Abstract
The impact of intestinal helminth infection on Mycobacterium tuberculosis (MTB)-specific immune responses during active tuberculosis (TB) is not known. We investigated the role of intestinal helminth infection in anti-MTB immunity by evaluating both cellular phenotype and cytokine profiles in patients with TB and patients with concomitant TB and intestinal helminth infection (TB + Helm) during TB therapy. Twenty-seven per cent of TB patients enrolled for the study were co-infected with at least one intestinal helminth. At baseline, absolute frequencies of leucocytes, monocytes and eosinophils from TB and TB + Helm patients differed from healthy subjects. Concomitant intestinal helminth infection in TB + Helm patients had a negative impact (P < 0·05) on absolute frequencies of CD3+, CD4+, CD8+, natural killer (NK) T and CD4+ CD25high T cell subsets when compared to either TB patients or healthy controls. Differences in CD4+ T cell frequencies were accompanied by lower interferon (IFN)-γ and elevated and sustained interleukin (IL)-10 levels in whole blood (WB) cultures from TB + Helm compared to TB patients. In addition to a depressed anti-MTB immunity, TB + Helm patients also presented with more severe radiological pulmonary disease, with a significant difference (P = 0·013) in the number of involved lung zones at the end of TB treatment. The above data may indicate that concomitant intestinal helminth infection in patients with newly diagnosed TB skews their cytokine profile toward a T helper 2 response, which could favour persistent MTB infection and a more protracted clinical course of the disease.
Keywords: anti-TB therapy, IFN-γ, IL-10, immune response, intestinal helminths, tuberculosis
Introduction
Tuberculosis (TB) remains the leading cause of morbidity and mortality due to any one infectious agent worldwide [1]. It has also been estimated that approximately 1·2 billion people harbour at least one species of intestinal parasite worldwide. In fact, 50–100 million people are infected with Strongyloides stercoralis, with a high prevalence in tropical regions of Africa, Asia and South America, particularly in Brazil [2–4]. It has been suggested that helminthic infections in humans are associated with a significant hyperactive humoral immunity and a depressed cellular immune response. Ultimately, this immunological dysregulation may facilitate concomitant infection or increase pathogenicity of other microbes; however, this is not fully understood. Co-infection with Mycobacterium tuberculosis (MTB) is common in the developing world; however, whether helminthic infection worsens immune responses to TB is unclear, as evidenced by recent studies [5–8].
Active TB is characterized by a profound and prolonged suppression of MTB-specific T cell responses, as evidenced by decreased production of the T helper 1 (Th1) cytokines, interleukin (IL)-2 and interferon (IFN)-γ [9–13]. Overproduction of immunosuppressive cytokines [IL-10 and transforming growth factor (TGF)-β] by mononuclear phagocytes has been implicated in decreased T cell function during TB [8,14–16]. Intestinal helminth infections in both human and experimental animal models are, generally, associated with a strong Th2-like immune response and significant suppression of Th1-type responses [2,6,7,17–20]. Considering that effective anti-MTB immunity is dependent upon an intact Th1-type immune response, it is possible that pre-existing infection with intestinal helminths may down-regulate the required Th1-type immune response via up-regulation of Th2-type cytokine production, thus ultimately facilitating progressive mycobacterial infection [5–7,21,22].
Active TB has been associated with the expansion of CD4+ CD25high+ subset of CD4 T cells (regulatory T cells, Treg) which may also participate, at least in part, in the suppression of MTB-specific T cell responses during active TB [23,24]. Interestingly, it has been demonstrated that Treg frequencies are augmented in nematode infections [25,26] and that antigen-specific Treg cells contribute to the immunosuppression associated with chronic onchocerciasis [25]. However, whether Treg cells in dually infected TB + Helm patients are further expanded is still not known.
The present study was conducted to evaluate cellular phenotype and cytokine profiles in patients with newly diagnosed active pulmonary tuberculosis with or without concomitant intestinal helminth infection and to evaluate the effect of helminth infection on the clinical course of TB.
