Abstract
Inflammatory immune reactions in response to periodontopathogens are thought to protect the host against infection, but may trigger periodontal destruction. Thus, we examined the mechanisms by which the proinflammatory cytokine tumour necrosis factor (TNF)-α modulates the outcome of Actinobacillus actinomycetemcomitans-induced periodontal disease in mice. Our results showed that TNF-α receptor p55-deficient mice [p55TNF-knock-out (KO)] developed a less severe periodontitis in response to A. actinomycetemcomitans infection, characterized by significantly less alveolar bone loss and inflammatory reaction. Real-time polymerase chain reaction (PCR) demonstrated that levels of chemokines (CXCL1, 3 and 10; CCL3 and 5) and their receptors (CXCR2 and 3, CCR5) were lower in p55TNF-KO mice, as were matrix metalloproteinase (MMP)-1, 2 and 9 and receptor activator of nuclear factor kB ligand (RANKL) mRNA levels. However, the absence of the TNF-α p55 results in an impairment of protective immunity to A. actinomycetemcomitans infection, characterized by increased bacterial load and higher levels of C-reactive protein during the course of disease. Such impaired host response may be the result of the reduced chemoattraction of lymphocytes, neutrophils and macrophages, and reduced inducible nitric oxide synthase expression (iNOS) and myeloperoxidase (MPO) production in periodontal tissues of p55 TNF-KO mice. Our results demonstrate the mechanisms involved determining periodontal disease severity by TNF-α receptor p55, and its role in providing immune protection to A. actinomycetemcomitans periodontal infection.
Keywords: Actinobacillus actinomycetemcomitans, chemokines, inflammation, periodontal diseases, TNF receptor p55, TNF-α
Introduction
Periodontal diseases (PD) are chronic inflammatory diseases of the attachment structures of the teeth and are one of the most significant causes of tooth loss in adults and the most prevalent form of bone pathology in humans. The bacterial biofilm attached to the tooth surface hosts a wide diversity of potentially hazardous bacterial species, which trigger local and systemic inflammatory and immune responses [1,2]. The host response to periodontopathogens is thought to protect against infection, but if pathogens persist the inflammatory mediators themselves may damage periodontal tissues. In fact, the local action of proinflammatory cytokines, including tumour necrosis factor (TNF)-α, are important aetiological factors of PD [3].
TNF-α plays a critical role in the resistance to a wide range of microbial pathogens, contributing to both innate and adaptive immune responses [3]. This cytokine acts in several steps of leucocyte recruitment mechanisms, inducing the up-regulation of adhesion molecules, and the production of chemokines and matrix metalloproteinases (MMP) [4,5]. In addition, TNF-α up-regulates antigen presentation by, and the expression of, co-stimulatory molecules and the bactericidal activity of phagocytes [4,5]. The majority of anti-microbial and inflammatory effects of TNF-α are mediated through the TNF-α receptor p55 (p55TNF-R1), while the TNF-α receptor p75 signalling acts to attenuate the inflammatory response [6]. Mice homozygous for the p55TNF-R1 targeted mutation are characterized by abnormal immune system physiology, showing decreased inflammatory response and defects in resistance to intracellular pathogens. There are also defects in Peyer's patch development, splenic architecture and formation of germinal centres [4–6].
Regarding human PD, the TNF-α p55 receptor is expressed widely in diseased periodontal tissue [7]. Furthermore, TNF-α is present at high levels in both gingival crevicular fluid and diseased periodontal tissues [7,8]. Studies in rats and primates demonstrated clearly that TNF-α plays a central role in the inflammatory reaction, alveolar bone resorption and in the loss of connective tissue attachment in experimental PD, leading to the proposal of anti-TNF therapies to PD [9,10].
However, the molecular mechanisms linking TNF-α to inflammatory cell migration and to periodontal tissue destruction have not been established. In addition, the theoretical role exerted by TNF-α in the control of periodontal infection remains unknown. To clarify these questions, we infected mice that were genetically deficient in p55TNF-R1 with the periodontopathogen Actinobacillus actinomycetemcomitans in order to evaluate the mechanisms involved in the control of PD severity and A. actinomycetemcomitans infection.
Material and methods
Experimental groups
Experimental groups comprised 8-week-old male wild-type C57BL/6 mice, and mice with targeted disruption of the TNF-α receptor p55 [p55TNF-knock-out (KO)] bred and maintained in the animal facilities of the Department of Biochemistry and Immunology − FMRP/USP. Throughout the period of the study the mice were fed with sterile standard solid mice chow (Nuvital, Curitiba, PR, Brazil) and sterile water. Experimental groups comprised eight (1, 7, 30 and 45-day periodontal infection; pi) or 15 (0, 15 and 60 days pi) mice, depending upon the analyses performed at each time-point [four mice were employed for histological analysis, five for both flow cytometry and alveolar bone loss measurement, three for real-time polymerase chain reaction (PCR) analyses, and three for enzyme-linked immunosorbent assay (ELISA) and myeloperoxidase (MPO) measurements; for C reactive protein (CRP) and antibody analyses samples were taken from the same mice used for flow cytometry and real-time PCR]. The experimental protocol was approved by the local Institutional Committee for Animal Care and Use.
