Abstract
Lyme borreliosis (LB) can, despite adequate antibiotic treatment, develop into a chronic condition with persisting symptoms such as musculoskeletal pain, subjective alteration of cognition and fatigue. The mechanism behind this is unclear, but it has been postulated that an aberrant immunological response might be the cause. In this study we investigated the expression of the T helper 1 (Th1) marker interleukin (IL)-12Rβ2, the marker for T regulatory cells, forkhead box P3 (FoxP3) and the cytokine profile in patients with a history of chronic LB, subacute LB, previously Borrelia-exposed asymptomatic individuals and healthy controls. Fifty-four individuals (12 chronic LB, 14 subacute LB, 14 asymptomatic individuals and 14 healthy controls) were included in the study and provided a blood sample. Mononuclear cells were separated from the blood and stimulated with antigens. The IL-12Rβ2 and FoxP3 mRNA expression was analysed with real-time reverse transcription–polymerase chain reaction (RT–PCR). The protein expression of IL-12Rβ2 on CD3+, CD4+, CD8+ and CD56+ cells was assessed by flow cytometry. Furthermore, the secretion of interferon (IFN)-γ, IL-4, IL-5, IL-10, IL-12p70 and IL-13 was analysed by enzyme-linked immunospot (ELISPOT) and/or enzyme-linked immunosorbent assay (ELISA). Chronic LB patients displayed a lower expression of Borrelia-specific IL-12Rβ2 on CD8+ cells and also a lower number of Borrelia-specific IFN-γ-secreting cells compared to asymptomatic individuals. Furthermore, chronic LB patients had higher amounts of Borrelia-specific FoxP3 mRNA than healthy controls. We speculate that this may indicate that a strong Th1 response is of importance for a positive outcome of a Borrelia infection. In addition, regulatory T cells might also play a role, by immunosuppression, in the development of chronic LB.
Keywords: chronic, cytokine, FoxP3, IL-12Rβ2, Lyme borreliosis
Introduction
Lyme borreliosis (LB) is the most common vector-borne disease in Europe [1]. A first sign of LB can be the circular bluish-red patch, erythema migrans (EM) [2]. Late manifestations of the disease include neurological symptoms such as radiculitis, paresis and headache [3]. Although LB is treatable with antibiotics there are patients who, despite completing treatment, have persistent symptoms, including musculoskeletal pain, paraesthesia, fatigue and subjective alteration of cognition [4–6]. They are diagnosed with chronic LB [7]. The mechanism behind these persisting symptoms is unclear. Studies show that long-term antibiotic therapy does not improve the clinical picture of these patients [8–10]. The chronic manifestations might be the result of injury caused by the immune response [11].
According to previous studies the most efficient way to eradicate the Borrelia spirochete seems to be by mounting a strong T helper 1 (Th1)-type immune response early in the infection, i.e. interferon (IFN)-γ-mediated activation of macrophages and CD8+ T cells [12–15]. Later this response should be switched over to a Th2-type response, which suppresses the Th1-type inflammation by the antagonistic effect interleukin (IL)-4 exerts on IFN-γ. If this switching is delayed, there might be a risk of tissue damage and the development of chronic LB [16,17]. Children diagnosed with neuroborreliosis display both a Th1 and a Th2 response in cerebrospinal fluid (CSF), whereas adults with the same diagnosis have a more Th1-deviated response [18]. Chronic disease is rarely seen in children [19], possibly the result of a more balanced immune response to the Borrelia spirochete. Furthermore, the ability to establish strong Th1 responses in mice is known to depend on the genetic background and similar differences in humans have been reported [20].
IL-12, in its active form termed IL-12p70, is a key cytokine in the mounting of strong Th1-responses, as it induces the differentiation of naive Th cells into Th1 and stimulates the secretion of IFN-γ[21]. The functional high-affinity IL-12 receptor is a heterodimer consisting of two chains, IL-12Rβ1 and IL-12Rβ2, the latter being the primary signal transduction component [22]. IL-12Rβ1 is expressed constitutively on activated T and natural killer (NK) cells [23], whereas IL-12Rβ2 is found only on cytotoxic T cells, Th1 and NK cells [24,25]. Decreased capacity to induce expression of IL-12Rβ2 has been reported in blood mononuclear cells from atopic individuals, who mount Th2-responses preferentially upon antigenic stimulation in vitro[26]. Conversely, increased expression of IL-12Rβ2 mRNA has been reported in patients with Crohn's disease, which is believed to be Th1-mediated [27].
Regulatory T cells (Treg), characterized by the expression of CD4 and high CD25 levels, are believed to play a significant role in the regulation of inflammatory responses by inhibiting T cells by cell–cell interaction and by secretion of anti-inflammatory cytokines, mainly IL-10 and transforming growth factor (TGF)-β[28,29]. Treg require the transcription factor forkhead box p3 (FoxP3) for development and function [30]. Mice lacking the functional FoxP3 are unable to regulate their lymphocyte activity and thereby do not survive, emphasizing the importance of Treg cells in controlling the immune system [29].
The aim of this study was to determine if there were constitutive differences in the ability to mount a strong Th1-type response between patients with chronic LB, subacute LB and Borrelia seropositive asymptomatic individuals, and whether the Borrelia-induced Treg response was altered in chronic LB. Differences in the ability to mount Th1-type responses were studied both by analyses of the induction of IL-12Rβ2, determined at mRNA and protein levels, and by assessing the following secretion of Th1/Th2 associated cytokines at single cell and protein levels. Borrelia-induced Treg responses were determined by analysis of FoxP3 mRNA expression.
