2169/internalmedicine.38.717. [DOI] [PubMed] [Google Scholar]
- 19.Santos SB, Porto AF, Muniz AL, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carries. BCM Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umehara FS, Izumo S, Ronquillo AT, Matsumuro K, Sato E, Osame M. Cytokine expression in the spinal cord lesions in HTLV-associated myelopathy. J Neuropathol Exp Neurol. 1994;53:72–7. doi: 10.1097/00005072-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene. 2005;24:6035–46. doi: 10.1038/sj.onc.1208970. [DOI] [PubMed] [Google Scholar]
- 22.Bieganowska K, Hollsberg P, Buckle GJ, et al. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T cell lymphotropic virus-associated myelopathy. J Immunol. 1999;162:1765–71. [PubMed] [Google Scholar]
- 23.Biddison WE, Kubota R, Kawanishi T, et al. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J Immunol. 1997;159:2018–25. [PubMed] [Google Scholar]
- 24.Denys A, Udalova IA, Smith C, et al. Evidence for a dual mechanism for IL-10 suppression of TNF-alpha production that does not involve inhibition of p38 mitogen-activated protein kinase or NF-kappa B in primary human macrophages. J Immunol. 2002;168:4837–45. doi: 10.4049/jimmunol.168.10.4837. [DOI] [PubMed] [Google Scholar]
- 25.Rajasingh J, Bord E, Luedemann C, et al. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB J. 20:2112–14. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 26.Sabouri AH, Saito M, Lloyd AL, et al. Polymorphism in the interleukin-10 promoter affects both provirus load and the risk of human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2004;190:1279–85. doi: 10.1086/423942. [DOI] [PubMed] [Google Scholar]