Periodontal infection
Bacterial culture and periodontal infection were performed as described previously [11]. In brief, the animals received an oral delivery of 1 × 109 colony-forming units (CFU) of a diluted culture of A. actinomycetemcomitans JP2 (grown anaerobically in supplemented agar medium, TSBV), in 100 µl of phosphate-buffered saline (PBS) with 2% of carboxymethylcellulose, placed in the oral cavity of mice with a micropipette. After 48 and 96 h, this procedure was repeated. Negative controls included sham-infected mice, which received PBS with carboxymethylcellulose in solution without A. actinomycetemcomitans, and non-infected animals.
Histological analysis
Four animals, selected at random from each group, were sacrificed at 0 and 60 days after infection. The periodontal tissues obtained were then fixed with paraformaldehyde at 4% in PBS, pH 7·4, for 12 h at room temperature. The specimens were demineralized thoroughly in 10% ethylenediaminetetraacetic acid disodium salt (EDTA) for 1–2 weeks. The decalcified mouse periodontal tissues were trimmed, dehydrated in graded ethanol and embedded in paraffin. Serial sections (5 µm) were cut and mounted on glass slides precoated with 0·1% poly l-lysine (Sigma, St Louis, MS, USA). Histological assessment was carried out following routine haematoxylin and eosin staining.
Isolation of inflammatory cells from periodontal tissues and flow cytometric analysis
The isolation and characterization of leucocytes present in the lesion site were performed as described previously [11]. The whole buccal and palatal periodontal tissues of upper molars were collected, weighed and incubated for 1 h at 37°C, dermal side down, in RPMI-1640, supplemented with NaHCO3, penicillin/streptomycin/gentamycin and 0·28 Wunsch units/ml of liberase blendzyme CI (Roche–F. Hoffmann-La Roche Ltd, Basel, Switzerland). The tissues of five mice, at each time-point per group, were processed in the presence of 0·05% DNase (Sigma-Aldrich, Steinhein, Germany) using Medimachine (BD Biosciences PharMingen, San Diego, CA, USA), according to the manufacturer's instructions. After processing, cell viability was assessed by Trypan blue exclusion, and the cell count was performed in a Neubauer chamber. For immunofluorescence staining, after cell counting the cells were stained for 20 min at 4°C with the optimal dilution of each antibody; phycoerythrin (PE)- and fluorescein isothiocyanate (FITC)-conjugated antibodies against CD3, CD4, CD8, GR1 and F4/80, and respective isotype controls were employed (BD Biosciences PharMingen). Cells were washed again and analysed by flow cytometry (fluorescence activated cell sorter (FACScan) and cellquest software; BD Biosciences PharMingen). Results represent the number of cells [± standard deviation (s.d.)] in the periodontal tissues of each mouse, normalized by the tissue weight, for two independent experiments.
Quantification of alveolar bone loss
Evaluation of the extent of alveolar bone loss was performed as described previously [11]. The maxillae were hemisected, exposed overnight in 3% hydrogen peroxide and defleshed mechanically. The palatal faces of the molars were photographed at 20× magnification using a dissecting microscope (Leica, Wetzlar, Germany), with the occlusal face of the molars positioned perpendicularly to the base. The images were digitized and analysed using ImageTool 2·0 software (University of Texas Health Science Center, San Antonio, TX, USA). Quantitative analysis was used for the measurement of the area between the cement–enamel junction (CEJ) and the alveolar bone crest (ABC) in the three posterior teeth, in arbitrary units of area (AUA). At each time-point five animals were analysed, and for each animal the alveolar bone loss was defined as the average of CEJ–ABC between the right and the left arch.
Real-time PCR reactions
The extraction of total RNA from periodontal tissues was performed with Trizol reagent following the protocol recommended by the manufacturer (Life Technologies, Rockville, MD, USA), and the complementary DNA was synthesized using 3 µg of RNA through a reverse transcription reaction (Superscript III, Invitrogen Corporation, Carlsbad, CA, USA). For the quantification of A. actinomycetemcomitans, DNA extraction from periodontal tissue samples was performed with DNA Purification System (Promega Biosciences Inc., San Luis Obispo, CA, USA), as described previously [12]. Real-time PCR quantitative mRNA or DNA analyses were performed in an ABI Prism 7000 Sequence Detection System using the SybrGreen system (Applied Biosystems, Warrington, UK). SybrGreen PCR MasterMix (Applied Biosystems), 100 nM specific primers and 2·5 ng of cDNA (or 5 ng of DNA) were used in each reaction. The primer sequences, the predicted amplicon sizes and the annealing and melting temperatures, designed using the PrimerExpress software (Applied Biosystems) are depicted in Table 1. The standard PCR conditions were 95°C (10 min), followed by 40 cycles of 94°C (1 min), 56°C (1 min) and 72°C (2 min), and by the standard denaturation curve. For mRNA analysis, the relative level of gene expression was calculated in reference to beta-actin expression in the sample, using the cycle threshold (Ct) method. For DNA analysis, gene expression levels were determined using the Ct method and normalized by the tissue weight. Negative controls without cDNA or DNA and without reverse transcriptase were also performed. A representative experiment of three is presented in the Results.