Materials and methods
Subjects
Fifty-four individuals were included in the study. They were divided into four groups (Table 1) based on their diagnosis; patients with a history of chronic LB (n = 12), patients with a history of subacute LB (n = 14), Borrelia seropositive asymptomatic individuals (n = 14) and healthy controls seronegative for Borrelia (n = 14). Patients with LB were recruited from the Department of Infectious Diseases at the University Hospital in Linköping and the healthy controls comprised staff at the same hospital. The asymptomatic individuals were located by screening people who attended the Blood Centre of the Department of Transfusion Medicine, University Hospital in Linköping.
Table 1.
Description of the diagnostic groups.
| Chronic LB n = 12 | Subacute LB n = 14 | Asymptomatic n = 14 | Healthy control n = 14 | |
|---|---|---|---|---|
| Age, mean (range) years | 62 (27–82) | 53 (25–70) | 54 (38–73) | 50 (23–72) |
| Sex, F : M | 6 : 6 | 2 : 12 | 2 : 12 | 9 : 5 |
LB, Lyme borreliosis; F, female; M, male.
LB was diagnosed according to the European clinical case definition [2], i.e. defined as an EM ≥ 5 cm or clinically relevant neurological symptoms, mononuclear pleocytosis in CSF (≥ 5 × 106 cells/l) and Borrelia-specific antibodies in CSF or serum. Patients diagnosed with chronic LB had had symptoms for longer than 6 months and patients with subacute LB had had symptoms for less than 6 months [3]. All LB patients received antibiotic therapy (Table 2).
Table 2.
Characteristics of patients with chronic and subacute Lyme borreliosis.
| CSF | |||||||
|---|---|---|---|---|---|---|---|
| No. | Diagnosis | Known EM | Known tick bite | MNC- pleocytosis | Borrelia antibody IgG and/or IgM | Clinical manifestations | Therapy |
| 1 | Chronic NB | + | – | + | + | Disturbed balance and hearing, speech impairment | Doxycycline, Ceftriaxone |
| 2 | Chronic NB | – | – | + | + | Disturbed balance, nausea, back pain, vertigo | Doxycycline |
| 3 | Chronic NB | – | – | + | + | Facial palsy, headache | Ceftriaxone |
| 4 | Chronic NB | – | – | + | + | Facial palsy, headache, radiculitis, back pain, fever | Doxycycline |
| 5 | Chronic NB | – | – | + | + | Facial palsy, headache, back and neck pain | Doxycycline |
| 6 | Chronic NB | – | – | + | + | Headache, numbness | Doxycycline |
| 7 | Chronic ACA | – | + | n.d. | n.d. | Headache, radiculitis, knee pain, vertigo | Doxycycline |
| 8 | Chronic NB | – | – | – | + | Neck stiffness, arthralgia, fatigue | Doxycycline, Ceftriaxone |
| 9 | Chronic NB | – | – | + | + | Pain in legs, numbness | Ceftriaxone |
| 10 | Chronic NB | – | + | + | + | Facial palsy, radiculitis, vertigo, fatigue | Doxycycline, Ceftriaxone |
| 11 | Chronic NB | – | + | + | + | Headache, fatigue, athralgia, neck and joint pain, vertigo, cognitive impairment | Doxycycline |
| 12 | Chronic NB | – | – | + | + | Facial palsy, head ache, fever, fatigue | Doxycycline |
| 13 | Subacute NB | – | + | + | + | Facial palsy, numbness, migrating pain | Doxycycline |
| 14 | Subacute NB | – | – | + | + | Facial palsy, headache, radiculitis, numbness | Doxycycline |
| 15 | Subacute NB | – | – | – | + | Headache, fiver, fatigue, weight lost, vertigo | Doxycycline, Ceftriaxone |
| 16 | Subacute NB | – | + | + | + | Radiculitis, numbness, pain in legs | Doxycycline |
| 17 | Subacute NB | – | – | + | + | Facial palsy, double vision | Doxycycline |
| 18 | Subacute NB | – | + | + | + | Headache, cognitive impairment, vertigo | Doxycycline |
| 19 | Subacute NB | – | – | – | + | Facial palsy, headache, radiculitis, fatigue, nausea | Doxycycline |
| 20 | Subacute NB | – | + | + | + | Facial palsy, numbness, memory deficit | Doxycycline |
| 21 | Subacute NB | – | + | + | + | Facial palsy, numbness, back pain, vertigo | Ceftriaxone |
| 22 | Subacute NB | – | – | + | + | Radiculitis, fever, fatigue, pain, weakness, tremor, disturbed balance | Doxycycline |
| 23 | Subacute NB | + | – | + | + | Facial palsy, headache, radiculitis | Doxycycline |
| 24 | Subacute NB | – | – | + | + | Fever, fatigue, neck pain, myalgia | Doxycycline |
| 25 | Subacute NB | – | – | + | + | Facial palsy, radiculitis, back and neck pain | Doxycycline |
| 26 | Subacute LB | + | + | n.d. | n.d. | Eye pain | Doxycycline |
EM, erythema migrans; CSF, cerebrospinal fluid; MNC, mononuclear cells; NB, neuroborreliosis; ACA, acrodermatitis chronica atrophicans; LB, Lyme borreliosis; n.d., not done.
The asymptomatic individuals had no recollection of an EM and no other borreliosis-related symptoms. They all had positive Borrelia serology and positive T cell reaction to Borrelia-antigen, analysed by enzyme-linked immunospot assay (ELISPOT) [31]. Seven of 14 individuals remembered being bitten by a tick.
To evaluate the possible confounding effect of atopy, the participants were asked to complete a questionnaire on atopic diseases. The response frequency was 87% (47/54) and the response rate was similar among the groups. Atopic diseases were diagnosed by an experienced allergologist in 36% of chronic LB, 46% of subacute LB, 42% of asymptomatic individuals and 36% of healthy controls (no statistically significant differences).