Methods
Subjects
The present study protocol was reviewed and approved by the Institutional Review Boards both at Case Western Reserve University (CWRU) and Universidade Federal do Espírito Santo (UFES), and complies with human experimentation guidelines of the US Department of Health and Human Services. Forty consecutive, HIV-uninfected, acid-fast bacilli (AFB) smear-positive adult patients with newly diagnosed pulmonary TB attending the TB clinic at the Hospital Universitário Cassiano Antonio de Morais (HUCAM) in Vitória, Brazil were invited to participate in the study. Informed consent was obtained from each participant prior to their enrolment. A diagnosis of TB was confirmed in all patients by mycobacterial culture. Using chest X-ray, four TB patients had minimal disease, eight moderately advanced and 25 far advanced TB [27]. All TB patients were treated with standard short-course anti-MTB chemotherapy as described previously [28]. Twenty-five HIV-uninfected, intestinal-parasite-free, purified protein derivative (PPD)-positive healthy control subjects, with a positive skin test (> 10 mm) but not active TB, were recruited among staff at HUCAM. In order to investigate the presence of intestinal helminth infections, both healthy control subjects and TB patients provided at least three consecutive stool samples for parasitological diagnosis. Intestinal parasitism was diagnosed by three different techniques, using the methods of Lutz [29], Kato-Katz [30] and Baerman-Moraes [31,32]. TB patients positive for intestinal helminths are referred to as TB + Helm patients. All TB + Helm patients received standard therapy appropriate for the species of intestinal helminth they harboured: patients with S. stercoralis received thiobendazole, whereas mebendazole was given to patients infected with either Ascaris lumbricoides or Trichuris trichiura.
Samples
Blood samples were obtained from healthy control subjects, TB and TB + Helm patients. Procedures for collection and processing of sputum and blood samples from TB patients and healthy controls were as described previously [28]. Blood samples were obtained at baseline from healthy control subjects, and at both the beginning and the completion of anti-MTB therapy from TB and TB + Helm patients. Blood samples were used for cell phenotyping, flow cytometry analysis and for the establishment of whole blood (WB) cultures. Spontaneous sputum samples were also obtained from both TB and TB + Helm patients at multiple time-points during the course of anti-MTB therapy, and used for AFB smears, qualitative and quantitative mycobacterial cultures.
Analysis of cell phenotype by flow cytometry
Ethylenediamine tetraacetic acid (EDTA) blood was used for surface staining and analysis of T cell markers [three-colour analysis with combinations of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- and peridinin chlorophyll (PerCP)-conjugated antibodies]. Antibodies used included: anti-CD3 (SK7), anti-CD4 (SK3), anti-CD8 (SK1), anti-CD25 (2A3), anti-CD56 (My31), anti-CD16 (B73·1), anti-CD19 (4G7) and anti-IgG1 (X40) (Becton Dickinson Biosciences, Mountain View, CA, USA). Samples were analysed using a fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson Biosciences, Mountain View, CA, USA). Flow cytometry data were analysed in one session at study completion using CellQuest software (Becton Dickinson), to ensure that surface marker expression at all study time-points could be compared directly.
WB culture
Heparinized blood was diluted 10-fold and dispensed into 24-well tissue culture plates (1 ml aliquots per well). Wells remained unstimulated or received MTB (H37Rv) culture filtrate (CF) (5 µg/ml, provided under NIH contract). Cell-free supernatants were collected following 5-day incubation at 37°C, 5% CO2 and stored frozen at −70°C until use.
Cytokine assays
IFN-γ, IL-5 and IL-10 levels in WB culture supernatants were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits from Endogen (Woburn, MA, USA), R&D Systems (Minneapolis, MN, USA) and Biosource (Camarillo, CA, USA), respectively. The lower limit of detection for the assays was < 2 pg/ml, < 5 pg/ml and < 11 pg/ml, respectively.
Statistics
Normally distributed data sets were analysed by Student's t-test, paired t-test and correlation analysis. The Mann–Whitney U-test was used for data sets that were not normally distributed. Calculations were performed using both spss 10·0 for Windows (SPSS Inc., Chicago, IL, USA) and stata version 7 (STATA Corporation, College Station, TX, USA) statistical software programs. P ≤ 0·05 was considered significant.