Table 1.
Primer sequences and reaction properties.
| Target | Sense and anti-sense sequences | tA (°C) | tM (°C) | bp |
|---|---|---|---|---|
| CXCL1 | ATTGTATGGTCAACACGCACG | 58 | 79 | 134 |
| TTTGAACGTCTCTGTCCCGAG | ||||
| CXCL3 | CCTTGACCCTGAAGCTCCCTTGGTTC | 60 | 80 | 221 |
| CGTGCGTGTTGACCATACAATATG | ||||
| CXCR2 | GAGAACCTGGAAATCAACAGTT | 58 | 85 | 521 |
| GTACTTGTGGCATGTACAATGG | ||||
| CXCL10 | CA GCACCATGAA CCCAAGTGC | 58 | 80 | 431 |
| GGT CTTCTGAAAG GTGACCAGC | ||||
| CXCR3 | ATC TACCTATCAGCCAACTACGA | 60 | 79 | 379 |
| TCAGAGAGCAA ATGTGGATGT | ||||
| CCL3 | TTCTGCTGACAAGCTCACCCT | 60 | 79 | 322 |
| ATGGCGCTGAGAAGACTTGGT | ||||
| CCL5 | TTCCCTGTCATCGCTTGCTCT | 60 | 81 | 433 |
| CGGATGGAGATGCCGATTTT | ||||
| CCR5 | TTCCCTGTCATCGCTTGCTCT | 60 | 81 | 433 |
| CGGATGGAGATGCCGATTTT | ||||
| CCL1 | ATTGTATGGTCAACACGCACG | 58 | 79 | 134 |
| TTTGAACGTCTCTGTCCCGAG | ||||
| CCR4 | CTT GCACCAAGGA AGGTAT | 58 | 81 | 116 |
| AG CATAGACAGA TACCTAGG | ||||
| MMP-1 | TGGACCTGGAGGAAATCTTGC | 58 | 79 | 155 |
| AGAGTCCAAGAGAATGGCCGA | ||||
| MMP-2 | CTGATGGCACCCATTTACACCT | 60 | 82 | 186 |
| GATCTGAGCGATGCCATCAAA | ||||
| MMP-9 | AGAGATGCGTGGAGAGTCGAA | 65 | 85 | 162 |
| AAGGTTTGGAATCTGCCCAGG | ||||
| TIMP-1 | ACTGCAGGATGGACTCTTGCA | 30 | 82 | 206 |
| TTTCAGAGCCTTGGAGGAGCT | ||||
| TIMP-2 | CAAGTTCTTCGCCTGCATCAA | 61 | 84 | 155 |
| TCGAAACCCTTGGAGGCTT | ||||
| TIMP-3 | TTCTCAGCGAGGATGGCACTT | 60 | 81 | 200 |
| AAACACGGTTCAGGATGCTGG | ||||
| RANKL | CAGAAGATGGCACTCACTGCA | 65 | 73 | 203 |
| CACCATCGCTTTCTCTGCTCT | ||||
| OPG | GGAACCCCAGAGCGAAATACA | 57 | 77 | 225 |
| CCTGAAGAATGCCTCCTCACA | ||||
| IL-1β | GGAAGATTCTGAAGAAGAGACGG | 58 | 79 | 329 |
| TGAGATTTTTAGAGTAACAGG | ||||
| IFN-γ | ATGAAATATACAAGTTATATCATG | 58 | 77 | 501 |
| TGTTTCGAGGTCGAAGAGCATCCC | ||||
| IL-4 | GCGATA TCACCTTACAGGAG | 58 | 82 | 308 |
| TGTCCTGTGAAGGAAGCCAAC | ||||
| IL-10 | AGATC TCCGAGATGC CTTCA | 58 | 85 | 307 |
| CCGTGGAGCAGGTGAAGAAT | ||||
| iNOS | CGTCATTTCTGTCCGTCTCT | 56 | 82 | 390 |
| TTGCTGGCTGATGGCTGGCG | ||||
| β-actin | ATGTTTGAGACCTTCAACA | 56 | 75 | 495 |
| CACGTCAGACTTCATGATGG | ||||
| Actinobacillus actinomycetemcomitans | ATGCCAACTTGACGTTAAAT | 60 | 78 | 557 |
| AAACCCATCTCTGAGTTCTTCTTC |
At: annealing temperature; Mt: melting temperature; bp: base pairs of amplicon size. IFN: interferon; IL: interleukin; iNOS: inducible nitric oxide synthase; MMP: metalloproteinase; OPG: osteoprotegerin; TIMP: tissue inhibitor of metalloproteinase; RANKL: receptor activator of nuclear factor kB ligand.