Preparation and stimulation of mononuclear cells
Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood using gradient centrifugation on Ficoll Paque (Pharmacia Biotech, Sollentuna, Sweden) as described previously [32]. The cell density was adjusted to 1 × 106 lymphocytes/ml.
PBMC were cultured in RPMI-1640 (Life Technologies AB, Täby, Sweden) with 10% heat-inactivated fetal calf serum (FCS) (Sigma Aldrich, Stockholm, Sweden) and stimulated with an outer surface protein-enriched fraction of Borrelia garinii strain Ip90 (OF) [16], with a final concentration of 10 µg/ml, purified protein derivate of tuberculin (PPD) in a final concentration of 10 µg/ml (Statens Serum Institut, Copenhagen, Denmark) or phytohaemagglutinin (PHA) in a final concentration of 2 µg/ml (Sigma Aldrich). The cells were incubated at 37°C with 5% CO2 and 95% humidity. Cells used for polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) were cultured for 44 h (PHA) or 92 h (OF and PPD) and for flow cytometry incubation times were 20 h (PHA) and 44 h (OF). For each stimulation and time, control cells were also cultured, i.e. only medium was added.
The supernatants were collected after centrifugation and stored at −70°C. The stimulated cells were lysed with RLT lysis buffer (RNeasy 96 RNA extraction kit; Qiagen, Hilden, Germany) and stored at −70°C for later RNA extraction.
Flow cytometry analysis of IL-12β2 receptor
After stimulation, as described above, cells were centrifuged and resuspended in fluorescence activated cell sorter (FACS) medium consisting of phosphate-buffered saline (PBS) with 2% FCS (Sigma Aldrich). Cells were labelled with primary monoclonal antibodies: rat anti-human IL-12Rβ2, clone 2B6/12β2 (BD, Stockholm, Sweden) and mouse anti-human CD3-fluorescein isothiocyanate (FITC), clone UCHT1 (Dako, Solna, Sweden), mouse anti-human CD4-FITC, clone MT310 (Dako), mouse anti-human CD8-FITC, clone DK25 (Dako) or mouse anti-human CD56-FITC, clone NCAM16.2 (BD). As negative controls isotype-matched antibodies were used, mouse IgG2b-FITC, clone MPC-11 (BD) for CD56, rat IgG2a, clone R35-95 (BD) for IL-12Rβ2 and mouse IgG1-FITC, clone DAK-G01 (Dako) for CD3, CD4 and CD8. After washing, cells were resuspended in FACS medium and incubated with fragment (Fab′2) mouse anti-rat IgG conjugated with biotin (Jackson ImmunoResearch Laboratories, Baltimore, MD, USA), to label the antibodies of anti-IL-12Rβ2. The cells were washed three times in FACS medium, incubated with streptavidin-R-phycoerythrin (Dako) and then washed twice before final resuspention in FACS medium.
Two-colour flow cytometry was performed using FACSCalibur flow cytometry (BD) and results were analysed using CellQuest Pro.
RNA extraction
Total RNA was extracted according to the RNeasy 96 Protocol (Qiagen). In brief, cells were lysed, mixed with ethanol and applied to a RNeasy 96-well plate. Contaminations were washed away, the membrane was dried and the RNA was eluted in Rnase-free water.
Reverse transcription (RT)
RNA was converted to cDNA using the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions.
Real-time polymerase chain reaction (PCR)
The amount of IL-12Rβ2 mRNA and rRNA was quantified as described previously [26]. FoxP3 mRNA was quantified using TaqMan® Gene Expression Assay (assay i.d.: Hs00203958_m1, Applied Biosystems) according to the manufacturer's instructions.
Standards from which a standard curve was calculated were included in each run. All samples were run in duplicate and rRNA and mRNA were analysed separately.
ELISPOT
The ELISPOT assay used was performed as described in detail elsewhere [12,33]. In brief, plates were coated with monoclonal antibodies, anti-IFN-γ, anti-IL-4, anti-IL-5, anti-IL-10, anti-IL-12p70 and anti-IL-13 (Mabtech AB, Nacka, Sweden) incubated overnight and then frozen at −20°. The coated plates were thawed, 100 000 lymphocytes/well were added and stimulated with OF (final concentration of 10 µg/ml) or, as positive control, PHA (final concentration of 20 µg/ml; Sigma Aldrich). To detect the spontaneous secretion cells were not stimulated; only medium was added to these wells. The spontaneous secretion and stimulations were performed in triplicate. As a negative control, wells were filled with medium only (i.e. no cells). The plates were then incubated at 37°C with 5% CO2 and 95% humidity for 48 h.
Developing was performed with paired biotin-conjugated monoclonal antibodies (Mabtech AB), streptavidin conjugated with alkaline phosphatase (Mabtech AB) and finally AP colour development reagent nitroblue tetrazolium (NBT) and bromo-chloro-indolyl phosphate (BCIP) diluted in AP-buffer (AP conjugate substrate kit; Bio-Rad Laboratories AB, Sundbyberg, Sweden), with washings between all steps.
The spots were counted by the same person (S. J.) using the AID EliSpot Reader System version 2.6 (AID, Strassberg, Germany).
ELISA
The ELISA assay for detection of IFN-γ, IL-5 and IL-10 was performed as described previously by Jenmalm et al. [34]. In brief, plates were coated with antibodies, anti-IFN-γ (PeliPair; Sanquin Reagents, Amsterdam, the Netherlands), anti-IL-5 (R&D Systems, Abingdon, Oxon, UK) or anti-IL-10 (PeliPair; Sanquin Reagents). Samples and standards, diluted in RPMI-1640 (Life Technologies AB) with 10% FCS (Sigma Aldrich), were added in duplicate wells. Medium only was used as a negative control. After washing, biotinylated antibodies were added followed by streptavidin–horseradish peroxidase (Sanquin Reagents) and 3,3′, 5,5′-tetrametylbenzidine (Sigma Aldrich). The reaction was stopped by adding 1·8 M H2SO4. The amount of substrate converted to product was detected as absorbance at 450 nm in a Multiskan Ascent V1·24 ELISA reader (Therma Labsystems, Helsinki, Finland).
