Abstract
CD81 is a member of the tetraspan superfamily and plays a role in immune responses and in hepatitis C virus (HCV) pathogenesis. We analysed CD81 cell surface and mRNA expression in different lymphocytic subpopulations in human immunodeficiency virus (HIV)-1, HCV and dually infected subjects. CD81 cell surface expression was evaluated with fluorescence activated cell sorter (FACS) analysis; mRNA quantification was performed with semiquantitative polymerase chain reaction (PCR). CD81 cell surface expression on CD4+ T lymphocytes was significantly different by analysis of variance (anova) test (P < 0·001), with reduced expression in HIV-1+ patients. In B lymphocytes, higher cell surface expression was present in HIV-1, in HCV and in dually infected subjects compared to healthy controls. CD81 expression on B lymphocytes showed a positive correlation with plasma HIV-RNA. CD81 mRNA levels in B lymphocytes were significantly higher in HIV-1+ patients compared to healthy controls. The potential consequence of the down-regulation of CD81 in CD4+ cells during HIV-1 infection in conjunction with diverted CD28, CD4 and CD3 expression is the disruption of T cell function. Increased CD81 expression on B lymphocytes might explain the higher prevalence of lymphoproliferative disorders in HIV-1 and HCV infection. Up-regulation of CD81 mRNA on CD4+ T cells indicates that down-regulation of CD81 occurs at the post-transcriptional/translational level.
Keywords: B lymphocytes, CD81, HCV infection, HIV infection, lymphoproliferative disorders, T lymphocytes
Introduction
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) share the same parenteral route of transmission; as a consequence the prevalence of chronic HCV infection is higher among HIV patients than in the healthy population [1,2]. Co-infection with HIV appears to affect adversely the natural history of HCV infection, as it has been associated with higher HCV viral load, more rapid progression to cirrhosis, liver failure and hepatocellular carcinoma [3–7]. Among HIV-infected Italian patients, the prevalence of HIV/HCV co-infection is approximately 50% [1] and as anti-retroviral therapy has dramatically improved morbidity and mortality of HIV infection, HCV-related liver disease has become a major cause of hospitalization and death in such patients and often leads to difficulties in anti-retroviral therapy management [8]. Moreover, HCV infection was shown to be associated with an impaired recovery of CD4+ cell count in HCV/HIV co-infected patients receiving highly active anti-retroviral therapy (HAART). In particular, HCV genotype 1b may possibly be involved in progression of HIV infection to AIDS and death in haemophilic subjects [9,10].
CD81 is a member of the tetraspan superfamily of proteins and is expressed widely on a variety of cell types, including hepatocytes, B and T lymphocytes. The tetraspanins exert their biological function as a consequence of their association with other cell surface molecules. In B cells CD81 is associated with the CD19/CD21/Leu13 molecular complex and co-stimulation of this complex together with CD81 lowers the threshold for B cell activation and promotes cell proliferation [11,12]. In T cells CD81 is associated with CD4, CD8, CD82 and integrins and plays a role in T cell activation [13–16]. Engagement of CD81 enhances anti-CD3 stimulation of T cells with regard to interleukin (IL)-2 and interferon (IFN)-γ production and increases superantigen-induced T cell activation and proliferation [17–20]. CD81 has been demonstrated to bind HCV envelope 2 glycoprotein in vitro and is a potential co-receptor for HCV, even though its role in cellular uptake of HCV is still debated [21]. HCV can modulate CD81 expression on CD19 cells; it was suggested that HCV-mediated stimulation of CD81/CD19/CD21 complex triggers B cell proliferation [22–24]. HCV genotype 1 determines an increase of cell surface CD81 expression [25]. IFN-α is able to down-regulate CD81 expression in vitro in peripheral blood mononuclear cells (PBMC) and in hepatocytes and in vivo in HCV-infected patients with virological response to therapy, while higher levels of CD81 cell surface expression correlate with genotype 1 and resistance to IFN and ribavirin therapy [25].
Given the relevance of CD81 in immune responses and in HCV pathogenesis, and considering the profound deregulation of the immune system determined by HIV, we decided to investigate CD81 expression on different lymphocytic subpopulations in HIV-1 infected patients with or without HCV infection in order to evaluate a possible role of CD81 modulation in the interaction between HIV and HCV infection.