Protein extraction and cytokine ELISA
Measurements of cytokines in periodontal tissues were performed as described previously [12]. For protein extraction, palatal periodontal tissue was homogenized in phosphate-buffered saline (PBS) pH 7·4, centrifuged at 100 g at 4°C and the supernatants were stored at −70°C. The concentrations of cytokines in periodontal extracts were determined by ELISA using commercially available kits (R&D Systems, Minneapolis, MN, USA), as follows: interleukin (IL)-1β (sensitivity > 3 pg/ml), TNF-α (> 3·4 pg/ml), interferon (IFN)-γ (> 2 pg/ml), IL-4 (> 2 pg/ml) and IL-10 (> 4 pg/ml). All assays were carried out according to the manufacturer's instructions. The results were expressed as picograms of cytokine (± s.d.) per milligram of periodontal tissue, for one experiment representative of three.
Serum C reactive protein (CRP) measurement
The levels of serum CRP were determined using a commercially available agglutination kit (Labtest Diagnóstica, São Paulo, Brazil). In brief, 50 µl of serum samples (diluted 4, 16, 64, 128 and 256 times), 50 µl of 0·9% NaCl and 50 µl of a solution containing latex beads coated with anti-CRP antibodies were dispensed in 96-well plates. The plate was agitated with circular movements for 2 min, and the macroscopic evidence of agglutination was observed. For the semiquantification of CRP levels, the level of assay sensitivity (> 6 mg/l) were multiplied by the titre of CRP of each sample. One experiment representative of three is presented in the results.
Periodontal tissue MPO activity
The activity of MPO in periodontal tissue was measured as described previously [13]. Briefly, periodontal tissues were homogenized in ice-cold buffer (0·1 M NaCl, 20 mM NaPO4, 15 mN Na EDTA), pH 4·7, and centrifuged at 3000 g for 15 min. The pellet was then subjected to hypotonic lysis (900 µl of 0·2% NaCl solution for 30 s followed by addition of an equal volume of a solution containing 1·6% NaCl and 5% glucose). After further centrifugation, the pellet was resuspended in 50 mM NaPO4 buffer, pH 5·4, containing 0·5% hexadecyltrimethylammonium bromide (H-TAB) and rehomogenized. The homogenate was then frozen and thawed three times and centrifuged again at 10 000 g for 15 min at 4°C. MPO activity in the resuspended pellet was assayed by measuring the change in absorbance at 450 nm using tetramethylbenzidine (1·6 mM) and H2O2 (0·5 mM). A unit of MPO activity was defined as that converting 1 µmol of hydrogen peroxide to water in 1 min at 22°C. One experiment representative of three is presented in the results.
Serum antibody to A. actinomycetemcomitans
The levels of serum antibody specific to A. actinomycetemcomitans were measured as described previously [14]. Briefly, 96-well microtitre plates (Corning Incorporated, Corning, NY, USA) were coated with formalin-fixed whole bacterial cells in 0·1 M sodium carbonate buffer, incubated at room temperature for 4 h, washed three times with PBS (0·05% Tween 20) and then blocked with PBS containing 5% bovine serum albumin (BSA) for 30 min. Serial serum dilutions were added, incubated at room temperature for 2 h and, after washing the wells three times, 100 µl of peroxidase-conjugated anti-mouse IgG (Zymed; Invitrogen Life Technologies, Carlsbad, CA, USA) were added as a detection antibody. After washing three times, 100 µl of substrate buffer (o-phenylenediamine dihydrochloride) was added and incubated for 45 min. The enzymatic reaction was stopped by adding 50 µl of 3 N HCl and the absorbances were measured at 492 nm with a microtitre plate reader (EMAX; Molecular Devices Corporation, Sunnyvale, CA, USA). Non-infected mice serum was used as a control for non-specific binding. The IgG levels are expressed as the antibody serum titre; data are presented from one experiment that is representative of two.
Statistical analysis
The number of inflammatory cells and the CEJ–ABC area values were submitted to the one-way analysis of variance (anova) statistical test, followed by Bonferroni's post-test analysis. Differences in the relative intensity of mRNA expression, bacterial load, cytokine, CRP, MPO and antibody levels, and mice weight between p55TNF-KO and wild-type mice groups at each time-point were analysed by the unpaired t-test. Values of P < 0·05 were considered statistically significant. All statistical tests were performed with the GraphPad Prism version 3·0 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Alveolar bone loss and inflammatory reaction in response to A. actinomycetemcomitans infection
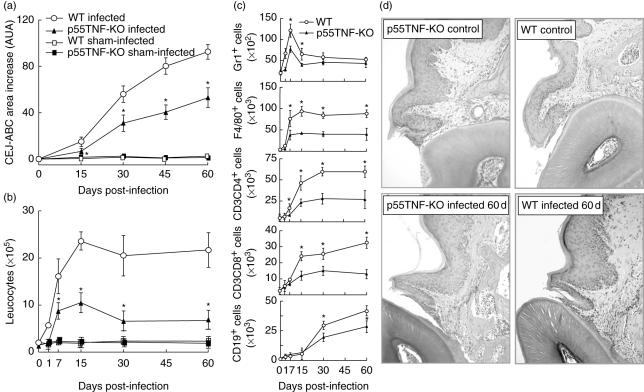
We first evaluated the role of TNF-α in the modulation of the inflammatory reaction and bone resorption in mice infected with A. actinomycetemcomitans. With regard to alveolar bone resorption, we found significantly less bone resorption post-infection in p55TNF-KO when compared with wild-type mice (Fig. 1a). The analysis of the inflammatory cells extracted from the gingival tissue of A. actinomycetemcomitans-infected p55TNF-KO mice also showed significantly fewer leucocytes compared to wild-type infected mice (Fig. 1b). The number of GR1+ cells (more than 85% of neutrophils, according to the size and complexity scatter analysis) was lower in p55TNF-KO mice in the initial phase of disease (1, 7 and 15 days pi) when compared with wild-type mice (Fig. 1c). Our results also demonstrate significantly fewer F4/80+, CD3+CD4+ and CD3+ CD8+ cells in the gingival tissue of p55TNF-KO mice (Fig. 1c). The number of CD19+ cells was lower in periodontal tissues of p55TNF-KO mice at 30 and 60 days pi (Fig. 1c).