Results
Characteristics of study subjects
The current study was conducted to evaluate cellular phenotype and cytokine profiles in patients with newly diagnosed active pulmonary tuberculosis with or without concomitant intestinal helminth infection. Demographic data from control subjects, TB and TB + Helm patients are summarized in Table 1. The majority of both TB and TB + Helm patients had extensive pulmonary involvement radiographically; although not statistically significant, a trend to a higher frequency of patients with advanced disease among TB + Helm compared to TB patients was observed (data not shown). Interestingly, the number of disease-involved zones at completion of TB treatment was significantly higher (P = 0·013) in TB + Helm as opposed to TB patients.
Table 1.
Gender and age of tuberculosis (TB) patients, patients with both TB and intestinal helminth infection and healthy control subjects.
| Age | |||||
|---|---|---|---|---|---|
| Groups | Gender | n | Mean | Median | Range |
| Healthy | 25 | 30·5 | 26·0 | 20–50 | |
| Male | 15 | 32·5 | 27·5 | 22–50 | |
| Female | 10 | 28·2 | 26·0 | 20–46 | |
| TB | 29 | 33·3 | 34·0 | 18–53 | |
| Male | 21 | 33·8 | 35·0 | 18–53 | |
| Female | 8 | 32·1 | 30·5 | 21–52 | |
| TB + Helm | 11 | 37·4 | 41·0 | 22–50 | |
| Male | 10 | 37·8 | 41·0 | 22–50 | |
| Female | 1 | 33·0 | 33·0 | n.a. | |
n.a.: Not applicable.
Concomitant intestinal helminth infection (TB + Helm patients) was found in 27·5% (11/40) of patients with TB. Parasitological findings showed that S. stercoralis was the most prevalent (72·7%) intestinal helminth among TB + Helm patients (eight of 11, data not shown).
Cellular distribution and phenotype are distinct between healthy subjects and TB and TB + Helm patients
First we examined absolute frequencies of white blood cells as a whole, and of lymphocyte, monocyte and eosinophil subsets (Table 2). While TB and TB + Helm patients displayed significant leucocytosis when compared to healthy control subjects (P < 0·02 for both), absolute leucocyte numbers were comparable between the two groups of TB patients. By contrast, absolute lymphocyte counts were reduced significantly between TB and TB + Helm patients when compared to healthy control subjects (P < 0·01 for both groups). Importantly, however, absolute lymphocyte numbers were significantly lower in blood from TB + Helm patients as opposed to TB patients (P = 0·006). Numbers of monocytes were significantly higher in blood from TB and TB + Helm patients than controls (P = 0·000). Interestingly while eosinophil counts were elevated in both TB and TB + Helm patients, when compared to controls differences in eosinophils counts were significant only between TB patients and healthy control subjects. These data indicate that patients with both active tuberculosis and concomitant intestinal helminth infection are characterized by a profound lymphopenia, as well as expansion of monocytes and eosinophils.
Table 2.
Differential white cell counts (cells/mm3) in blood from tuberculosis (TB) patients, patients with both TB and intestinal helminth infection and healthy control subjects.
| Healthy | TB | TB + Helminth | P-values* | |
|---|---|---|---|---|
| Leucocytes | 0·000a, 0·017b, 0·814c | |||
| Mean | 6667 | 9469 | 9936 | |
| Median | 6670 | 8920 | 9430 | |
| Range | 3840–8980 | 4870–17440 | 5950–15810 | |
| Lymphocytes | 0·010a, 0·000b, 0·006c | |||
| Mean | 2132 | 1734 | 1238 | |
| Median | 2180 | 1740 | 1320 | |
| Range | 1240–3110 | 940–2730 | 800–1820 | |
| Monocytes | 0·000a, 0·000b, 0·153c | |||
| Mean | 402 | 880 | 1075 | |
| Median | 380 | 800 | 998 | |
| Range | 120–600 | 464–1943 | 517–1597 | |
| Eosinophils | 0·015a, 0·187b, 0·987c | |||
| Mean | 144 | 279 | 281 | |
| Median | 140 | 197 | 179 | |
| Range | 40–340 | 13–1196 | 15–908 |
Significant differences between groups are depicted with their respective P-values and were calculated using independent-samples t-test.
TB patients compared to healthy controls.
TB + Helminth patients compared to healthy controls.
TB patients compared to TB + Helminth patients.
