Abstract
Sjögren's syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration, destruction of the salivary and lacrimal glands and production of autoantibodies against a variety of cellular proteins. The aberrant immune response against these autoantigens may begin or extend to other proteins that are not yet defined. Several studies have shown that autoantibody production is taking place in the affected salivary glands. In the present study, using proteomic approaches, we aimed to: (a) identify new autoantigens in the salivary glands of primary SS (pSS) patients and (b) evaluate the epigenetic changes of known autoantigens. Total parotid gland extracts of pSS patients were analysed using two-dimensional gel electrophoresis, sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot with pSS patients' sera or purified autoantibodies and immunoprecipitation using homologous IgG. Identification of the unknown proteins was performed using mass spectrometry (MS). Immunoblot analysis on two-dimensional gels using purified anti-La/SSB antibodies revealed that pSS salivary glands contain high levels of post-translationally modified La/SSB autoantigen, while the native form of the protein is recognized faintly, in contrast to normal controls. Moreover, salivary glands of pSS patients contain post-translationally modified actin that becomes immunogenic in the microenviroment of the affected tissue. The alteration of the physicochemical properties of self-proteins could thus contribute to the break of immune tolerance against them.
Keywords: La/SSB, actin, autoantigens, Sjögren's syndrome
Introduction
Sjögren's syndrome (SS) is a slowly progressive inflammatory autoimmune disease, characterized by hypergammaglobulinaemia, which affects primarily the salivary and lacrimal glands and leads to decreased exocrine secretions [1]. Immunoglobulins of SS patients cover a wide spectrum of autoantibodies targeting organ- and non-organ-specific autoantigens. The most common and specific autoantibodies in SS are directed against the ribonucleoprotein particle Ro/La RNP [2]. Circulating anti-La/SSB antibodies are considered to be more specific for SS because they are rarely detected in other autoimmune diseases [1]. A large body of knowledge, over the past several years, suggests that autoantibodies characterizing primary SS (pSS) are produced mainly in the affected salivary glands. Antibody-producing plasma cells with specificity for Ro/SSA and La/SSB are detected among the infiltrating lymphocytes of salivary glands; the saliva of patients with pSS presents high levels of anti-Ro/La autoantibodies [3]. Finally, autoantibodies against La/SSB are also associated with up-regulation of La/SSB mRNA in minor salivary gland biopsies of patients with pSS [4], indicating an overproduction of the protein, due possibly to an increased turnover of the autoantigen.
Despite progress in understanding of the local autoimmune response, questions regarding autoantigen processing and presentation within the inflammatory microenvironment of parotid glands in pSS are still unanswered. The mechanisms by which autoantigens within the pathological lesion of SS bypass the tolerance required for autoantibody formation, as well as reasons for the perpetuated autoimmune response, are largely unknown. Indeed, the inflammatory environment may change the physicochemical properties of the autoantigens in several ways. Enzymes can cleave the autoantigens, releasing new epitopes (cryptotopes) or induce post-translational modifications (PTMs) [5–7]; furthermore, proteins in the extracellular milieu can form complexes with chaperones, creating larger antigenic complexes [8]. In previous studies it has been shown that autoantigens are clustered in apoptotic blebs, and it has been proposed that cell death and ineffective clearance of apoptotic cells can contribute to the breakdown of immune tolerance [9].
The aim of this study was to evaluate modifications of known autoantigens and determine new targets of the autoimmune response in the parotid glands of pSS patients, using an immunomic approach (a combination of conventional immunological methods along with proteomic technologies such as mass spectrometry).
Materials and methods
Protein extraction from parotid glands and human salivary gland epithelial cells (HSG)
Parotid glands of three patients with pSS were excised due to persistent parotid gland enlargement. Two patients had lymphadenopathy, purpura and mucosa-associated lymphoid tissue (MALT) lymphoma and the third presented with Raynaud's phenomenon and sclerodactyly. Parotid gland specimens (strictly from unaffected regions) were also taken from two patients with mixed parotid tumour and an HSG epithelial cell line (purchased from European Collection of Cell Cultures, ECACC Salisbury, UK, no. 95031024). All surgical specimens were received after informed consent and local ethical approval. Parotid gland specimens were stored immediately after excision at −80°C until use. For protein extraction each gland was homogenized in 1–2 ml of lysis buffer (50 mM Tris-HCl pH 7·4, 1% IGEPAL, 1% Triton X-100, 1% sodium deoxycholate, 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid (EGTA), 1 mM phenylmethylsulphonyl fluoride (PMSF), 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM Na3VO4, 1 mM NaF) using a Dounce homogenizator on ice. All reagents were purchased from Sigma-Aldrich (Munich, Germany). The lysate was centrifuged twice for 15 min at 6500 g at 4°C. The protein concentration of the final supernatant was determined using the Bradford method (Coomassie Plus Protein Assay reagent Kit-Pierce, Etten-Leur, the Netherlands).