Fig. 1.
p55TNF-R1 modulates alveolar bone loss and inflammatory cell migration after Actinobacillus actinomycetemcomitans oral inoculation of mice. C57BL/6 and TNF-α receptor p55-deficient mice [p55TNF-knock-out (KO)] mice were infected orally with A. actinomycetemcomitans and evaluated for: (a) alveolar bone loss quantification, performed through the measurements of cement–enamel junction–alveolar bone crest (CEJ–ABC) area in the palatal face of maxillary molars; (b) total leucocyte counts of the inflammatory infiltrate, performed in a Neubauer chamber; and (c) GR1+, F4/80+, CD3CD4+, CD3CD8+, CD19+ cell counts, analysed by flow cytometry, as described in Material and methods. Values (mean ± s.d.) obtained from five animals at each time-point, from one experiment representative of three. *P < 0·05 versus wild-type, (a) and (b) one-way analysis of variance (anova) with Bonferroni's post-test, (c) unpaired t-test. (d) Histological sections of periodontal tissues of control and infected wild-type and p55TNF-KO mice (haematoxylin and eosin staining; magnification, ×400).
In agreement, histological analysis of periodontal tissues showed an inflammatory cell infiltrate in the connective tissue and surrounding bone tissue and apical proliferation of the junctional epithelium of A. actinomycetemcomitans-infected mice (Fig. 1d). In contrast, only a slight inflammatory reaction was observed in p55TNF-KO mice at 60 days of infection when compared to wild-type mice (Fig. 1d). Inflammatory cells were almost absent in both non-infected wild-type and p55TNF-KO mice. Control mice (sham- and non-infected groups) of both strains did not present evidence of any inflammatory reaction or alveolar bone loss (Fig. 1a,b) or other histological alterations in the period analysed (data not shown). These results demonstrate that p55TNF-R1 present an important role in the determination of experimental PD severity.
Chemokines, chemokine receptors and cytokines in periodontal tissues
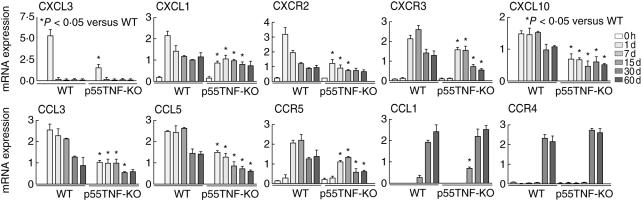
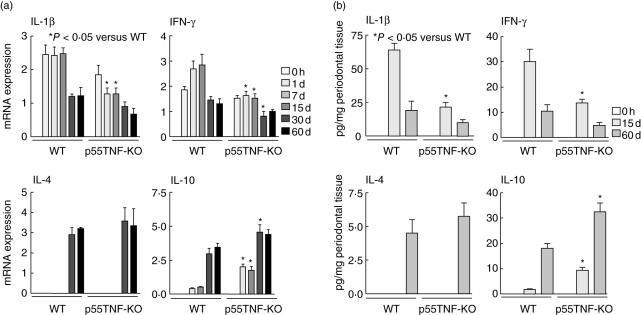
Because p55TNF-KO mice present a significant decrease in the inflammatory reaction in periodontal tissues, we next investigated the expression of chemokines and their respective receptors (Fig. 2), as these are considered the key mediators of the cell migration process. Our data demonstrate lower expression of neutrophil chemoattractants CXCL3 (1 day pi) and CXCL1 (1, 7, 15 and 30 days pi) and their receptor CXCR2 in p55TNF-KO mice when compared to wild-type mice. The expression levels of Th1-type chemokines CCL3, CCL5 and CXCL10, and their receptors CCR5 and CXCR3, were also significantly lower in p55TNF-KO mice between 1 and 60 days pi. With regard to the expression of the Th2-type chemokine CCL1 and its receptor CCR4, no major differences were found between p55TNF-KO and wild-type-infected mice. ELISA analyses demonstrate that IL-1β and IFN-γ levels were significantly lower in p55TNF-KO mice at 15 days pi. In contrast, the levels of IL-10 were significantly higher than in wild-type mice at 15 and 60 days pi. No significant differences were found in the levels of IL-4 in periodontal tissues of p55TNF-KO and wild-type-infected mice at the analysed times. Similarly, real-time PCR demonstrate a reduction in the levels of IL-1β and IFN-γ mRNA and a significant increase in IL-10 mRNA expression in p55TNF-KO mice (Fig. 3). Comparing the levels of IL-4 mRNA expression in p55TNF-KO and wild-type-infected mice, no significant differences were found in the time-points studied. Thus, the absence of p55TNF-R1 results in a lower production of inflammatory and Th1-type chemokines and cytokines and higher levels of IL-10 in response to A. actinomycetemcomitans infection.