Patients and methods
Patients
We performed a cross-sectional analysis of CD81 antigen expression on the surface of different lymphocytic subpopulations in 80 out-patients attending our institute. Of these patients, 27 were dually infected with HIV and HCV (HIV+ HCV+); 42 had single HIV infection (HIV+ HCV–); and 11 were chronically infected with HCV (HIV– HCV+). Nineteen healthy volunteers were studied as controls (HIV– HCV–). None of the HCV-infected patients had ever been treated with interferon and/or ribavirin before the study. A total of 56 HIV-1-infected patients were receiving treatment with anti-retroviral therapy, while 13 were treatment-naive.
HCV genotype was determined by means of a commercial assay (Inno-Lipa HCV, Innogenetics, Gent, Belgium). HIV RNA plasma levels were measured by using the branched chain DNA (bDNA) technique (Versant HIV-1 bDNA, Bayer, Milan, Italy), which has a lower detection threshold of 50 copies per ml. Patients infected with HBV (i.e. positive for hepatitis B surface antigen: HBsAg) were excluded from the study.
Flow cytometry
A total of 100 μl of fresh blood, collected in ethylenediamine tetraacetic acid (EDTA)-treated tubes, were double-stained with either CD4, CD8, CD19 or CD16 fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MoAb) (Becton Dickinson, Mountain View, CA, USA) and with CD81 R-phycoerythrin (PE)-conjugated MoAb (Pharmingen, San Diego, CA, USA). Appropriate isotypic controls were used for all the antibodies used. Stained specimens were fixed with Immunoprep kit reagents (Beckman Coulter Immunotech, Marseille, France) by means of Q-Prep workstation (Beckman Coulter Immunotech) and analysed by means of an EPICS XL flow cytometer (IL Coulter, Milan, Italy). As most lymphocytes are CD81-positive we measured the channel of mean fluorescence intensity (MFI) of the CD81-positive peak on a logarithmic scale.
The expression of CD81 antigen on the cell surface of gated CD4, CD8, CD16 and CD19 lymphocytes was also quantified by means of a commercially available kit (Quantum Simply Cellular Microbeads Kit; Sigma, St Louis, MO, USA), measuring the number of antibody molecules bound per cell (antibody binding capacity: ABC). This kit provides a mixture of four populations of microbeads coated with different amounts of goat anti-mouse immunoglobulin with a precalibrated ABC. The microbeads, reacting in a separate tube with directly labelled mouse MoAb, are used to calibrate the fluorescence scale of the flow cytometer for each antibody, thus converting the mean fluorescence intensity measured on stained lymphocytes into the number of molecules of antigen expressed per cell.
Cell separation
PBMC were isolated from freshly drawn heparinized blood by Ficoll density gradient separation. CD4+, CD8+ and CD19+ cells were positively selected from PBMC by means of immunomagnetic beads ((Dynal ASA, Oslo, Norway), according to the manufacturer's instructions. This separation technique routinely yielded populations that were > 95% pure as verified by cytofluorimetry.
RNA extraction and reverse transcription
CD4+, CD8+ and CD19+ cells isolated from the patients were lysed by addition of EUROzol (EUROClone Ltd, West York, UK) and total RNA was extracted according to the manufacturer's instructions. Purified RNA was quantified by measuring the absorbance at 260 nm. cDNA was synthesized from 1 µg of total RNA by priming with 40 µg of oligo(dT) primer (Finnzymes Oy, Espoo, Finland), 1 mM of each deoxynucleotide triphosphate and 200 units of AMV reverse transcriptase (Finnzymes Oy). The reaction was first incubated for 10 min at 70°C and then at 42°C for 60 min.
Real-time polymerase chain reaction (PCR) analysis
Six µl of cDNA were used in a 50-µl reaction with TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) to analyse target and reference gene [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] expression. Cycling conditions were 2 min at 50°C, 10 min at 95°C followed by 40 repeats of 15 s at 95°C and 1 min at 60°C. Sequence-specific fluorescent signal was detected by an ABI prism 7007 Sequence Detection System. Ct values above a set threshold were defined using the sequence detection software supplied with the instrument. mRNA data were normalized relative to the GAPDH and then used to calculate expression levels according to the ΔΔCt method.