The cell line (HSG) was cultured according to ECACC instructions [10]. Cells (1 × 106) were washed with ice-cold phosphate-buffered saline (PBS) twice and the cells were then suspended in 0·5 ml of lysis buffer and incubated on ice for 30 min. The cells were homogenized by passing through a 1-ml syringe. The lysate was centrifuged for 15 min at 3000 g at 4°C. Protein concentration was determined at the supernatant using the Bradford method mentioned above.
Purification of total IgG and specific anti-La antibodies
Total IgG from two pSS patients with parotid gland enlargement were purified using a Protein A-Sepharose affinity chromatography column (Sigma-Aldrich). Sepharose beads were swelled according to the manufacturer's instructions. Serum samples were diluted in PBS pH 7·4 and were applied to the column. The latter was washed with PBS and bound immunoglobulins were eluted with 0·1 M HCl-glycine pH 2·7. The pH was fixed immediately to 7·4 using 0·5 M NaOH in PBS. Immunoglobulins were dialysed versus PBS O/N at 4°C. The amount of total IgG in each sample was determined using the Bradford method mentioned above.
Anti-La antibodies were purified using a synthetic peptide spanning the sequence 349–364 of La/SSB (major linear B cell epitope of the protein). The synthetic peptide was coupled (coupling buffer: 0·1 M NaHCO3−0·5 M NaCl pH 9·9) to CNBr-activated sepharose 4B gel at a concentration of 10 mg/g of dried sepharose powder according to the manufacturer's instructions (Pharmacia Biotech, Uppsala, Sweden). Total IgG from one pSS patient was used for the isolation of specific anti-La antibodies. The purification procedure of specific anti-La antibodies was performed as described above for total IgG isolation. The amount of anti-La antibodies was determined using the Bradford method mentioned above. The specificity of the anti-La antibodies was determined by Western blot on a HeLa extract.
Two-dimensional gel electrophoresis and Western blotting
Parotid gland extract (400 μg) from each (two) pSS patient (one from MALT lymphoma and the other without) and two controls as well as the HSG cell line protein sample were diluted to a final volume of 340 μl to a solution with a final concentration of 8 M urea, 0·5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulphonate (CHAPS), 0·2% (w/v) dithiothreitol (DTT), 0·5 (v/v) immobilized Ph gradients (IPG) buffer pH 3–10 (Pharmacia Biotech, Uppsala, Sweden), 0·002% bromophenol blue and 18 cm Immobiline dry strips pH 3–10 (Pharmacia Biotech, Uppsala, Sweden) were rehydrated with the diluted protein samples for 20 h at 20°C using cup loading strip holders. Proteins were focused using the Ettan IPGphor platform according to the following protocol: 1 h at 500 V, 1 h at 1000 V and 4 h at 8000 V.
After isoelectric focusing, strips were equilibrated in 50 mM Tris-HCl pH: 6·8, 6 M urea, 30% glycerol, 2% sodium dodecyl sulphate (SDS), 1% DTT and 0·002% bromophenol blue for 15 min at room temperature (RT). Strips were then loaded onto 13% SDS–polyacrylamide gel electrophoresis (PAGE) (acrylamide : bisacrylamide ratio 40 : 0·232) and were run O/N at 90 V.
Proteins were then transferred to 0·45 micron nitrocellulose membranes (Pierce, Etten-Leuer, the Netherlands) according to standard procedures. Afterwards themembranes were blocked with 10% non-fat dry milk in Tris-buffered saline-Tween (TBS-T) (0·1%) for 2 h at RT and then incubated with 5 μg/ml of purified anti-La antibodies in blocking buffer O/N at 4°C. Membranes were washed four times with TBS-T (0·1%) at RT and were then incubated for 1 h at RT with a horseradish peroxidase (HRP)-conjugated goat anti-human IgG Fcγ-specific antibody (Jackson ImmunoResearch, West Grove, PA, USA) diluted 1 : 2000 in blocking buffer. Membranes were washed four times with TBS-T (0·1%) at RT and peroxidase reaction was visualized using the ECL Plus System (Pharmacia Biotech, Uppsala, Sweden).