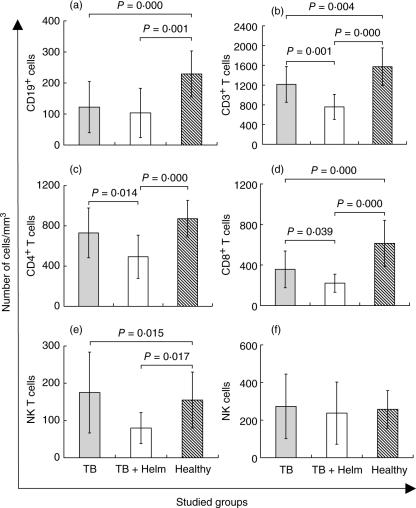
To investigate whether the observed lymphopenia was a result of a proportional reduction in all lymphocyte subpopulations, we next enumerated absolute counts of B, T and natural killer (NK) T cells. CD19+ B cell counts were significantly lower in both TB and TB + Helm patients as opposed to controls (P < 0·001 for both; Fig. 1a), but comparable between both groups of TB patients. Numbers of CD3+ T cells also were lower in blood from both TB patient groups compared to controls (P < 0·004; Fig. 1b). However, in contrast to findings for B cells, absolute frequencies of CD3+ T cells from TB + Helm patients were significantly decreased when compared to TB patients (P < 0·001). The distribution of CD4+, CD8+ and NK+ T cell subsets followed the same pattern observed for CD3+ T cells (Fig. 1c–e). However, when NK cells were evaluated no significant differences were observed between the three groups (Fig. 1f). CD4+ T cells are considered pivotal for the establishment of an effective anti-TB immune response. Considering that their absolute numbers were reduced significantly among TB + Helm patients (Fig. 1c), we next evaluated their frequency at both time of diagnosis and completion of TB treatment (Fig. 2a) in both TB and TB + Helm patients. In contrast to findings for absolute numbers, both CD4+ and CD8+ T cell frequencies at baseline were comparable between TB patient groups. However, at completion of anti-MTB therapy (week 24 of treatment) CD4+ T cell frequencies were significantly lower in blood from TB + Helm compared to TB patients (P = 0·019), whereas CD8+ T cell frequencies were similar (data not shown). These data suggest that TB + Helm patients undergo a defect in their T cell compartment that remains sustained by completion of TB treatment.
Fig. 1.
Absolute frequencies (cells/mm3) of T, B and natural killer (NK) cells and T cell subpopulations in blood from healthy control subjects, tuberculosis (TB) and TB and intestinal helminth infection (TB + Helm) patients. Results are expressed as mean average ± s.d. Significant differences between groups are depicted in the figure with their respective P-values and were calculated using independent-samples t-test.
Fig. 2.
(a) CD4+ T cell frequencies in blood from tuberculosis (TB) ( ) and TB and intestinal helminth infection (TB + Helm) (□) patients at the beginning and at the completion of TB therapy. Cytokine profile (b) interferon (IFN)-γ and (c) interleukin (IL-10), in Mycobacterium tuberculosis culture filtrate (MTB-CF)-stimulated whole blood (WB) culture supernatants from TB (
) and TB and intestinal helminth infection (TB + Helm) (□) patients at the beginning and at the completion of TB therapy. Cytokine profile (b) interferon (IFN)-γ and (c) interleukin (IL-10), in Mycobacterium tuberculosis culture filtrate (MTB-CF)-stimulated whole blood (WB) culture supernatants from TB ( ) and TB + Helm (□) patients at the beginning and at the completion of TB therapy. Results are expressed as mean average ± s.d. Significant differences between groups are depicted in the figure with their respective P-values and were calculated using paired-samples t-test.
) and TB + Helm (□) patients at the beginning and at the completion of TB therapy. Results are expressed as mean average ± s.d. Significant differences between groups are depicted in the figure with their respective P-values and were calculated using paired-samples t-test.