Values were calculated from the absorbance of the standard curve after subtracting the negative control. The sensitivity limit for quantitative determinations was 3 pg/ml for IFN-γ, 4 pg/ml for IL-5 and 3 pg/ml for IL-10.
Data handling
Flow cytometry
A total of 20 000 events were counted. Lymphocytes were gated based on size and granularity. Percentages of cells co-expressing antigen-induced IL-12Rβ2 and CD3, CD4, CD8 or CD56 were calculated by setting the detection limit for non-stimulated cells.
Real-time PCR
The amount of IL-12Rβ2 and FoxP3 mRNA was normalized by division of the amount of rRNA. To obtain the Borrelia-specific and PHA-induced quantity, the amount of the non-stimulated cells was subtracted.
ELISPOT and ELISA
For the ELISPOT assay, the mean value of number of spots in the wells, spontaneous and stimulated, was calculated from the triplicate. The mean value was also calculated from the duplicate wells in the ELISA assay. To obtain the specific antigen-induced secretion, both for the ELISPOT and ELISA assays, the mean value of the spontaneous secretion was subtracted from the mean value of the stimulated secretion.
Statistics
The data were not normally distributed; hence to compare values between groups non-parametric tests were used. The Kruskal–Wallis test was used as a pretest and the Mann–Whitney U-test as post-hoc when comparing data regarding IL-12Rβ2 and cytokine secretion. No corrections for multiple comparisons were made as the parameters analysed were viewed as part of a pattern and not as separate events. The results were therefore not evaluated as separate but seen as being interconnected. For the previously uninvestigated expression of FoxP3, a lower level of testing was applied using only the Mann–Whitney U-test. Correlation analysis was performed using Spearman's rank correlation and the differences in frequency of atopy were evaluated using Fisher's exact test. Statistical calculation was performed with spss version 11·5 for Windows (SPSS Inc., Chicago, IL, USA). A P-value of < 0·05 was considered significant.
Ethics
The study was approved by The Regional Ethical Review Board in Linköping, Sweden. All participants gave informed consent.
Results
Cell surface expression of IL-12Rβ2
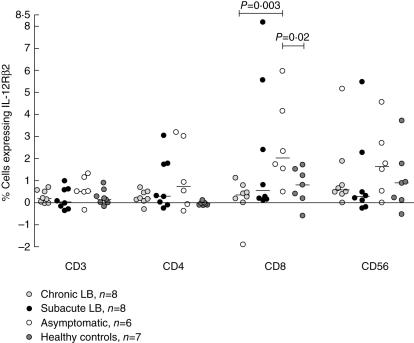
Asymptomatic individuals displayed a higher level of Borrelia-specific IL-12Rβ2 on CD8+ cells than patients with chronic LB (P = 0·003, Fig. 1) and healthy controls (P = 0·02, Fig. 1). No difference was seen between the groups for the expression of IL-12Rβ2, after Borrelia stimulation, on CD3+, CD4+ or CD56+ cells (Fig. 1) or on PHA-induced expression on CD3+, CD4+, CD8+ or CD56+ (Table 3).
Fig. 1.
Percentage of peripheral blood lymphocytes expressing interleukin (IL)-12Rβ2 in response to Borrelia stimulation detected by flow cytometry. P-values show statistically significant differences from comparison with the Mann–Whitney U-test. Each point represents one individual and the lines mark the median values. LB, Lyme borreliosis.
Table 3.
Percentage of peripheral blood lymphocytes expressing interleukin (IL)-12 receptor β2 detected with flow cytometry, ratio of mRNA/rRNA in peripheral blood mononuclear cells quantified using real-time polymerase chain reaction (PCR) and cytokine secretion detected with enzyme-linked immunospot or enzyme-linked immunosorbent assay.