Immunoprecipitation (IP) assay for the identification of new autoantigens
Parotid gland protein extracts from two pSS patients (24 mg total protein) were precleared with 0·5 ml preswelled Protein A-Sepharose beads for 2 h. Beads were removed by centrifugation and the extracts were incubated with 0·5 ml of normal human serum overnight and were finally incubated with 0·5 ml preswelled Protein A-Sepharose beads for 1 h. All the preclearing procedures took place at 4°C. Six mg of total IgG from the two pSS patients were coupled covalently to Protein A-Sepharose beads according to standard procedures [11] and incubated with the patients' homologous precleared parotid gland protein extracts, end-over-end, overnight at 4°C. After extensive washes of the beads with PBS, captured proteins were eluted using 0·1 M HCl-glycine pH 2·7. The eluents were neutralized immediately using 0·5 M NaOH in PBS and dialysed versus PBS pH 7·4 O/N at 4°C and then concentrated using polyethylene glycol. Eluted proteins were analysed on a 13% polyacrylamide gel. Gels were either silver-stained or transferred to nitrocellulose membranes for immunoblot analysis. The membranes were blocked with 10% non-fat dry milk in TBS-T (0·1%) for 2 h at RT and then incubated with patients' (two) serum and a healthy donor's serum diluted 1 : 100 in blocking buffer O/N at 4°C. Membranes were washed four times with TBS-T (0·1%) at RT and were then incubated for 1 h at RT with an HRP-conjugated goat anti-human IgG Fcγ specific antibody (Jackson ImmunoResearch) diluted 1 : 2000 in blocking buffer. Membranes were washed four times with TBS-T (0·1%) at RT and peroxidase reaction was visualized using the ECL Plus system (Pharmacia Biotech, Uppsala, Sweden). Immunoreactive bands were cut off from the corresponding silver-stained gels, and after overnight in-gel digestion with sequence grade trypsin (Promega, Madison, WI, USA) the resulting peptides were extracted from the gel. The eluant was analysed on an ion trap mass spectrometer with a nanospray source (LCQ Deca; ThermoFinnigan, San Jose, CA, USA). Mass spectrometry (MS) and MS/MS data were used to search protein databases (SwissProt) with the Turbo-sequest search engine.
Identification of actin isoforms in parotid gland extract (PGE)
Total PGE 20 (μg) were run on a commercially available vertical gel of isoelectric focusing (IEF) (pI range 3–10) according to the manufacturer's instructions (Biorad Laboratories Inc., Milan, Italy). This system separates proteins on a vertical gel according to their isoelectric point and not their molecular mass. Moreover, total PG extracts were run on a 15% SDS-PAGE (acrylamide : bis acrylamide ratio 29 : 1). Proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Bedford, MA, USA) according to the manufacturer's instructions. Membranes were then blocked with 5% non-fat dry milk in TBS-Tween-20 0·1% for 1 h at RT, followed by incubation with a polyclonal goat anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) targeting the conserved carboxy-terminus of the human protein [1 : 1000 in 1% bovine serum albumin (BSA)-TBS-Tween-20 0·05%] or with an HRP-conjugated rabbit anti-goat IgG O/N at 4°C (1 : 2000 in 1% BSA in TBS-Tween-20 0·05%). Membranes were washed four times for 15 min with TBS-T (0·1%) at RT and were then incubated for 1 h at RT with an HRP-conjugated rabbit anti-goat IgG (Vector Laboratories, Burlingame, CA, USA) (1 : 2000 in 1% BSA in TBS-Tween-20 0·05%). Membranes were washed four times for 15 min with TBS-T (0·1%) at RT and peroxidase reaction was detected using the ECL Plus System (Pharmacia Biotech, Uppsala, Sweden).
Results
Specificity of purified anti-La/SSB antibodies
Purified antibodies against the major B cell epitope of La/SSB 349–364aa specifically recognize recombinant La/SSB. They react with a single band at 48 kDa, identical to that recognized by monoclonal anti-La antibodies in Western blot on HeLa cells extract (Fig. 1). They also recognize recombinant La/SSB, but not recombinant Ro60 or Ro52 in enzyme-linked immunosorbent assays (ELISA) (data not shown). Moreover, previous studies from our laboratory have demonstrated the specific recognition of La/SSB by these autoantibodies [12].
Fig. 1.
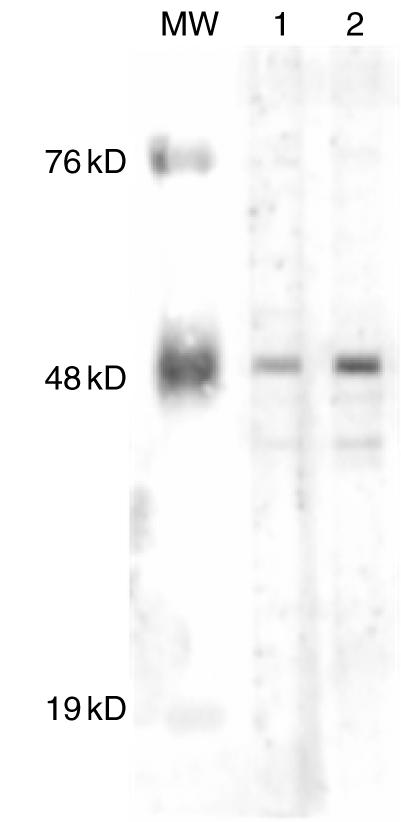
Specificity of affinity purified anti-La/SSB antibodies, as assessed by immunoblot on HeLa cells protein extract. MW: molecular weight markers, lane. 1: affinity purified anti-La/SSB antibodies, lane 2: monoclonal anti-La/SSB antibody.