Cytokine profiles in MTB-CF stimulated WB culture supernatants differ between TB and TB + Helm patients
To establish a functional correlate for differences found in CD4+ T cell counts between TB and TB + Helm patients, we evaluated cytokine levels in MTB-CF-stimulated WB culture supernatants at the beginning [day 0 (D0)] and completion (week 24) of anti-MTB therapy. First, we evaluated IFN-γ immunoreactivity in culture supernatants from TB and TB + Helm patients at baseline. Although frequencies of CD4+ T cells were not significantly different (Fig. 2a), MTB-CF-induced production of IFN-γ was significantly lower in supernatants from TB + Helm compared to TB patients (Fig. 2b). At the end of TB treatment, IFN-γ levels remained suppressed in TB + Helm compared to TB patients, and followed the profile of CD4+ T cell frequencies.
As stated, IL-10 is induced strongly in response to intestinal helminth infections and also plays a role in immune suppression associated with active TB. In order to establish further the basis for depressed IFN-γ levels, we evaluated IL-10 immunoreactivity in culture supernatants in parallel. Interestingly, IL-10 levels were not significantly different in culture supernatants of TB and TB + Helm patients at baseline (Fig. 2c). However, at completion of treatment, IL-10 levels decreased significantly in culture supernatants from TB patients (P = 0·05) but remained elevated in supernatants from TB + Helm patients. These findings suggest that at least at the end of TB treatment, persistently low IFN-γ production may be a result of a sustained production of IL-10. Interestingly, IL-5 was not detectable in culture supernatants from either TB patients or TB + Helm patients (data not shown). Neither IL-4 nor TGF-β were evaluated in the current study. Preliminary data demonstrated that soluble IL-4 was not detectable in supernatants from WB cultures stimulated with MTB culture filtrate, and that TGF-β was not considered, because high background levels of TGF-β in WB cultures (released by platelets during degranulation) preclude meaningful analysis of antigen-induced changes in production of the cytokine.
Findings described above do not explain fully the basis for depressed IFN-γ production observed in supernatants of TB + Helm patients at the time of TB diagnosis. However, recently, another mode of immune suppression involving CD4 CD25high T cells (i.e. Treg) was described in patients with active pulmonary TB [24]. To determine whether Treg were involved in regulation of IFN-γ production in WB cultures, we enumerated absolute counts of CD4 CD25high T cells (Fig. 3). Surprisingly, although significantly higher when compared to healthy individuals (data not shown), numbers of Treg were lower in TB + Helm compared to TB patients (P = 0·051), suggesting that Treg suppressor function was probably not the only mechanism responsible for the observed low IFN-γ levels in MTB-CF-stimulated WB culture supernatants.
Fig. 3.
CD4+ CD25high T cell absolute counts in blood from tuberculosis (TB) ( ) and TB and intestinal helminth infection (TB + Helm) (□) patients at the beginning of TB therapy. Results are expressed as mean average ± s.d. Significant differences between groups are depicted in the figure with their respective P-values and were calculated using independent-samples t-test.
) and TB and intestinal helminth infection (TB + Helm) (□) patients at the beginning of TB therapy. Results are expressed as mean average ± s.d. Significant differences between groups are depicted in the figure with their respective P-values and were calculated using independent-samples t-test.
Discussion
Research over the last decade has identified a multitude of mechanisms for depressed MTB-antigen-stimulated production of IFN-γ at the time of diagnosis of active TB [9,10,14–16,33]. However, mechanisms responsible for the prolonged delay in recovery of MTB-specific T cell IFN-γ production by peripheral blood mononuclear cells (PBMC) of TB patients [9] remain unclear. The impact of intestinal helminth infections on susceptibility to M. tuberculosis is still controversial. Recently, Brown et al. [34] reported that nematode infection and progression of active tuberculosis among HIV-1 infected patients were not correlated. However, a significant association between intestinal helminthic infections and mycobacterial diseases, such as pulmonary tuberculosis and multi-bacillary leprosy, has been demonstrated by several authors [8,17,35,36]. In the present study, 27·5% (11 of 40) of all patients with TB, were positive for at least one intestinal helminth, confirming previous findings [35,36]. Interestingly, infection with S. stercoralis was found in 72·7% (eight of 11) of TB + Helm patients. Infection with S. stercoralis has been associated with a strong Th2-type immune response [4].