| Chronic LB | Subacute LB | Asymptomatic | Healthy control | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (min–max) | n | Median (min–max) | n | Median (min–max) | n | Median (min–max) | ||
| Cell phenotype | Antigen | ||||||||
| CD3+ | PHA | 9 | 4·3 (0·0–14·0) | 8 | 0·4 (− 0·2–33·3) | 6 | 3·0 (0·2–12·1) | 8 | 2·5 (0·0–10·2) |
| CD4+ | PHA | 9 | 2·9 (0·2–13·7) | 8 | 0·2 (− 0·4–39·7) | 6 | 4·1 (0·1–13·5) | 8 | 3·7 (0·6–11·0) |
| CD8+ | PHA | 9 | 2·9 (− 0·3–6·9) | 8 | 1·7 (0·6–26·5) | 6 | 0·3 (0·0–9·5) | 8 | 1·3 (− 0·5–6·0) |
| CD56+ | PHA | 9 | 0·9 (− 0·4–8·3) | 7 | − 0·2 (− 0·9–26·2) | 5 | 0·3 (0·0–2·6) | 8 | 1·7 (0·0–4·2) |
| mRNA | Antigen | ||||||||
| IL-12Rβ2 | OF | 9 | 0·3 (− 1·1–3·0) | 13 | 0·5 (0·1–4·7) | 13 | 0·2 (− 1·5–2·6) | 12 | 0·1 (− 0·3–9·7) |
| PHA | 10 | 9·2 (0·3–66·1) | 13 | 1·2 (0·2–21·6) | 13 | 4·4 (0·2–66·7) | 13 | 2·2 (0·4–60·7) | |
| PPD | 3 | 2·6 (0·9–4·7) | 9 | 1·1 (0·1–14·3) | 9 | 1·2 (− 1·8–1·8) | 7 | 2·2 (0·0–37·2) | |
| FoxP3 | PHA | 10 | 1·3 (− 1·8–7·2) | 13 | 1·8 (0·7–12·1) | 13 | 2·5 (0·8–8·6) | 13 | 1·7 (0·7–11·9) |
| PPD | 3 | 1·0 (0·9–2·5) | 9 | 0·6 (− 5·7–2·0) | 9 | 0·2 (− 4·8–1·4) | 7 | − 0·8 (− 4·6–1·5) | |
| Antigen | Cytokine | ||||||||
| OF (ELISPOT) | IL-4 | 9 | 2·0 (− 5·6–36·2) | 9 | 0·9 (− 7·4–4) | 12 | 2·2 (− 3·3–24·2) | 13 | ·0 (− 14·7–3·7) |
| IL-5 | 7 | 3·9 (− 3·2–40·6) | 7 | 1·4 (− 7·3–17·8) | 4 | 10·9 (− 9·2–56·5) | 4 | 2·6 (2·0–11·0) | |
| IL-10 | 9 | 323·5 (− 33·5–821·7) | 6 | 109·3 (20·5–1063·3) | 6 | 325·3 (9·0–962·4) | 7 | 236·0 (4·1–956·2) | |
| IL-13 | 7 | 2·3 (− 0·7–20·7) | 5 | −· 0·5 (− 6·2–15·6) | 4 | 4·0 (− 82·8–19·6) | 4 | − · 0·2 (− 2·3–4·0) | |
| OF (ELISA) | IFN-γ | 11 | 177·9 (− 37·0–1111·8) | 11 | 88·4 (− 5·4–544·1) | 13 | 67·7 (− 273·8–1013·5) | 12 | 106·1 (− 1102·7–943·8) |
| IL-5 | 11 | ·0 (− 8·1–27·7) | 11 | ·0 (− 11·1–10·2) | 13 | 0·6 (− 43·6–25·0) | 12 | ·0 (− 4·2–35·7) | |
| IL-10 | 11 | 61·2 (37·7–170·0) | 11 | 59·5 (4·8–153·8) | 13 | 58·0 (3·9–443·1) | 12 | 110·0 (− 6·3–646·6) | |
| PPD (ELISA) | IFN-γ | 5 | 5099·5 (920·6–27846·1) | 7 | 4913·5 (133·3–28650·0) | 10 | 1841·8 (82·2–23320·1) | 9 | 4461·0 (− 181·0–52009·7) |
| IL-5 | 5 | 9·4 (− 1·6–60·8) | 7 | 16·7 (− 6·0–43·7) | 10 | 9·9 (− 29·6–52·1) | 9 | ·0 (− 4·2–21) | |
| IL-10 | 5 | 27·7 (26·8–45·1) | 7 | 32·0 (4·6–76·5) | 10 | 23·5 (10·7–67·5) | 9 | 68·2 (− 21·1–249·0) | |
| PHA (ELISA) | IFN-γ | 11 | 627·5 (39·1–14322·7) | 11 | 1176·9 (29·0–8047·9) | 13 | 2543·4 (46·8–5899·2) | 13 | 574·9 (17·6–4039·4) |
| IL-5 | 12 | 61·4 (10–159·3) | 11 | 30·8 (4·1–485·2) | 13 | 40·0 (3·9–510·0) | 13 | 5·3 (0–164·5) | |
| IL-10 | 12 | 250·8 (41·3–435·4) | 11 | 294·1 (24·7–1207·6) | 13 | 309·6 (42·6–716·1) | 13 | 264·3 (73·3–674·7) | |
LB, Lyme borreliosis; PHA, phytohaemagglutinin; OF, outer surface protein enriched fraction of Borrelia garinii strain Ip90; PPD, purified protein derivate of tuberculin; Fox, forkhead box; IL, interleukin; IFN, interferon.
Quantification of IL-12Rβ2 and FoxP3 mRNA
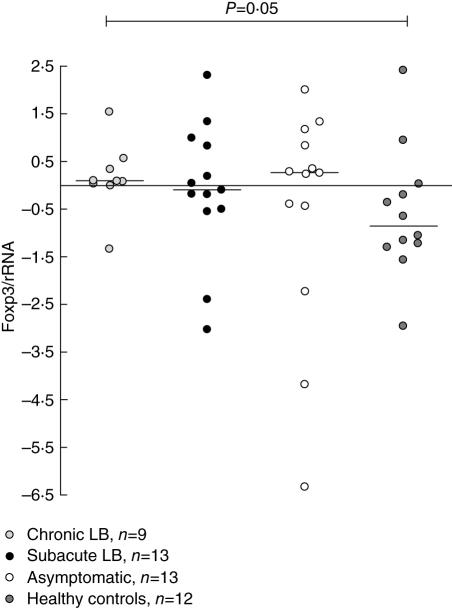
The Borrelia-specific FoxP3 mRNA expression was higher in patients with chronic LB compared with healthy controls (P = 0·05, Fig. 2), whereas the PPD-specific or PHA-induced FoxP3 mRNA expression was similar among the groups (Table 3), nor was any statistical difference found between the groups for the Borrelia-specific, PPD- or PHA-induced IL-12Rβ2 mRNA expression (Table 3).
Fig. 2.
Ratio of Borrelia-specific forkhead box P3 (FoxP3) mRNA/rRNA in peripheral blood mononuclear cells, quantified using real-time polymerase chain reaction (PCR). P-values show statistically significant differences from comparison with the Mann–Whitney U-test. Each point represents one individual and the lines mark the median values. LB, Lyme borreliosis.