Detection of La/SSB isoforms in parotid glands of pSS patients
La/SSB presents in several tissues and cell lines with eight isoforms, described previously in the literature. The isoelectric point of these isoforms ranges from 6 to 7 [13]. In order to identify the isoforms of this protein in parotid glands of pSS patients, immunoblot with purified anti-La antibodies was performed after two-dimensional gel electrophoresis of parotid gland extracts from two pSS patients. An extract from a human salivary gland epithelial cell line and a parotid gland extract from a patient with mixed parotid tumour were used as controls. This procedure revealed that the majority of La/SSB in parotid glands of pSS patients is degraded from 48 kDa to 34 kDa. In order to eliminate quantitative differences between the parotid gland specimens and controls, the identified spots were compared using the Image ProPlus software. Quantification analysis revealed that 76% of total La/SSB detected in pSS parotid gland specimens was truncated in contrast to control parotid gland specimens, where only 26% of total La/SSB was found truncated at 34 kDa, and to the control HSG cell line where only 5·6% of total La/SSB detected was found to be truncated. Moreover, only five distinct La/SSB isoforms were detected in pSS patients' specimens, in contrast to seven isoforms in controls. The intensity of the spots that were identified towards the acidic end of the IEF gel was higher in the pSS parotid extract. These spots occur most probably by a post-translational modification, which increases the total negative charge of La/SSB or decreases the total positive charge compared to the native protein (Fig. 2).
Fig. 2.
Identification of La/SSB isoforms in parotid gland extracts of primary Sjögren's syndrome (pSS) patients (a) or control patient (b) and in the human salivary gland (HSG) cell line (c). Images were calibrated using Image ProPlus software. Total La/SSB (76%) was found truncated in parotid gland extract (PGE) from pSS patients (a), by contrast to 26% in the control specimen (b), or 5·6% in the HSG cell line (c). The native isoform of La/SSB is depicted with an arrow in (c) and was detected faintly in PGE of pSS patients (a). (d) Percentage of La/SSB degradation in pSS patients' parotid glands (black bars), normal parotid glands (grey bars) or in the HSG cell line (white bars).
Identification of a 45-kDa protein as a possible autoantigen
A protein at around 45 kDa was detected as a target of autoantibodies in the parotid gland extract of one pSS patient. This protein was identified by performing immunoprecipitation with the patient's total IgG incubated with homologous parotid gland protein extract (Fig. 3a) and was recognized by homologous serum by Western blot. The band of interest was analysed with mass spectrometry and the protein was identified as human actin (Fig. 4).
Fig. 3.
(a) Immunoprecipitation of actin (black arrow) from a primary Sjögren's syndrome (pSS) patient parotid gland extract (PGE) with homologous IgG (lane 1, silver-stained gel). The bands in lane 1 disclose the proteins obtained after immunoprecipitation with total IgG from a patient. All bands appearing in the silver-stained gels were subjected to mass spectrometry (MS)/MS analysis. Some of the bands were identified as antibody heavy and light chains, deriving most probably from the IgG-coated sepharose beads. Some others failed to be identified. Lanes 2, 3: immunoblot of the immunoprecipitated proteins with homologous serum (2) or normal human serum (3). Actin was the only band, which was recognized differentially by the pSS patient's serum and not by normal human serum (a, lane 2). (b) Immunoprecipitaton of actin (black arrow) from a second pSS patient's PGE with homologous IgG (lane 1, silver-stained gel). MWM = molecular weight markers.
Fig. 4.
(a) Identification of actin by MS/MS analysis. MS/MS spectrum for one of the identified peptides (b′ and y′ fragment values shown in the inset). (b) Actin peptides identified by mass spectrometry in bold and underlined. Amino acid residues highlighted in grey represent possible sites of post-translational modifications, but no modifications were observed in the identified peptides. As shown, six of the 26 possible modified amino acid residues reside within the sequences of the identified peptides, but none of them was actually found to hold a post-translational modification.
This observation prompted us to repeat the immunoprecipitation experiment using total PGE from another pSS patient. Homologous IgG again immunoprecipitated actin, as shown in Fig. 3b.