Statistical analysis
Data were analysed using the spss version 11 for Windows XP statistical software package. CD81 expressions on CD4+ and CD8+ T lymphocytes, B lymphocytes and natural killer (NK) cells, as well as CD4+, CD8+, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), were analysed by means of univariate and multivariate analyses of variance (anova). Non-parametric correlation (Spearman's rho) and the Kruskal–Wallis test were used when investigating HIV plasma viraemia. For absolute levels Student's t-test was performed to compare the mRNA levels in the studied groups.
Results
Patients
The characteristics of the patients studied are summarized in Table 1. No significant difference emerged among the four groups for gender, while a significant difference was present regarding age, as HCV-infected patients were older, reflecting the epidemiology of HCV in Italy (P = 0·009).
Table 1.
Characteristics of the patients.
| HIV– HCV– | HIV– HCV+ | HIV+ HCV– | HIV+ HCV+ | |
|---|---|---|---|---|
| Number of patients | 19 | 11 | 42 | 27 |
| Age* | 38 ± 10 | 52 ± 12 | 42 ± 12 | 41 ± 8 |
| Male/female | 12/7 | 6/5 | 25/17 | 16/11 |
| Years of HIV infection | 5 ± 4 | 13 ± 5 | ||
| Antiretroviral therapy with PI | 13 | 11 | ||
| Antiretroviral therapy PI sparing | 19 | 13 | ||
| No therapy | 10 | 3 | ||
| CD4+ cell count/mm3 | 910 ± 344 | 889 ± 348 | 379 ± 252 | 470 ± 322 |
| HIV-RNA (copies/ml) | 24 979 ± 88 047 | 28 621 ± 97 886 | ||
| ALT** | 22 ± 12 | 81 ± 51 | 28 ± 23 | 66 ± 58 |
| AST*** | 21 ± 10 | 73 ± 62 | 26 ± 17 | 53 ± 39 |
| HCV RNA (copies/ml) | 949 046 ± 1 448 910 | 1 116 710 ± 1 680 410 | ||
| HCV genotype 1 | 5 | 15 | ||
| HCV genotypes 2, 3 and 4 | 6 | 12 |
Data are expressed as mean values ± standard deviation.
P = 0·009
P = 0·001
P = 0·001.
AST: aspartate aminotransferase; ALT: alanine aminotransferase.
As expected, CD4+ cell counts were significantly lower in HIV+ HCV+ and in HIV+ HCV– than in HIV– HCV+ and in HIV– HCV– patients (P < 0·05); while CD8+ absolute count and percentage were significantly higher in HIV+ HCV+ and in HIV+ HCV– than in HIV– HCV+ and in HIV– HCV– patients (P < 0·05). No differences in HIV RNA levels were observed between HIV+ HCV+ and HIV+ HCV– patients.
ALT serum levels were significantly higher in HIV+ HCV+ and in HIV– HCV+ than in HIV+ HCV– and in HIV– HCV– (P = 0·001).
CD81 surface expression on CD4+ T lymphocytes derived from peripheral blood mononuclear cells
CD81 cell surface expression on CD4+ T lymphocytes in the four groups of patients according to HIV and HCV infection was significantly different by anova test (P < 0·001) (Fig. 1). HIV-1 infection was correlated strongly with a significantly reduced CD81 surface expression (P < 0·001); in fact, CD81 antigen was significantly lower both in HIV+/HCV+ patients (median 3·80) and HIV+/HCV– patients (3·60) compared to HIV–HCV+ subjects (5·00) and healthy controls (5·30). When the studied population was analysed according to HCV infection, we did not observe any significant difference between HCV-infected and HCV-negative patients on CD4+ T cells (Fig. 1). In multivariate analysis no correlation was found between CD81 MFI on CD4+ T lymphocytes, sex and age, whereas the correlation was confirmed regarding HIV-1 infection (P < 0·001; R2 = 0·3). Considering only HIV-1-infected patients, we did not find any correlation between CD81 MFI and CD4 cell count or plasma HIV-RNA. HIV-1 infected patients receiving anti-retroviral therapy with HIV-1 viraemia < 50 copies/ml did not show a significant difference in the expression of CD81 on CD4+ T lymphocytes compared to viraemic untreated patients (median MFI values: 3·82 and 4·27). In order to further confirm our data we decided to evaluate CD81 cell surface expression on CD4+ T lymphocytes using a commercially available method with high sensitivity. We therefore performed ABC with FACS analysis. ABC for CD81 on CD4+ lymphocytes was significantly lower in HIV+ HCV– patients (median value 29152, range 8957–31 240) and in HIV+ HCV+ patients (median value 27 151, range 10 030–35 703) compared both to HIV– HCV+ patients (median value 37 683, range 22 697–69 476) and to HIV–HCV– healthy controls (median value 39 568, range 21 784–80 451) (P < 0·05).