Fig. 2.
p55TNF-R1 modulates the expression of chemokines and chemokine receptors during the course of experimental periodontal disease. Periodontal tissues of C57BL/6 and tumour necrosis factor (TNF)-α receptor p55-deficient mice [p55TNF-knock-out (KO)], infected orally with Actinobacillus actinomycetemcomitans, were harvested from day 0 (before infection) until 60 days of infection. The levels of CXCL3, CXCL1, CXCR2, CXCL10, CXCR3, CCL3, CCL5, CCR5, CCL1 and CCR4 mRNA were quantified by real-time polymerase chain reaction, using the SybrGreen System and the cycle threshold (Ct) method, as described in Material and methods. The results are presented as the expressions of the target mRNAs with normalization to β-actin, mean ± s.d. from duplicate measurements, one experiment representative of three. *P < 0·05 compared to wild-type, unpaired t-test.
Fig. 3.
p55TNF-R1 modulates the production of cytokines during the course of experimental periodontal disease. Periodontal tissues of C57BL/6 and TNF-α receptor p55-deficient mice [p55TNF-knock-out (KO)] mice, infected orally with Actinobacillus actinomycetemcomitans, were harvested from day 0 (before infection) until 60 days of infection. Cytokine productions were analysed at both mRNA and protein levels. (a) The levels of interleukin (IL)-1β, interferon (IFN)-γ, IL-10 and IL-4 mRNA were quantified by real-time polymerase chain reaction, using the SybrGreen System and the cycle threshold (Ct) method. The results are presented as the expressions of the target mRNAs with normalization to β-actin, mean ± s.d. from duplicate measurements, one experiment representative of three. (b) The levels of IL-1β, IFNγ, IL-10 and IL-4 protein in periodontal tissues were determined at the indicated times by enzyme-linked immunosorbent assay (ELISA), as described in Material and methods. The results are presented as picograms of cytokine per milligram of tissue, mean ± s.d. from duplicate measurements, one experiment representative of three. *P < 0·05 compared to wild-type, unpaired t-test.
MMPs/tissue inhibitor of metalloproteinases (TIMPs) and receptor activator of nuclear factor kB ligand (RANKL)/osteoprotegerin (OPG) expression
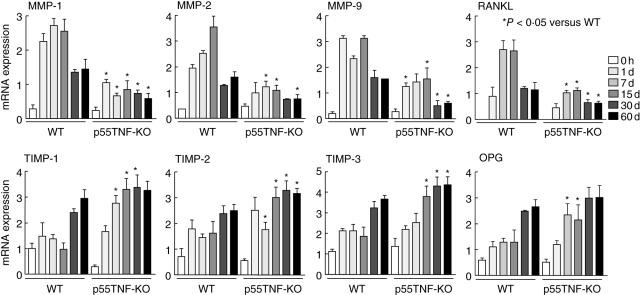
We next investigated whether the lower PD severity seen in p55TNF-KO mice could be due to a modulation in the balance between MMPs/TIMPs and RANKL/OPG expression (Fig. 4). The quantitative analysis of MMPs (MMP-1, MMP-2 and MMP-9) mRNA expression in gingival tissues from infected p55TNF-KO mice showed a significantly lower expression when compared to wild-type-infected mice during the entire course of disease (Fig. 4). Conversely, the expression of TIMPs (TIMP-1, 2 and 3) was found to be generally increased in p55TNF-KO (Fig. 4). Investigating the expression of osteoclast regulatory factors, we found that RANKL expression was significantly lower in p55TNF-KO, while OPG expression was increased in p55TNF-KO mice (Fig. 4). Taken together, these results demonstrate the molecular pathways involved in the determination of alveolar bone loss severity by p55TNF-R1.
Fig. 4.
p55TNF-R1 modulates the expression of matrix metalloproteinases (MMPs), tissue inhibitor of metalloproteinases (TIMPs), receptor activator of nuclear factor kB ligand (RANKL) and osteoprotegerin (OPG) during the course of experimental periodontal disease. Periodontal tissues of C57BL/6 and TNF-α receptor p55-deficient mice [p55TNF-knock-out (KO)] mice, infected orally with Actinobacillus actinomycetemcomitans, were harvested from day 0 (before infection) until 60 days of infection. The levels of MMPs, TIMPs, RANKL and OPG mRNA were quantified by real-time polymerase chain reaction, using the SybrGreen System and the cycle threshold (Ct) method. The results are presented as the expressions of the target mRNAs with normalization to β-actin, mean ± s.d. from duplicate measurements, one experiment representative of three. *P < 0·05 compared to wild-type, unpaired t-test.