Cytokine secretion, ELISPOT and ELISA
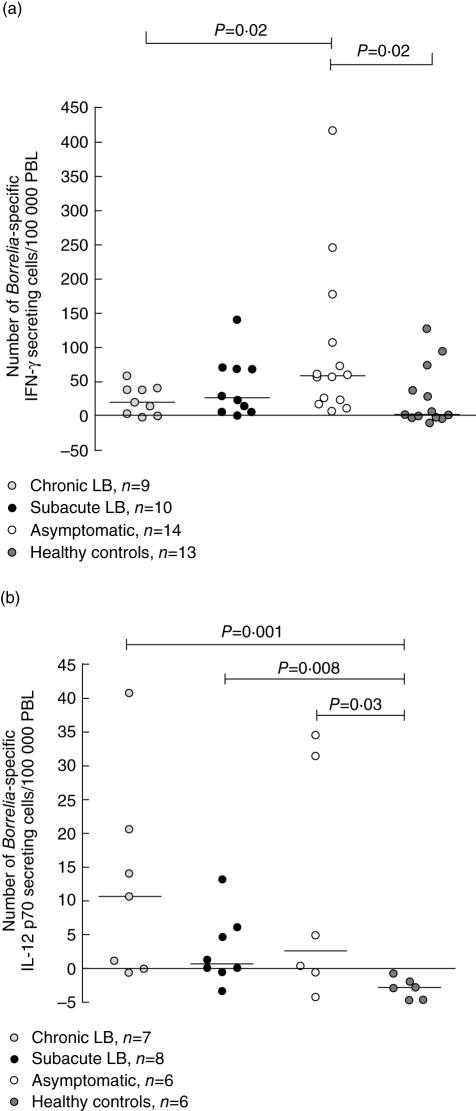
Asymptomatic individuals had higher number of Borrelia-specific cells secreting IFN-γ detected with ELISPOT, compared with chronic LB (P = 0·02, Fig. 3a) and healthy controls (P = 0·02, Fig. 3a). Chronic LB, subacute LB and asymptomatic individuals had a significantly higher number of Borrelia-specific cells secreting IL-12p70 compared to healthy controls (P = 0·001, P = 0·008, respectively, P = 0·03, Fig. 3b).
Fig. 3.
Number of Borrelia-specific interferon (IFN)-γ (a) and interleukin (IL)-12p70 (b) secreting cells/100 000 peripheral blood lymphocytes (PBL) detected by enzyme-linked immunospot assay (ELISPOT). Values are net values, thus the number of spontaneously cytokine-secreting cells has been subtracted. P-values show statistically significant differences from comparison with the Mann–Whitney U-test. Each point represents one individual and the lines mark the median values. LB, Lyme borreliosis.
No statistical difference was found between the groups for Borrelia-specific secretion of IL-4, IL-5, IL-10 or IL-13 detected by ELISPOT, or for IFN-γ, IL-5 or IL-10 measured with ELISA (Table 3). PHA-induced and PPD-specific secretion of IFN-γ, IL-5 or IL-10, as measured by ELISA, was also similar among the groups (Table 3).
IFN-γ secretion correlated with IL-12Rβ2 mRNA expression
The PHA-induced IFN-γ secretion, measured with ELISA, correlated with the amount of PHA-induced IL-12Rβ2 mRNA (rho = 0·50, P = 0·0004). The same was also seen for the Borrelia-specific (rho = 0·60, P < 0·0001) and PPD-specific stimulation (rho = 0·39, P = 0·04). There was no correlation between the Borrelia-specific IFN-γ secretion when detected with ELISPOT and IL-12Rβ2 mRNA (data not shown).
Correlation of IL-12Rβ2 protein and mRNA expression
The expression of IL-12Rβ2 on CD3+ cells on a protein level did not correlate with the quantity of IL-12Rβ2 mRNA after Borrelia or PHA stimulation (data not shown).
IL-10 secretion correlated with FoxP3 mRNA expression
No correlation was found between the amount of IL-10, measured with ELISA and FoxP3 mRNA, PHA-, Borrelia- or PPD-induced expression (data not shown); nor did the number of Borrelia-specific IL-10-secreting cells, detected by ELISPOT, and FoxP3 mRNA correlate (data not shown).
Discussion
Our main finding was that chronic LB had a lower expression of Borrelia-specific IL-12Rβ2 on CD8+ cells than asymptomatic individuals. This difference was not seen on the transcriptional level, possibly because mRNA was not analysed in CD4+ and CD8+ cells separately. The same pattern seen for IL-12Rβ2 on CD8+ cells between the groups was also found for IFN-γ, detected by ELISPOT. Thus, chronic LB patients responded to stimulation with Borrelia antigen with a lower number of IFN-γ-secreting cells than asymptomatic individuals. We have reported earlier that CD8+ cells are the main producers of Borrelia-specific IFN-γ in human chronic LB [14], indicating that cytotoxic responses are involved in the eradication of Borrelia. The present finding supports this assumption, and needs to be investigated further.
Previous research has led to the hypothesis that a strong Th1-type immune response early in the course of the disease is the best strategy to eradicate promptly the Borrelia spirochete and also to avoid persisting symptoms [15,35]. We have demonstrated previously that patients with EM have an IFN-γ-dominated immune response to Borrelia antigen [16]. Furthermore, we have found that the spontaneous cytokine secretion in patients with a history of chronic LB is Th2-dominated. Thus, the ratio of IL-4/IFN-γ secretion was higher in chronic LB patients than in individuals with a history of asymptomatic infection [31], which may reflect a decreased ability to mount strong Th1 responses in patients who develop chronic LB. This is in line with findings from experimental studies in mice, where resistant mice showed strong Th1 responses early in infection whereas the susceptible mice, which did not eradicate the spirochetes, showed weak Th1 responses [35]. The present findings that patients with a chronic disease course have lower expression of IL-12Rβ2 on CD8+ cells and a lower number of IFN-γ-secreting cells, after stimulation with Borrelia-antigen, supports the theory that a Th1-type response is of importance for the outcome of LB. Furthermore, despite the higher number of cells secreting IL-12p70, most probably antigen-presenting cells, in response to Borrelia-stimulation seen in chronic LB, compared to healthy controls, these patients do not accumulate an IFN-γ response to the same antigen. This can be a reflection of the low IL-12Rβ2 expression which will result in the cells' inability to mount a Th1-type response.