Fig. 1.
CD81 expression by mean fluorescence intensity on CD4+ T lymphocytes derived from peripheral blood mononuclear cells of healthy controls (a), HIV–/HCV+ (b), HIV+/HCV– (c) and HIV+/HCV+ (d) patients. (a) versus (c): P < 0·001; (a) versus (d): P < 0·001; (b) versus (c): P < 0·001; (b) versus (d): P < 0·001.
CD81 surface expression on CD19+ lymphocytes derived from peripheral blood mononuclear cells
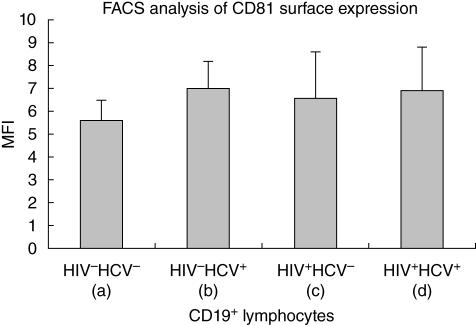
The four groups had significantly different levels of surface CD81 expression on CD19+ lymphocytes (P = 0·01). Patients with single HCV-infection presented higher levels of membrane CD81 expression compared to healthy controls (median values: 6·80 and 5·60, respectively) (P < 0·001). Surface levels of CD81 in both HIV+/HCV– (median MFI 6·60; P = 0·04) and HIV+/HCV+ (median MFI 6·90; P < 0·001) patients were significantly higher than healthy controls and similar to HCV single-infected subjects (Fig. 2).
Fig. 2.
CD81 expression by mean fluorescence intensity on CD19+ lymphocytes derived from peripheral blood mononuclear cells of healthy controls (a), HIV–/HCV+ (b), HIV+/HCV– (c) and HIV+/HCV+ (d) patients. (a) versus (b): P < 0·001; (a) versus (c): P = 0·04; (a) versus (d): P < 0·001.
In multivariate analysis no significant correlation was found between CD81 MFI on B cells, age and sex, while a correlation was confirmed with both HIV and HCV infection status (P = 0·036, r2 = 0·1). Interestingly, regarding CD81 MFI on B lymphocytes in HIV infected patients, a positive correlation was identified between CD81 cell surface expression and HIV-RNA (Spearman's rho: P = 0·019, with a strength of correlation of r = 0·08), while no correlation was present with the CD4 cell count.
CD81 surface expression on CD8+ T lymphocytes and natural killer cells
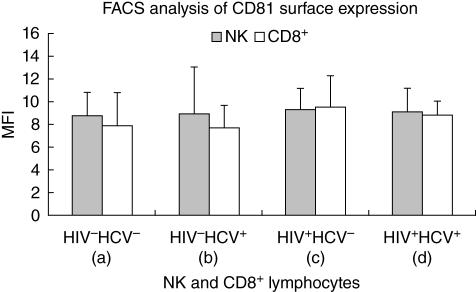
Finally we analysed CD81 cell surface expression on CD8+ T lymphocytes and NK cells in the four groups of patients. We did not observe any statistical difference either on CD8+ T cells or on NK cells (Fig. 3). A lower percentage of CD16 NK cells were evidenced in HIV-infected patients compared to healthy controls and single HCV-infected patients (P = 0·006).
Fig. 3.
CD81 expression by mean fluorescence intensity on CD8+ lymphocytes and natural killer cells derived from peripheral blood mononuclear cells of healthy controls, HIV–/HCV+, HIV+/HCV– and HIV+/HCV+ patients.