Previous studies indicate that active pulmonary TB is associated with a reduction in absolute numbers of both circulating CD4 and CD8 T cells [23]. Further, lymphopenia has also been described in patients with chronic intestinal helminth infection [37]. Consistent with these findings, we found that T cell lymphopenia was even more pronounced among TB + Helm patients compared to TB alone. Therefore, it is possible that the significant decline in lymphocyte counts in TB + Helm patients may be the result of additive effects of both TB and intestinal helminthiasis on the host immune system. Surprisingly, and despite the fact that eosinophilia has been associated mainly with helminth infections, absolute counts of eosinophils were elevated and comparable in both TB and TB + Helm patients. One possible explanation for this finding could be that TB patients harboured occult helminth infection, despite the fact that all control subjects and patients underwent extensive examination for helminths in at least three consecutive stool samples investigated by the Lutz [29], Kato-Katz [30] and Baerman-Moraes [31,32] methods. However, in a previous study we have demonstrated that up to 25% of TB patients without intestinal helminths had eosinophil counts higher than 600 cells/mm3 [35]. Here, IL-5, an important cytokine in helminth-associated eosinophilia was undetectable in WB culture supernatants from both TB and TB + Helm patients. Recently, data from a longitudinal evaluation of immune responses during an experimental infection of a human volunteer with Necator americanus demonstrated that elevated eosinophilia following the primary infection (up to 815 days post-infection) occurred in the absence of detectable IL-5 production in WB cultures stimulated with worm-soluble antigens [38]. These authors suggested that focal presentation of adult worm antigens in the intestinal mucosa-associated lymphoid tissue may be able to maintain local IL-5 production by antigen-specific T cells, but these are not recruited to the systemic circulation.
TB immune suppression has been associated with both low IFN-γ production and depression of (MTB) antigen-driven T cell proliferative responses [9–14]. Interestingly, IFN-γ and lymphoproliferative responses to mycobacterial antigens appear to be suppressed in a correlated manner [14]. Thus, T cell responses could be assessed by antigen-specific IFN-γ production in WB culture.
Here, we used MTB-CF-stimulated WB cultures to extend these findings to patients with dual TB and intestinal helminthic infections. In fact, IFN-γ levels in CF-stimulated WB cultures from TB + Helm patients were fivefold lower compared to TB patients at baseline and remained depressed at the end of MTB treatment. While this reduction of cytokine production may be solely the result of lower absolute numbers of CD4+ T cells (Fig. 1c) in TB + Helm patients, it is also possible that this profound and persistent suppression of IFN-γ production may be due to the expansion of MTB-specific memory T cells primed to produce Th2-type cytokines [39]. Previous studies from our group support the concept that IL-10 and TGF-β mediate antigen hyporesponsiveness, as evidenced by suppression of antigen-driven blastogenesis and IFN-γ production, which are characteristics of tuberculosis [14,40]. In the current study, we found persistently elevated IL-10 levels in CF-stimulated WB culture supernatants from TB + Helm patients compared to TB patients at the end of TB treatment. As noted above, preliminary results from the current study showed the absence of detectable of IL-4 levels in WB culture supernatants form both TB and TB + Helm patients. This finding concurs with previous data from our group, showing that IL-4 was undetectable in PBMC culture from both TB patients and matched household contacts [14]. Whether this indicates that IL-4 does not play a role in suppression of IFN-γ production in our culture system or was not detected due to limitation in the immunoassay used is still unclear. Future studies are needed to resolve this question.
Another mechanism for depressed T cell function in TB, described recently, involves expansion of CD25high CD4+ T cells with a regulatory phenotype (Treg) [23,24]. We have demonstrated that the immunosupression observed normally during tuberculosis was associated with a threefold increase in the frequency of circulating Treg cell in the blood of patients with TB when compared to healthy controls [23]. In the same study we also demonstrated that depletion of CD4 CD25high cells from PBMC cultures led to an increase in IFN-γ levels in culture supernatants. Data from Guyot-Revol et al. [24] confirmed our findings and demonstrated that CD4 CD25high expressed higher levels of forkheard box P3 (FoxP3) mRNA, suggesting that they were indeed Treg cells [24].