Identification of distinct actin isoforms in parotid glands from pSS patients
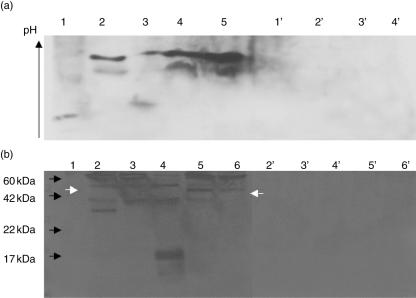
Immunoblot analysis of PGE after isoelectric focusing revealed four different isoforms of actin in parotid gland specimens of pSS patients. All different isoforms migrated towards the acidic end of the gel, suggesting that actin is modified in parotid glands of pSS patients (Fig. 5a). These modifications can explain the fact that actin was precipitated from PGE specimens from pSS patients and recognized by homologous serum. In contrast, actin from control parotid specimens was not recognized by pSS patients' sera. The molecular weight of actin in pSS patients was found slightly increased from 43 to 45 kDa in parotid gland specimens from pSS patients, indicating the addition of a chemical group to the protein or an insufficient cleavage of the precursor molecule of the protein (Fig. 5b).
Fig. 5.
Detection of actin in parotid gland specimens. (a) Isoforms of actin after isoelectric focusing on a pI gradient gel. Lanes 1–3: primary Sjögren's syndrome (pSS) parotid gland extracts (PGEs), 4–5: normal PGEs. Lanes 1′–4′: isotype control (PGEs 1–4). (b) Detection of actin after sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE). Lanes 2–4: pSS PGEs, 5–6: normal PGEs. Lanes 2′–6′: isotype control. Black arrows depict the position of molecular weight markers. The white arrow in the left depicts the larger isoform of actin detected in PGEs from pSS patients, compared to healthy individuals (white arrow in the right).
Discussion
SS is an autoimmune disorder which affects primarily the exocrine glands. The functional epithelium of the affected tissues is replaced gradually by lymphocytic infiltrates, resulting in a decreased exocrine function of the glands. Previous studies have shown that the B cells in the lesion are activated, because the IgG and IgM isotypes predominate in plasma cells infiltrating the salivary glands of SS patients [1].
Autoantibodies in the sera of SS patients target proteins with no apparent common biophysical and biochemical characteristics [6]. The most common autoantibodies of SS patients target the Ro/La RNP complex. This response is an antigen-driven process and results in the formation of autoantibodies against several epitopes of each protein component (intramolecular spreading), as well as to the other protein-members of the complex (intermolecular spreading) [2]. Several reports have described various novel targets of autoantibodies in SS, such as the nuclear mitotic apparatus (NuMA) [14], members of the golgin family [15], α- and β- fodrin [16,17], the 90 kDa nucleolar organizer region (NOR-90), p80 coilin [18] and centromere protein-C (CENP-C) [19].
Although it is generally accepted that the tissue target in SS is the epithelium of salivary and lacrimal glands, to date, cells from these sources have not been used in studies involving the identification of as-yet unknown targets of the autoimmune response [6]. The definition of specific autoantigens within the epithelial salivary gland cells and the detection of altered, already known autoantigens remains challenging, in order to understand the aetiopathogenetic mechanisms of the syndrome and to improve therapeutic interventions. Therefore our aim was to investigate alterations of the already known autoantigens and to define unknown targets of the autoimmune response within the tissue lesion of SS. Parotid gland protein extracts were analysed in order to illustrate the actual autoantigen modifications in the microenviroment of the autoimmune response.
Previous studies using conjunctiva tissue from pSS patients demonstrated that La/SSB is detected in the cytoplasm and on the membrane of epithelial cells from pSS patients. In contrast, La/SSB was expressed weakly in the nucleus of normal cells [20]. In our study the majority of La/SSB protein, the main autoantibody target in SS, was found to be truncated in salivary gland extracts. The native isoform of La/SSB was detected only faintly in PGE from pSS patients. As shown in Fig. 2, La autoantigen is partially degraded in fragments appearing to migrate at 43 and 34 kDa. The fragment at 43 kDa is most propably the 1–374aa region, which has been described previously to be produced during apoptosis [21] This region contains the major La/SSB epitope (349–364aa). The other cluster of spots at 34 kDa appear at a different pI range compared to the 43 kDa fragment and most probably depicts a truncated La/SSB molecule, lacking approximately the first 120aa residues from the –NH2 terminus and retaining the epitope 349–364 in the –COOH terminus. The possible mechanisms for the cleavage of La/SSB include: (1) apoptosis, during which La/SSB is cleaved at position 374 [21] and (2) viral infection, which results in the cleavage of La/SSB at position 358 [22]. However, the latter assumption is less probable, as the antigenic determinant 349–364 is disrupted. Moreover, the spots at 34 kDa were also detected in the HSG cell line but these spots represented a small proportion of the total La/SSB autoantigen, in contrast to the PGE of pSS patients where La/SSB was accumulated in this region. The detected isoforms of La/SSB most probably correspond to modified isoforms of the protein with increased total negative charge. Phosphorylation is a protein modification adding negative charge without significantly affecting the molecular weight of a molecule. La/SSB is phosphorylated primarily at the serine 366 amino acid residue, resulting in inhibition of the polymerase III transcription factor activity of the protein; this chemical modification results in the decreased ability of the molecule to bind and protect the 5′ end of nascent RNA molecules [23]. In our laboratory the major linear B cell epitope of the molecule has been defined previously in the amino acid region 349–368aa of the protein [24,25]. The phosphorylated form of this epitope was found to be recognized more frequently and with a higher relative avidity by autoantibodies derived from sera of patients with pSS [26], compared with the unmodified form of the epitope.