CD81 mRNA expression in CD4+ T lymphocytes and in B cells
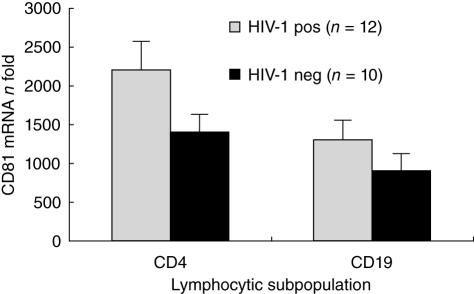
We assessed CD81 mRNA expression in CD4+ T cells and in B cells in a subgroup of single HIV-1-infected patients (n = 12) and in healthy controls (n = 10) with real-time PCR. CD81 mRNA resulted in significantly up-regulated HIV-infected patients compared to HIV– patients in both cellular populations (P < 0·05 in both cell types) (Fig. 4).
Fig. 4.
CD81 mRNA expression on CD4+ T cells and CD19+ lymphocytes of HIV+/HCV– patients and HIV–/HCV– healthy controls with real-time polymerase chain reaction. CD4+ T cells, P = 0·008; CD19+ cells, P = 0·010.
Discussion
We have demonstrated that CD81 expression is modulated by HIV-1 on CD4+ T lymphocytes and by both HIV-1 and HCV on B lymphocytes. CD81 is expressed widely and forms complexes with different antigens on the surface of various cell types in a cell type-specific manner; such associations influence its capacity to deliver signals to target cells [26]. In human T cell lines CD81 can associate with CD4 and CD8 co-receptors and it has been demonstrated that in murine αβ T cells CD81 functions as a T cell co-stimulatory signal, leading to strong proliferation of CD4+ and CD8 + cells independently from CD28 [27]. Moreover, CD81 cross-linking greatly enhances anti-CD3 activation of both T cell receptor (TCR)αβ (CD4+ and CD8+) and TCRγδ T cells with regard to IFN-γ production [18,19].
CD81 is down-regulated on CD4+ T lymphocytes during HIV infection independently from HCV infection. It has been demonstrated that HIV Nef protein disrupts T cell receptor machinery by down-modulating cell surface expression of CD4 and expression or signalling of CD3-TCR; Nef also down-modulates class I major histocompatibility complex (MHC) surface expression [28–31]. Finally Nef down-modulates CD28 by accelerating endocytosis [32]. The potential consequence of the concerted down-regulation of CD28, CD4 and/or CD3 and CD81 is disruption of antigen-specific signalling machineries in CD4+ T cells following a productive antigen recognition event. Moreover, CD81 is involved in the induction of strong virus-specific T helper responses that are required for maintenance of effective cytotoxic T lymphocyte (CT) function in chronic viral infections [27], therefore down-regulation on CD4+ T cells might impair T cell responses and favour HIV disease progression and possibly the emergence and worsening of other diseases such as HBV, EBV, CMV and other opportunistic infections. In particular, the down-regulation of CD81 expression on CD4+ T cells in HIV/HCV co-infected individuals might explain the more rapid progression of HCV chronic hepatitis [33].
It is relevant to point out that no correlation between CD81 expression and CD4+ cell count was found; down-regulation of CD81 expression is probably an early and persistent immunological defect related to HIV infection.
Compared with healthy donors, CD4+ T lymphocytes from HIV-infected patients showed lower CD81 cell surface expression and higher CD81 mRNA, indicating a post-transcriptional/translational or a recycling mechanism for CD81 down-regulation during HIV-1 infection.
Recently, it has been demonstrated that engagement of CD81 provides a co-stimulatory signal resulting in increased expression of HIV in vitro, both in primary CD4+ T lymphocytes and in Jurkat cells [34]; HIV-1 might interfere with CD81 expression in vivo to ensure efficient viral transcription and regulation.
Regarding B lymphocytes, we have confirmed previous data showing a significant up-regulation of CD81 on B cells in HCV-infected patients [25], even though we did not detect a significant correlation between CD81 levels of expression and HCV-RNA, as already reported by others [35]. More interestingly, we have observed a significant up-regulation of CD81 on B cells in HIV-1 infected patients independently of HCV infection.
The issue of dysregulation of CD81 expression on CD19 cells during HCV infection has not yet been clarified completely; in fact, different papers have reported that expression of CD81 on peripheral B cells is increased in HCV-infected patients [35–38], while Cacoub et al. [39] have described down-regulation of CD81 expression; our data support the observations of increased CD81 expression on B lymphocytes during chronic HCV infection. Recent works have demonstrated that engagement of CD81 facilitates B cell activation and proliferation [40] and in particular HCV envelope 2 protein is able to induce hypermutation of the immunoglobulin gene and cellular proliferation through the mitogen-activated protein kinase/extracellular-regulated kinase (MAPK/ERK) signalling pathway via CD81 [41–43]; these data explain partially the linkage between HCV, B cell activation, autoimmune diseases and lymphoproliferative disorders [40,44].