Although monocytes–macrophages, T cells and B cells express IL-10 following activation in both human and experimental tuberculosis, its production has been associated mainly with monocytes [14,23,40]. However, it is possible that in the current study IL-10 production in WB cultures may be sustained by both T cells and monocytes. Specifically, it has been described that CD4 CD25high (Treg) cells produce substantial amounts of IL-10 in human tuberculosis [23,24] and in chronic helminth infection [25,26]. In a recent report, CD4 CD25high T cells in mice infected with Schistosoma mansoni produced large amounts of IL-10 [26], a finding that may explain the cytokine profile in CF-stimulated WB culture supernatants in the current study described above. To investigate this possibility further, we enumerated absolute numbers of CD4 CD25high T cells in blood from TB and TB + Helm patients. Interestingly, numbers of Treg were lower in TB + Helm as opposed to TB patients. One possible explanation for decreased Treg counts in peripheral blood of TB + Helm patients may be that these cells are sequestered at sites of both active helminth infection in the gut and the lung during active pulmonary TB. In addition, it is also possible that Treg frequencies in these patients are lower due to the extension of pulmonary disease observed during TB + Helm infection, which would sequester more cells in the lung. Support for this hypothesis stems from our own recent study demonstrating compartmentalization of Treg at sites of active TB in the lung [23]. In addition, Campanelli et al. [41] showed that functional Treg cells accumulate in skin lesions from patients with cutaneous leishmaniasis. Thus, taken together, it is possible that in TB + Helm patients Treg numbers are reduced due to sequestration of CD4 CD25high T cells at more sites of active helminth and mycobacterial infections, such as gut and lung, respectively.
Although not statistically significant, we also observed a tendency of more advanced disease among patients with dual infections when compared to TB patients (data not shown). As an efficient clinical response to MTB is dependent upon an effective Th1 response, the more protracted clinical course in TB + Helm patients in association with increased IL-10 levels may indicate that intestinal helminth infection in TB patients may skew their cytokine profile towards a Th2 response. Data from other authors, demonstrating that chronic immune activation associated with intestinal helminth infections results in a predominant Th2-type cytokine profile, impaired signal transduction and anergy [7,19,20,42] and that a nematode-derived homologue of the human macrophage migration inhibitory factor (MIF) could interfere with host immunity systemically, shifting it towards a Th2-type immune response [43], support our hypothesis.
In summary, data presented here make a strong case for the negative impact of intestinal helminth infection on both anti-MTB immunity and clinical response to TB therapy. Timely diagnosis and treatment of intestinal helminths may be important for a successful response to anti-TB treatment. This may be critical to the success of the global effort to control TB.
Acknowledgments
We would like to thank the physicians (Drs Valderio Dettoni, Ricardo Tristão-Sá and David J. Hadad) and nursing staff at the NDI clinical research centre (CPC) at UFES, Vitoria, Brazil for their involvement in patient recruitment, follow-up and specimen collection. In addition, we appreciate the assistance by the personnel at the NDI microbiology laboratory with sputum processing and culture. Most importantly, however, we would like to acknowledge all TB patients and healthy volunteers in Vitória enrolled in the present study. Without their participation the project would not have been possible. This study was supported by funding from NIAID NIH (RFP AI-45244/AI-95383, Tuberculosis Research Unit).