Post-translational modifications seem to play a pivotal role in autoimmune disorders and are considered increasingly as possible triggering factors for the breakdown of immune tolerance against self-proteins. Autoimmune sera of anti-nuclear antibody (ANA)-positive systemic lupus erythematosus (SLE) patients preferentially precipitate proteins which undergo phosphorylation in response to apoptosis [7]. Previous studies have shown that adenovirus infection increases the extent of phosphorylation of La/SSB as well as its antigenicity, and results in the translocation of the protein to the cell membrane [27–29]. Affected salivary gland epithelial cells from pSS patients bear an activated phenotype compatible with a virus infection [30,31]. In this regard, a virus infection of salivary glands could trigger the extended phosphorylation of La/SSB and increase the antigenicity of the protein by presenting the molecule to the immune system on the surface of the glandular epithelium. Moreover, La/SSB is required for the internal ribosome entry site (IRES)-mediated translation of Coxsackie virus B3 RNA [32]. The work by Trianatafyllopoulou et al. is consistent with a Coxsackie virus infection of epithelial cells of the salivary glands of pSS patients [30,31]. In summary, a viral infection of parotid glands could generate alterations of La/SSB that lead to the breaking of immune tolerance against the molecule.
In our study it was also shown that using sera of pSS patient-modified actin is immunoprecipitated from homologous salivary glands. Modified cytoskeleton proteins have been defined previously as targets of autoimmune responses. Early rheumatoid arthritis patients seem to have specific antibodies targeting citrullinated fillagrin and vimentin and their presence is correlated with aggressive and destructive disease [33]. α-Fodrin, a cytoskeleton protein that forms tetramers which bind to actin, calmodulin and microtubules [34], has been characterized as a putative autoantigen of SS [16]. Insights into the potential role of α-fodrin in the pathogenesis of SS were gained from a mouse model of Sjögren's syndrome. Immunoblot analysis of organ extracts with homologous mice sera revealed a 120-kDa band, which was subsequently sequenced and identified as a cleavage product of α-fodrin. The autoantibodies recognized only the cleavage product of α-fodrin and not the intact molecule [16]. Herein it was demonstrated that actin was recognized by SS patients' sera in homologous parotid gland extracts. Moreover, actin was found to be slightly larger in parotid gland specimens from pSS patients. This observation suggests a modification that increases the total mass of the molecule. Our data suggest that actin becomes antigenic after the modification, as it was not recognized by SS patients' sera in parotid gland extracts from control individuals (data not shown). Previous studies have shown that actin can be glycosylated (ADP-ribosylation) by numerous bacterial toxins [35]. Cleaved forms of actin were identified in parotid gland extracts from pSS patients with a molecular mass ranging from 7 to 37 kDa. Actin is one of the major components of exosomes and apoptotic blebs [36]. Exosomes are membrane vesicles of endosomal origin that are thought to represent an acellular mechanism for antigen transfer to classic antigen-presenting cells, as well as for direct antigen presentation with the capacity to induce immune response or tolerance [37]. Previous studies have shown that non-neoplastic salivary gland epithelial cell lines release exosomes that contain the Ro/SSA, La/SSB and Sm autoantigens. This mechanism may represent a pathway whereby intracellular autoantigens are presented to the immune system [38]. In addition, other studies involving apoptotic keratinocytes showed that the Ro/SSA, La/SSB and RNP autoantigens are clustered in surface apoptotic blebs [9]. The presence of altered proteins such as actin in exosomes or in apoptotic blebs may act as a boost to the break of the immune tolerance against self-proteins.
In conclusion, in this study parotid gland extracts from pSS patients were used for the first time in order to determine new autoantigenic molecules and/or autoantigen alterations. La/SSB, the major target of the autoimmune response, was found to be truncated in its majority, while the native form of the protein was expressed faintly. Moreover, it was shown that actin of parotid glands from SS patients is altered and this alteration breaks the tolerance against this self-protein. Currently, post-translational modified proteins are evaluated in order to estimate the prevalence of autoantibodies against them in Sjögren's syndrome.