In HIV infection, B lymphocytes are severely damaged and show signs of phenotypic and functional alteration [45,46]. A significant polyclonal B cell activation is observed commonly and associated with hypergammaglobulinaemia, autoantibodies and autoimmune diseases [47,48]. HIV-induced up-regulation of CD81 might contribute to such B cell deregulation and favour polyclonal B cell activation, as the CD19/CD21/Leu13/CD81 complex reduces the threshold for B cell activation by significantly enhancing B cell receptor signalling. Moreover, in HIV/HCV co-infected individuals, interaction of HCV envelope 2 glycoprotein with the second extracellular loop of CD81 might lead to B cell deregulation by interfering with the function of the CD19/CD21/Leu13/CD81 complex. CD81-mediated activation of B cells might explain both the frequent appearance and the occasional emergence of autoimmune diseases and lymphoproliferative disorders in HIV-1-infected patients, as has been described in HCV infection [48,49]. Treatment of HCV infection leads to normal levels of expression of CD81 [50]; it is therefore desirable to also treat HCV-related chronic hepatitis in co-infected patients to avoid emergence of autoimmune disorders and lymphoproliferative diseases.
CD81 up-regulation on B cells is correlated positively with HIV viraemia and might be due to a transcriptional mechanism; compared to healthy donors, CD19+ lymphocytes from HIV-1-infected patients display significantly higher levels of CD81 mRNA. HIV proteins exert important functions in their relevant target cells in vivo and have been shown to modulate expression of several cellular proteins [51]. In particular, tat can deregulate the expression of several heterologous cellular and viral genes and extracellular tat is mitogenic for mammary and amniotic epithelial cells and stimulates the expression of genes of pathogenetic interest in HIV infection [52,53].
A recent paper by Kronenberger et al. [38] has analysed the dynamics of CD81 cell surface expression on different PBMC populations in HCV-infected patients treated with IFN-α and found a significant up-regulation of CD81 on CD4, CD8, CD19 and CD56 cells in HCV patients before treatment compared to healthy controls. Our data confirm the up-regulation of CD81 on B cells, but we could not detect any significant up-regulation in CD4+, CD8+ and NK cells in the presence of HCV infection, even if a tendency towards higher expression was evidenced in NK cells. Our case file of HCV single-infected individuals is limited to 11 patients and it is possible that the low number of patients did not reach statistical significance, while in HIV/HCV co-infected subjects the presence of HIV-1 infection might interfere with the influence of HCV on CD81 expression. To validate our data further we also analysed CD81 expression with the ABC. This system allows conversion of the MFI into the number of molecules of antigen expressed per cell, and confirmed the results obtained with mean fluorescence. Therefore, the expression of CD81 on CD4+, CD8+ and NK cells requires further evaluation.
Altogether, our data suggest that both HIV-1 and HCV infection determine a profound dysregulation of the expression of the tetraspanin CD81 on B lymphocytes and HIV-1, and also on CD4+ T cells; such modification of expression might alter the signalling threshold required to stimulate both the T cell and B cell receptors and therefore affect HIV-1 and HCV disease progression and potentially cause lymphoproliferative disorders.
Acknowledgments
We are grateful to Bianca Ghisi for excellent editorial assistance, to all the patients participating in the study and to the staff at the Institute of Infectious Diseases and Tropical Medicine, ‘L. Sacco’ Hospital, who cared for the patients.