The secretion of IFN-γ, PHA-induced, Borrelia- and PPD-specific, correlated with the expression of IL-12Rβ2 mRNA, supporting the importance of IL-12Rβ2 expression for a Th1-type response. This has also been reported previously by Janefjord et al. [26]. We found no correlation between the percentages of IL-12Rβ2-expressing cells and the quantity of IL-12Rβ2 mRNA. This might be a reflection of regulation on a post-transcriptional level, due possibly to RNA-binding proteins [36].
In the present study, the diagnostic groups did not differ in their amount of Borrelia-specific IFN-γ secretion analysed with ELISA, but differences were seen in the number of cytokine-secreting cells detected by ELISPOT. The amount of cytokine does not necessarily correlate with the number of secreting cells [37,38]. The inconsistency of the results from the two methods might be due to in vitro consumption of IFN-γ, which is higher in cell culture supernatants than in the ELISPOT assay.
The balance of the immune system is also modulated by Treg. Stoop et al. have shown that patients with chronic hepatitis B virus infection show an increased percentage Treg in blood compared to non-chronic patients [39]. They speculate that the immunosuppressive effect of Treg could contribute to the inadequate immune response to the virus. In this study, we found that chronic LB patients had a higher amount of FoxP3 mRNA than healthy controls, after stimulation with Borrelia antigen. This difference was not seen between healthy controls and asymptomatic individuals or subacute LB patients. Indirectly, this could possibly indicate a stronger Treg expression in chronic LB patients than in patients with a more benign disease course. The finding needs to be investigated further before any definite conclusions could be drawn, for example analysing cells on a single-cell level.
In contrast, we did not find a difference between chronic LB, subacute LB and asymptomatic individuals in IL-10 secretion, a cytokine produced by Treg. Nor did we find a correlation between FoxP3 mRNA and the secretion of IL-10. However, cytokine secretion might not be the optimal approach to study Treg as these cells constitute a small percentage of the total number of T cells and IL-10 is produced more probably by other cells, e.g. macrophages and platelets, than by Treg. Also, Treg might induce inhibition by cell–cell contact and not by cytokine secretion, as seen in hepatitis C [40].
In conclusion, we found a lower expression of Borrelia-specific IL-12Rβ2 on CD8+ cells and also a lower number of Borrelia-specific IFN-γ-secreting cells from chronic LB patients compared to asymptomatic individuals. Possibly, this could indicate that a strong Th1 response is of importance for a positive outcome of a Borrelia infection. Furthermore, we showed that chronic LB had higher amounts of Borrelia-specific FoxP3 mRNA than healthy controls, which might imply that chronic LB patients have an immunosuppression caused by the increased Treg population.
Acknowledgments
The authors would like to thank Sven Bergström, Umeå University, Sweden for generously supplying us with the Borrelia spirochetes, Florence Sjögren for excellent advice on flow cytometry, Lotta Lindvall for helping with collecting blood samples and Anna Regnér for performing part of the ELISA analysis. The authors would also like to thank Karin Fält-Magnusson for evaluating the questionnaires on atopy. This work was supported by The County Council of Östergötland, The Swedish Research Council in Medicine, The Swedish Society of Medicine, Lions and University of Linköping.
References
- 1.Berglund J, Eitrem R, Ornstein K, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–27. doi: 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 2.Stanek G, O'Connell S, Cimmino M, et al. European Union Concerted Action on Risk Assessment in Lyme Borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–7. [PubMed] [Google Scholar]
- 3.Oschmann P, Dorndorf W, Hornig C, Schafer C, Wellensiek HJ, Pflughaupt KW. Stages and syndromes of neuroborreliosis. J Neurol. 1998;245:262–72. doi: 10.1007/s004150050216. [DOI] [PubMed] [Google Scholar]
- 4.Treib J, Fernandez A, Haass A, Grauer MT, Holzer G, Woessner R. Clinical and serologic follow-up in patients with neuroborreliosis. Neurology. 1998;51:1489–91. doi: 10.1212/wnl.51.5.1489. [DOI] [PubMed] [Google Scholar]
- 5.Vrethem M, Hellblom L, Widlund M, Ahl M, Danielsson O, Ernerudh J, Forsberg P. Chronic symptoms are common in patients with neuroborreliosis − a questionnaire follow-up study. Acta Neurol Scand. 2002;106:205–8. doi: 10.1034/j.1600-0404.2002.01358.x. [DOI] [PubMed] [Google Scholar]
- 6.Berglund J, Stjernberg L, Ornstein K, Tykesson-Joelsson K, Walter H. 5-y Follow-up study of patients with neuroborreliosis. Scand J Infect Dis. 2002;34:421–5. doi: 10.1080/00365540110080421. [DOI] [PubMed] [Google Scholar]
- 7.Klempner MS. Controlled trials of antibiotic treatment in patients with post-treatment chronic Lyme disease. Vector Borne Zoonotic Dis. 2002;2:255–63. doi: 10.1089/153036602321653842. [DOI] [PubMed] [Google Scholar]
- 8.Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60:1923–30. doi: 10.1212/01.wnl.0000071227.23769.9e. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan RF, Trevino RP, Johnson GM, et al. Cognitive function in post-treatment Lyme disease: do additional antibiotics help? Neurology. 2003;60:1916–22. doi: 10.1212/01.wnl.0000068030.26992.25. [DOI] [PubMed] [Google Scholar]