Acknowledgments
This work was supported by a grant (PENED 01ED164) from the Hellenic Secretariat for Research and Technology.
References
- 1.Tzioufas AG, Moutsopoulos HM. Sjögren's syndrome. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman M, editors. Rheumatology. Philadephia: Mosby; 2003. pp. 1431–43. [Google Scholar]
- 2.Routsias JG, Vlachoyiannopoulos PG, Tzioufas AG. Autoantibodies to intracellular autoantigens and their B-cell epitopes: molecular probes to study the autoimmune response. Crit Rev Clin Lab Sci. 2006;43:203–48. doi: 10.1080/10408360500523837. [DOI] [PubMed] [Google Scholar]
- 3.Horsfall AC, Rose LM, Maini RN. Autoantibody synthesis in salivary glands of Sjögren's syndrome patients. J Autoimmun. 1989;2:559–68. doi: 10.1016/0896-8411(89)90189-3. [DOI] [PubMed] [Google Scholar]
- 4.Tzioufas AG, Hantoumi I, Polihronis M, Xanthou G, Moutsopoulos HM. Autoantibodies to La/SSB in patients with primary Sjögren's syndrome (pSS) are associated with upregulation of La/SSB mRNA in minor salivary gland biopsies (MSGs) J Autoimmun. 1999;13:429–34. doi: 10.1006/jaut.1999.0333. [DOI] [PubMed] [Google Scholar]
- 5.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–9. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 6.Rosen A, Casciola-Rosen L. Altered autoantigen structure in Sjögren's syndrome. implications for the pathogenesis of autoimmune tissue damage. Crit Rev Oral Biol Med. 2004;15:156–64. doi: 10.1177/154411130401500304. [DOI] [PubMed] [Google Scholar]
- 7.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–54. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staikou EV, Routsias JG, Makri AA, et al. Calreticulin binds preferentially with B cell linear epitopes of Ro60 kD autoantigen, enhancing recognition by anti-Ro60 kD autoantibodies. Clin Exp Immunol. 2003;134:143–50. doi: 10.1046/j.1365-2249.2003.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Yoshida H, Hayashi Y, et al. Expression of epidermal growth factor and transforming growth factor-beta in human salivary gland adenocarcinoma cell line. Cancer Res. 1985;45:6160–7. [PubMed] [Google Scholar]
- 11.Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982;257:10766–9. [PubMed] [Google Scholar]
- 12.Routsias JG, Touloupi E, Dotsika E, et al. Unmasking the anti-La/SSB response in sera from patients with Sjogren's syndrome by specific blocking of anti-idiotypic antibodies to La/SSB antigenic determinants. Mol Med. 2002;8:293–305. [PMC free article] [PubMed] [Google Scholar]
- 13.Francoeur AM, Chan EK, Garrels JI, Mathews MB. Characterization and purification of lupus antigen La, and RNA-binding protein. Mol Cell Biol. 1985;5:586–90. doi: 10.1128/mcb.5.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price CM, McCarty GA, Pettijohn DE. NuMA protein is a human autoantigen. Arthritis Rheum. 1984;27:774–9. doi: 10.1002/art.1780270708. [DOI] [PubMed] [Google Scholar]
- 15.Griffith KJ, Chan EK, Lung CC, et al. Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjogren's syndrome. Arthritis Rheum. 1997;40:1693–702. doi: 10.1002/art.1780400920. [DOI] [PubMed] [Google Scholar]
- 16.Haneji N, Nakamura T, Takio K, et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjögren's syndrome. Science. 1997;276:604–7. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana M, Okano T, Ogawa Y, Kaburaki J, Kawakami Y. Autoantibodies to the amino-terminal fragment of beta-fodrin expressed in glandular epithelial cells in patients with Sjögren's syndrome. J Immunol. 2001;167:5449–56. doi: 10.4049/jimmunol.167.9.5449. [DOI] [PubMed] [Google Scholar]
- 18.von Muhlen CA, Tan EM. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 1995;24:323–58. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 19.Pillemer SR, Casciola-Rosen L, Baum BJ, Rosen A, Gelber AC. Centromere protein C is a target of autoantibodies in Sjögren's syndrome and is uniformly associated with antibodies to Ro and La. J Rheumatol. 2004;31:1121–5. [PubMed] [Google Scholar]
- 20.Yannopoulos DI, Roncin S, Lamour A, Pennec YL, Moutsopoulos HM, Youinou P. Conjunctival epithelial cells from patients with Sjögren's syndrome inappropriately express major histocompatibility complex molecules, La (SSB) antigen, and heat-shock proteins. J Clin Immunol. 1992;12:259–65. doi: 10.1007/BF00918149. [DOI] [PubMed] [Google Scholar]