References
- 1.De Luca A, Bugarini R, Lepri AC, et al. Co-infection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162:2125–32. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 2.Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C Virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 3.Sherman KE, O'Brien J, Gutierrez AG, et al. Quantitative evaluation of hepatitis C virus RNA in patients with concurrent human immunodeficiency virus infections. J Clin Microbiol. 1993;31:2679–82. doi: 10.1128/jcm.31.10.2679-2682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cribier B, Rey D, Schmitt C, et al. High hepatitis C viraemia and impaired antibody response in patients co-infected with HIV. AIDS. 1995;9:1131–6. doi: 10.1097/00002030-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou Y, Bochet M, Di Martino V, et al. The Multivirc Group. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus co-infected patients. Hepatol. 1999;30:1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Samaniego J, Rodriguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179–83. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 8.Soriano V, Sulkowski M, Bergin C, et al. Care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. AIDS. 2002;16:813–28. doi: 10.1097/00002030-200204120-00001. [DOI] [PubMed] [Google Scholar]
- 9.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus co-infection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–5. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 10.Piroth L, Duong M, Quantin C, et al. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–8. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–49. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 12.Levy S, Todd SC, Maecker HT. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey PW, Allison ME, Akkaraju S, et al. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 14.Imai T, Fukudome K, Takagi S, et al. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol. 1992;149:2879–86. [PubMed] [Google Scholar]
- 15.Imai T, Kakizaki M, Nishimura M, et al. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J Immunol. 1995;155:1229–39. [PubMed] [Google Scholar]
- 16.Mannion BA, Berditchevski F, Kraeft SK, et al. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1 (CD49d/CD29) J Immunol. 1996;157:2039–47. [PubMed] [Google Scholar]
- 17.Todd SC, Lipps SG, Crisa L, et al. CD81 expressed on human thymocytes mediates integrin activation and interleukin 2-dependent proliferation. J Exp Med. 1996;184:2055–60. doi: 10.1084/jem.184.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng CT, Miskovsky E, Klimpel GR. Crosslinking CD81 results in activation of TCRgammadelta T cells. Cell Immunol. 2001;207:19–27. doi: 10.1006/cimm.2000.1744. [DOI] [PubMed] [Google Scholar]
- 19.VanCompernolle SE, Levy S, Todd SC. Anti-CD81 activates LFA-1 on T cells and promotes T cell–B cell collaboration. Eur J Immunol. 2001;31:823–31. doi: 10.1002/1521-4141(200103)31:3<823::aid-immu823>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Wack A, Soldaini E, Tseng C, et al. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur J Immunol. 2001;31:166–75. doi: 10.1002/1521-4141(200101)31:1<166::aid-immu166>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Bartosch B, Cosset FL. Cell entry of hepatitis C virus. Virology. 2006;348:1–12. doi: 10.1016/j.virol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Franzin F, Efremov DG, Pozzato G, et al. Clonal B-cell expansions in peripheral blood of HCV-infected patients. Br J Haematol. 1995;90:548–52. doi: 10.1111/j.1365-2141.1995.tb05582.x. [DOI] [PubMed] [Google Scholar]
- 23.Tedder TF, Inaoki M, Sato S. The CD19–CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–18. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 24.Cherukuri A, Shoham T, Sohn HW, et al. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J Immunol. 2004 January 1;172:370–80. doi: 10.4049/jimmunol.172.1.370. [DOI] [PubMed] [Google Scholar]
- 25.Kronenberger B, Ruster B, Elez R, et al. Interferon alfa down-regulates CD81 in patients with chronic hepatitis C. Hepatology. 2001;33:1518–26. doi: 10.1053/jhep.2001.24668. [DOI] [PubMed] [Google Scholar]
- 26.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–48. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 27.Witherden DA, Boismenu R, Havran WL. CD81 and CD28 co-stimulate T cells through distinct pathways. J Immunol. 2000;165:1902–9. doi: 10.4049/jimmunol.165.4.1902. [DOI] [PubMed] [Google Scholar]