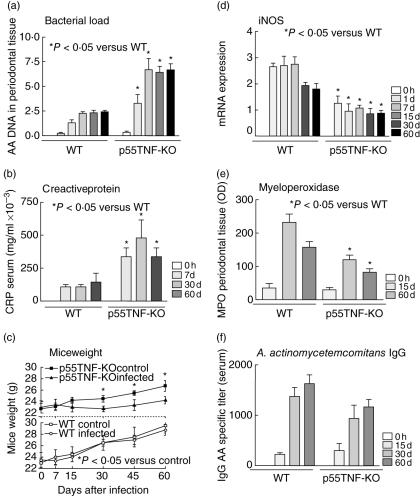
The control of experimental A. actinomycetemcomitans infection
In spite of the reduced severity of experimental PD, p55TNF-KO mice do not properly control the experimental A. actinomycetemcomitans infection (Fig. 5a). Our results demonstrate that p55TNF-KO mice had a significantly higher bacterial load in periodontal tissues when compared to wild-type mice. We also found that A. actinomycetemcomitans-infected p55TNF-KO mice presented a higher acute phase response (i.e. higher levels of CRP in the serum) (Fig. 5b) and a significantly lower weight gain during the course of infection, compared to p55TNF-KO non-infected mice (Fig. 5c). Conversely, in spite of an acute phase reaction in response to bacterial infection, wild-type mice do not present changes in weight gain during the course of disease.
Fig. 5.
The role of p55TNF-R1 in the control of Actinobacillus actinomycetemcomitans infection. C57BL/6 and tumour necrosis factor (TNF)-α receptor p55-deficient mice [p55TNF-knock-out (KO)] mice were infected orally with A. actinomycetemcomitans and were evaluated for: (a) A. actinomycetemcomitans load in periodontal tissues, quantified by real-time polymerase chain reaction (PCR), using the SybrGreen System, and normalized by tissue weight; (b) serum levels of C reactive protein, presented as mg/ml × 10−3; (c) weight gain of mice during the course of experimental periodontal disease; (d) levels of inducible nitric oxide synthase (iNOS) expression in periodontal tissues, quantified by real-time PCR, using the SybrGreen System and the cycle threshold (Ct) method; (e) levels of myeloperoxidase in periodontal tissues, presented as OD; and (f) the titres of A. actinomycetemcomitans-specific IgG in the serum, quantified by enzyme-linked immunosorbent assay; all performed as described in Material and methods. *P < 0·05 versus wild-type or control, unpaired t-test.
In order to investigate the mechanisms involved in the higher susceptibility of p55TNF-KO to A. actinomycetemcomitans infection, we found that the levels of the neutrophilic anti-microbial mediator myeloperoxidase (MPO) and expression of indicible nitric oxide synthase (iNOS) were lower in periodontal tissues of p55TNF-KO-infected mice (Fig. 5d,e). Finally, we also evaluated the levels of specific antibodies in the serum-infected mice, but no significant differences were found in the levels of A. actinomycetemcomitans-specific IgG in the serum of p55TNF-KO and wild-type-infected mice (Fig. 5f).
Discussion
Despite the clear cause-and-effect relationship demonstrated between TNF-α and the symptoms of experimental PD in rats and primates [3,9,10], the molecular mechanisms connecting this cytokine to the inflammatory reaction and tissue destruction that take place in periodontal tissues are not known. In addition, their putative role in the control of periodontal infection has not been explored. In this study, we demonstrate that TNF-α p55 receptor-deficient mice (p55TNF-KO), infected with A. actinomycetemcomitans, exhibit a significant decrease in both inflammatory cell migration and in the alveolar bone resorption when compared to wild-type mice. Accordingly, previous studies demonstrate that mice lacking the p55TNF receptor present a decrease in both the inflammatory reaction and bone loss in response to a wide a range of microbial stimulus and in different pathological processes [6,15].
In order to clarify the mechanisms by which TNF-α modulate the severity of experimental PD we evaluated the expression of chemokines, chemotactic cytokines supposed to be the main factors involved in the selective recruitment of inflammatory cells to the periodontal tissues [16]. Our results demonstrate that the messages encoding for neutrophil chemoattractants CXCL1 and CXCL3 and the number of neutrophils are diminished in periodontal tissues of p55TNF-KO mice. These chemokines are analogues of human IL-8, involved in neutrophil chemoattraction and found at high levels in diseased periodontal tissues [17]. Indeed, TNF-α modulates the production of both CXCL1 and CXCL3 and therefore the polymorphonuclear leucocyte (PMN) recruitment [18]. Our data also show that the expression of Th1-type chemokines CCL3, CCL5 and CXCL10 was lower in p55TNF-KO. Such chemokines and their receptors are expressed widely in human diseased periodontal tissues [16,19,20] and are typically involved in the chemoattraction of Th1 type cells, macrophages and dendritic cells [21]. In accordance, we found a significant reduction in the number of macrophages, CD4 and CD8 T cells in periodontal tissues of p55TNF-KO. In fact, TNF-α and their p55 receptor are described classically as inducers of cell migration process in multiple levels, including the regulation of chemokine expression [22–24]. Therefore, these broad-spectrum down-regulation of chemokine expression could explain the reduction in the inflammatory cell migration presented by p55TNF-KO mice.