- 10.Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Monco JC, Benach JL. Mechanisms of injury in Lyme neuroborreliosis. Semin Neurol. 1997;17:57–62. doi: 10.1055/s-2008-1040914. [DOI] [PubMed] [Google Scholar]
- 12.Ekerfelt C, Ernerudh J, Bunikis J, et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-gamma predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–62. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 13.Widhe M, Ekerfelt C, Forsberg P, Bergström S, Ernerudh J. IgG subclasses in Lyme borreliosis. a study of specific IgG subclass distribution in an interferon-gamma-predominated disease. Scand J Immunol. 1998;47:575–81. [PubMed] [Google Scholar]
- 14.Ekerfelt C, Jarefors S, Tynngård N, et al. Phenotypes indicating cytolytic properties of Borrelia-specific interferon-gamma secreting cells in chronic Lyme neuroborreliosis. J Neuroimmunol. 2003;145:115–26. doi: 10.1016/j.jneuroim.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Oksi J, Savolainen J, Pene J, Bousquet J, Laippala P, Viljanen MK. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun. 1996;64:3620–3. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widhe M, Jarefors S, Ekerfelt C, et al. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J Infect Dis. 2004;189:1881–91. doi: 10.1086/382893. [DOI] [PubMed] [Google Scholar]
- 17.Yin Z, Braun J, Neure L, et al. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 18.Widhe M, Skogman BH, Jarefors S, et al. Up-regulation of Borrelia-specific IL-4- and IFN-gamma-secreting cells in cerebrospinal fluid from children with Lyme neuroborreliosis. Int Immunol. 2005;17:1283–91. doi: 10.1093/intimm/dxh304. [DOI] [PubMed] [Google Scholar]
- 19.Huppertz HI. Lyme disease in children. Curr Opin Rheumatol. 2001;13:434–40. doi: 10.1097/00002281-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Bullens DM, Kasran A, Peng X, Lorre K, Ceuppens JL. Effects of anti-IL-4 receptor monoclonal antibody on in vitro T cell cytokine levels: IL-4 production from non-atopic donors. Clin Exp Immunol. 1998;113:320–6. doi: 10.1046/j.1365-2249.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manetti R, Gerosa F, Giudizi MG, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179:1273–83. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presky DH, Yang H, Minetti LJ, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–7. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–32. [PubMed] [Google Scholar]
- 24.Rogge L, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogge L, Papi A, Presky DH, et al. Antibodies to the IL-12 receptor beta 2 chain mark human Th1 but not Th2 cells in vitro and in vivo. J Immunol. 1999;162:3926–32. [PubMed] [Google Scholar]
- 26.Janefjord CK, Jenmalm MC. PHA-induced IL-12R beta(2) mRNA expression in atopic and non-atopic children. Clin Exp Allergy. 2001;31:1493–500. doi: 10.1046/j.1365-2222.2001.01206.x. [DOI] [PubMed] [Google Scholar]
- 27.Parrello T, Monteleone G, Cucchiara S, et al. Up-regulation of the IL-12 receptor beta 2 chain in Crohn's disease. J Immunol. 2000;165:7234–9. doi: 10.4049/jimmunol.165.12.7234. [DOI] [PubMed] [Google Scholar]
- 28.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408–14. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–99. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 30.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 31.Ekerfelt C, Forsberg P, Svenvik M, Roberg M, Bergström S, Ernerudh J. Asymptomatic Borrelia-seropositive individuals display the same incidence of Borrelia-specific interferon-gamma (IFN-gamma)-secreting cells in blood as patients with clinical Borrelia infection. Clin Exp Immunol. 1999;115:498–502. doi: 10.1046/j.1365-2249.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenmalm MC, Aniansson-Zdolsek H, Holt PG, Bjorksten B. Expression of and responses to CD2 and CD3 in 18-month-old children with and without atopic dermatitis. Pediatr Allergy Immunol. 2000;11:175–82. doi: 10.1034/j.1399-3038.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 33.Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergström S. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–60. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenmalm MC, Van Snick J, Cormont F, Salman B. Allergen-induced Th1 and Th2 cytokine secretion in relation to specific allergen sensitization and atopic symptoms in children. Clin Exp Allergy. 2001;31:1528–35. doi: 10.1046/j.1365-2222.2001.01190.x. [DOI] [PubMed] [Google Scholar]
- 35.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–11. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keene JD, Lager PJ. Post-transcriptional operons and regulons co-ordinating gene expression. Chromosome Res. 2005;13:327–37. doi: 10.1007/s10577-005-0848-1. [DOI] [PubMed] [Google Scholar]
- 37.Ekerfelt C, Ernerudh J, Jenmalm MC. Detection of spontaneous and antigen-induced human interleukin-4 responses in vitro: comparison of ELISPOT, a novel ELISA and real-time RT–PCR. J Immunol Meth. 2002;260:55–67. doi: 10.1016/s0022-1759(01)00520-8. [DOI] [PubMed] [Google Scholar]
- 38.Diaz I, Mateu E. Use of ELISPOT and ELISA to evaluate IFN-gamma, IL-10 and IL-4 responses in conventional pigs. Vet Immunol Immunopathol. 2005;106:107–12. doi: 10.1016/j.vetimm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology. 2005;41:771–8. doi: 10.1002/hep.20649. [DOI] [PubMed] [Google Scholar]
- 40.Cabrera R, Tu Z, Xu Y, et al. An immunomodulatory role for CD4(+) CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]