- 28.Iafrate AJ, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–84. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe AY, Jung JU, Desrosiers RC. Zeta chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J Virol. 1998;72:9827–34. doi: 10.1128/jvi.72.12.9827-9834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willard-Gallo KE, Furtado M, Burny A, et al. Down-modulation of TCR/CD3 surface complexes after HIV-1 infection is associated with differential expression of the viral regulatory genes. Eur J Immunol. 2001;31:969–79. doi: 10.1002/1521-4141(200104)31:4<969::aid-immu969>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz O, Marechal V, Le Gall S, et al. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–42. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 32.Swigut T, Shohdy N, Skowronski J. Mechanism for down-regulation of CD28 by Nef. EMBO J. 2001;20:1593–604. doi: 10.1093/emboj/20.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus co-infection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34:1193–9. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 34.Tardif MR, Tremblay MJ. Tetraspanin CD81 provides a co-stimulatory signal resulting in increased human immunodeficiency virus type 1 gene expression in primary CD4+ T lymphocytes through NF-kappaB, NFAT, and AP-1 transduction pathways. J Virol. 2005;79:4316–28. doi: 10.1128/JVI.79.7.4316-4328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kronenberger B, Herrmann E, Hofmann WP, et al. Dynamics of CD81 expression on lymphocyte subsets during interferon-alpha-based antiviral treatment of patients with chronic hepatitis C. J Leukoc Biol. 2006;80:298–308. doi: 10.1189/jlb.0106047. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman E. Expansion of CD5+ B-cell overexpressing CD81 in HCV infection: towards better understanding the link between HCV infection, B-cell activation and lymphoproliferation. J Hepatol. 2003;38:674–6. doi: 10.1016/s0168-8278(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann WP, Herrmann E, Kronenberger B, et al. Association of HCV-related mixed cryoglobulinemia with specific mutational pattern of the HCV E2 protein and CD81 expression on peripheral B lymphocytes. Blood. 2004;104:1228–9. doi: 10.1182/blood-2004-02-0644. [DOI] [PubMed] [Google Scholar]
- 38.Curry MP, Golden-Mason L, Doherty DG, et al. Expansion of innate CD5pos B cells expressing high levels of CD81 in hepatitis C virus infected liver. J Hepatol. 2003;38:642–50. doi: 10.1016/s0168-8278(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 39.Cacoub P, Bourliere M, Hausfater P, et al. Lower expression of CD81 B-cell receptor in lymphoproliferative diseases associated with hepatitis C virus infection. J Viral Hepatol. 2003;10:10–5. doi: 10.1046/j.1365-2893.2003.00380.x. [DOI] [PubMed] [Google Scholar]
- 40.Rosa D, Saletti G, De Gregorio E, et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci USA. 2005;102:18544–9. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Machida K, Cheng KT, Pavio N, et al. Hepatitis C virus E2–CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–89. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao LJ, Wang L, Ren H, et al. Hepatitis C virus E2 protein promotes human hepatoma cell proliferation through the MAPK/ERK signaling pathway via cellular receptors. Exp Cell Res. 2005;305:23–32. doi: 10.1016/j.yexcr.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 43.Carloni V, Mazzocca A, Ravichandran KS. Tetraspanin CD81 is linked to ERK/MAPKinase signaling by Shc in liver tumor cells. Oncogene. 2004;23:1566–74. doi: 10.1038/sj.onc.1207287. [DOI] [PubMed] [Google Scholar]
- 44.Zuckerman E, Slobodin G, Kessel A, et al. Peripheral B-cell CD5 expansion and CD81 overexpression and their association with disease severity and autoimmune markers in chronic hepatitis C virus infection. Clin Exp Immunol. 2002;128:353–8. doi: 10.1046/j.1365-2249.2002.01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 46.Moir S, Malaspina A, Ogwaro KM, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci USA. 2001;98:10362–7. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Milito A, Nilsson A, Titanji K, et al. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–6. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 48.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev. 2002;1:329–37. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 49.Takeshita M, Sakai H, Okamura S, et al. Splenic large B-cell lymphoma in patients with hepatitis C virus infection. Hum Pathol. 2005;36:878–85. doi: 10.1016/j.humpath.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Zuckerman E, Kessel A, Slobodin G, et al. Antiviral treatment down-regulates peripheral B-cell CD81 expression and CD5 expansion in chronic hepatitis C virus infection. J Virol. 2003;77:10432–6. doi: 10.1128/JVI.77.19.10432-10436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strebel K. Virus–host interactions: role of HIV proteins Vif, Tat, and Rev. AIDS. 2003;17(Suppl. 4):S25–34. doi: 10.1097/00002030-200317004-00003. [DOI] [PubMed] [Google Scholar]
- 52.Bettaccini AA, Baj A, Accolla RS, et al. Proliferative activity of extracellular HIV-1 Tat protein in human epithelial cells: expression profile of pathogenetically relevant genes. BMC Microbiol. 2005;5:20. doi: 10.1186/1471-2180-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huigen MC, Kamp W, Nottet HS. Multiple effects of HIV-1 trans-activator protein on the pathogenesis of HIV-1 infection. Eur J Clin Invest. 2004;34:57–66. doi: 10.1111/j.1365-2362.2004.01282.x. [DOI] [PubMed] [Google Scholar]