- 21.Ayukawa K, Taniguchi S, Masumoto J, et al. La autoantigen is cleaved in the COOH terminus and loses the nuclear localization signal during apoptosis. J Biol Chem. 2000;275:34465–70. doi: 10.1074/jbc.M003673200. [DOI] [PubMed] [Google Scholar]
- 22.Shiroki K, Isoyama T, Kuge S, et al. Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J Virol. 1999;73:2193–200. doi: 10.1128/jvi.73.3.2193-2200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan H, Sakulich AL, Goodier JL, Zhang X, Qin J, Maraia RJ. Phosphorylation of the human La antigen on serine 366 can regulate recycling of RNA polymerase III transcription complexes. Cell. 1997;88:707–15. doi: 10.1016/s0092-8674(00)81913-3. [DOI] [PubMed] [Google Scholar]
- 24.Tzioufas AG, Yiannaki E, Sakarellos-Daitsiotis M, et al. Fine specificity of autoantibodies to La/SSB. epitope mapping, and characterization. Clin Exp Immunol. 1997;108:191–8. doi: 10.1046/j.1365-2249.1997.d01-1003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yiannaki E, Vlachoyiannopoulos PG, Manoussakis MN, et al. Study of antibody and T cell responses in rabbits immunized with synthetic human B cell epitope analogues of La (SSB) autoantigen. Clin Exp Immunol. 2000;121:551–6. doi: 10.1046/j.1365-2249.2000.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terzoglou AG, Routsias JG, Avrameas S, Moutsopoulos HM, Tzioufas AG. Preferential recognition of the phosphorylated major linear B-cell epitope of La/SSB 349–368aa by anti-La/SSB autoantibodies from patients with systemic autoimmune diseases. Clin Exp Immunol. 2006;144:432–9. doi: 10.1111/j.1365-2249.2006.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizer LI, Deng JS, Stenberg RM, Tan EM. Characterization of a phosphoprotein associated with the SS-B/La nuclear antigen in adenovirus-infected and uninfected KB cells. Mol Cell Biol. 1983;3:1235–45. doi: 10.1128/mcb.3.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slobbe R, Van Esch B, Kveder T, Van Venrooij WJ. The use of adenovirus-infected HeLa cells for the detection of low titer autoantibodies. J Immunol Meth. 1991;138:237–44. doi: 10.1016/0022-1759(91)90172-c. [DOI] [PubMed] [Google Scholar]
- 29.Baboonian C, Venables PJ, Booth J, Williams DG, Roffe LM, Maini RN. Virus infection induces redistribution and membrane localization of the nuclear antigen La (SS-B): a possible mechanism for autoimmunity. Clin Exp Immunol. 1989;78:454–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Tapinos NI, Polihronis M, Tzioufas AG, Moutsopoulos HM. Sjögren's syndrome. Autoimmune epithelitis. Adv Exp Med Biol. 1999;455:127–34. [PubMed] [Google Scholar]
- 31.Triantafyllopoulou A, Tapinos N, Moutsopoulos HM. Evidence for coxsackievirus infection in primary Sjögren's syndrome. Arthritis Rheum. 2004;50:2897–902. doi: 10.1002/art.20463. [DOI] [PubMed] [Google Scholar]
- 32.Ray PS, Das S. La autoantigen is required for the internal ribosome entry site-mediated translation of Coxsackievirus B3 RNA. Nucl Acids Res. 2002;30:4500–8. doi: 10.1093/nar/gkf583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Gabalawy HS, Wilkins JA. Anti-Sa antibodies: prognostic and pathogenetic significance to rheumatoid arthritis. Arthritis Res Ther. 2004;6:86–9. doi: 10.1186/ar1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990;70:1029–65. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- 35.Richard JF, Petit L, Gibert M, Marvaud JC, Bouchaud C, Popoff MR. Bacterial toxins modifying the actin cytoskeleton. Int Microbiol. 1999;2:185–94. [PubMed] [Google Scholar]
- 36.Suarez-Huerta N, Mosselmans R, Dumont JE, Robaye B. Actin depolymerization and polymerization are required during apoptosis in endothelial cells. J Cell Physiol. 2000;184:239–45. doi: 10.1002/1097-4652(200008)184:2<239::AID-JCP12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 37.Thery C, Boussac M, Veron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 38.Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Salivary gland epithelial cell exosomes: a source of autoantigenic ribonucleoproteins. Arthritis Rheum. 2005;52:1517–21. doi: 10.1002/art.21005. [DOI] [PubMed] [Google Scholar]