References
- 1.World Health Organization (WHO) WHO annual report on global TB control - summary. Wkly Epidemiol Rec. 2003. pp. 122–8. [PubMed]
- 2.Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [Review] [DOI] [PubMed] [Google Scholar]
- 3.Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004;26:487–97. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 5.Bentwich Z, Weisman Z, Moroz C, Bar-Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections? Clin Exp Immunol. 1996;103:239–43. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalinkovich A, Weisman Z, Greenberg Z, et al. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–21. doi: 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borkow G, Leng Q, Weisman Z, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–60. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diniz LM, Zandonade E, Dietze R, Pereira FE, Ribeiro-Rodrigues R. Short report: do intestinal nematodes increase the risk for multibacillary leprosy? Am J Trop Med Hyg. 2001;65:852–4. doi: 10.4269/ajtmh.2001.65.852. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch CS, Toossi Z, Othieno C, et al. Depressed T cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–73. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 10.Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–72. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huygen K, Van Vooren JP, Turneer M, Bosmans R, Dierckx P, De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988;27:187–94. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres M, Mendez-Sampeiro P, Jimenez-Zamudio L, et al. Comparison of the immune response against Mycobacterium tuberculosis antigens between a group of patients with active pulmonary tuberculosis and healthy household contacts. Clin Exp Immunol. 1994;96:75–8. doi: 10.1111/j.1365-2249.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–8. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong JH, Zhang M, Modlin RL, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–18. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926–31. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, Bradley JE. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol. 1999;117:517–23. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–87. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Cooper PJ, Chico ME, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–13. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 20.Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthiasis and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–30. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulart IM, Penna GO, Cunha G. Immunopathology of leprosy: the complexity of the mechanisms of host immune response to Mycobacterium leprae. Rev Soc Bras Med Trop. 2002;35:365–75. doi: 10.1590/s0037-86822002000400014. [Review] [DOI] [PubMed] [Google Scholar]
- 22.Abulafia J, Vignale RA. Leprosy: pathogenesis updated. Int J Dermatol. 1999;38:321–34. doi: 10.1046/j.1365-4362.1999.00650.x. [Review] [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4+ CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 25.Satoguina J, Mempel M, Larbi J, et al. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes Infect. 2002;4:1291–300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 26.McKee AS, Pearce EJ. CD25+ CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–31. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous. Diagnostic standards and classification of tuberculosis. New York: National Tuberculosis and Respiratory Disease Association; 1969. Classification of pulmonary tuberculosis; pp. 68–76. [Google Scholar]
- 28.Ribeiro-Rodrigues R, Resende Co T, Johnson JL, et al. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002;9:818–23. doi: 10.1128/CDLI.9.4.818-823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz AV. Schistosoma mansoni and the schistosomiasis, according to observations made in Brazil. Mem Inst Oswaldo Cruz. 1919;11:121–5. [Google Scholar]
- 30.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 31.Baermann G. Mededeel mit h. Geneesk. Batavia: Laboratory Weltvreden Feestbundel; 1917. A simple method for the diagnosis of Ancylostomum (Nematode) in soil; pp. 41–7. [Google Scholar]
- 32.Moraes RG. Contribution to the study of Strongyloides stercoralis and strongyloidiasis in Brazil. Rev Saude Publ. 1948;1:507–624. [Google Scholar]
- 33.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 34.Brown M, Miiro G, Nkurunziza P, et al. Schistosoma mansoni, nematode infections, and progression to active tuberculosis among HIV-1-infected Ugandans. Am J Trop Med Hyg. 2006;74:819–25. [PubMed] [Google Scholar]
- 35.Tristao-Sa R, Ribeiro-Rodrigues R, Johnson LT, Pereira FE, Dietze R. Intestinal nematodes and pulmonary tuberculosis. Rev Soc Bras Med Trop. 2002;35:533–5. doi: 10.1590/s0037-86822002000500020. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health. 2006;11:551–8. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 37.Onyemelukwe GC, Musa BO. T-lymphocyte subsets in patients with hookworm infection in Zaria, Nigeria. Afr J Med Med Sci. 2001;30:255–9. [PubMed] [Google Scholar]
- 38.Wright W, Bickle Q. Immune responses following experimental human hookworm infection. Clin Exp Immunol. 2005;142:398–403. doi: 10.1111/j.1365-2249.2005.02945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pit DS, Polderman AM, Schulz-Key H, Soboslay PT. Prenatal immune priming with helminth infections: parasite-specific cellular reactivity and Th1 and Th2 cytokine responses in neonates. Allergy. 2000;55:732–9. doi: 10.1034/j.1398-9995.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- 40.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta1 and interleukin-10. Infect Immun. 1999;67:5730–5. doi: 10.1128/iai.67.11.5730-5735.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campanelli AP, Roselino AM, Cavassani KA, et al. CD4+ CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313–22. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]
- 42.Leng Q, Bentwich Z, Borkow G. Increased TGF-β, Cbl-b and CTLA-4 levels and immunosuppression in association with chronic immune activation. Int Immunol. 2006;18:637–44. doi: 10.1093/intimm/dxh375. [DOI] [PubMed] [Google Scholar]
- 43.Pastrana DV, Raghavan N, FitzGerald P, et al. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–63. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]