In addition to presenting a direct effect on the pathogenesis of PD, TNF-α can also down-regulate the production of other proinflammatory cytokines (IL-1β and IFN-γ) thought to be implicated in the immunoregulation of disease. IL-1β is an inflammatory mediator, associated classically with PD pathogenesis, which mediates TNF-α-induced osteoclastogenesis [3,10,25]. IFN-γ is a proinflammatory cytokine, involved typically in Th1-type responses, and can contribute to sustain inflammatory reactions through the up-regulation of inflammatory cytokine and chemokine production [22,24]. Thus, the down-regulation of IL-1β and IFN-γ could also contribute to attenuate the severity of the inflammatory reaction in p55TNF-KO mice.
The chronic inflammatory reaction is thought to trigger tissue destruction as a consequence of an imbalance in the expression of MMPs, which regulate the turnover of extracellular matrix degradation; RANKL, which controls osteoclast differentiation and activation; and their respective inhibitors TIMPs and OPG [26,27]. Our data show that the expression of both MMPs (MMP-1, 2 and 9) and RANKL were lower in p55TNF-KO. In fact, the TNF-α p55 receptor is involved in tissue damage driven by MMPs [28], and also contributes to bone resorption [29]. These results are also supported by previous findings showing positive correlations between the levels of TNF-α, MMPs and RANKL in diseased human and experimental periodontal tissues [12,30]. On the other hand, the expression of TIMPs and OPG in periodontal tissues was found to be higher in p55TNF-KO. Furthermore, the expression of IL-10, linked directly to the expression of TIMPs and OPG [26,27], was also found in higher levels in p55TNF-KO mice. Similarly, higher levels of IL-10, TIMPs and OPG expression have been correlated previously with lower severity in both human and experimental periodontal diseases [12,30]. In this context, the down-regulation of MMPs and RANKL and the up-regulation of TIMPs and OPG may be a mechanistic explanation for the significant attenuation in alveolar bone loss in p55TNF-KO mice.
However, in spite of the lower PD severity presented by p55 TNF-α receptor-deficient mice, their absence resulted in the impairment of protective immunity to A. actinomycetemcomitans infection, as demonstrated by the increased bacterial load and acute phase response presented by p55TNF-KO infected mice. Accordingly, TNFp55-KO mice characteristically present a severe impairment in the clearance of a diversity of pathogens [18,31,32], and can also develop CRP responses similar to wild-type mice [33,34]. Because increased levels of CRP in serum are correlated with weight loss and anorexia–cachexia syndrome and are an important risk factor for atherosclerosis, myocardial infarction and ischaemic stroke, it could be a link to the detrimental systemic effects attributed to periodontal diseases [35,36].
Investigating the reason of the impaired immunity to A. actinomycetemcomitans infection in p55TNF-KO mice, we found a reduced number of PMNs and macrophages in their periodontal tissues. Both cell types are components of the innate immunity and are involved directly in the killing of infectious agents through the production of anti-microbial reactants. Macrophages can be activated by TNF-α to produce nitric oxide (NO), a highly reactive anti-microbial radical, which is derived from l-arginine and molecular oxygen in a reaction catalysed by the enzyme iNOS [37]. In turn, PMNs have been demonstrated to also release NO and several other potent anti-microbial reactive oxygen species, such as MPO [38]. In fact, both MPO and iNOS levels were significantly reduced in gingival tissues of p55TNF-KO mice. Indeed, there is a complementary action between both oxygen and nitrogen reactive species, as mice deficient in both systems succumb quickly to infections by endogenous bacteria [39]. The lower levels of anti-microbial mediators found in the absence of TNF-α also can be influenced by the down-regulation of IFN-γ, as both cytokines contribute to the activation of both macrophages and neutrophils [40,41]. Furthermore, adaptative immunity to A. actinomycetemcomitans is also probably impaired in p55TNF-KO mice, as T CD4, T CD8 and B lymphocytes were found in lower numbers in periodontal tissues of these mice. CD4 T cells, polarized to a Th1 phenotype, classically amplify innate and cellular immunity through the activation of macrophages and PMNs [40,41] while Th2 cells also can play protective roles through the up-regulation of antibody production [41,42]. However, similar levels of A. actinomycetemcomitans-specific IgG and IL-4 were found of p55TNF-KO and wild-type mice, suggesting that the development of Th2 response was not impaired. In addition, the cytotoxic activity of CD8 T cells is supposed to play an important role in the control of invasive bacteria, such as A. actinomycetemcomitans [43]. Previous studies demonstrate that individual or combined deficiencies of CD4 and CD8 T cells resulted in lower PD severity in response to Porphyromonas gingivalis infection in mice, but no differences were found in the number of viable bacteria in oral cavity [44]. The combined deficiency of T and B cells in SCID mice also resulted in lower alveolar bone loss, but the effects of such immunodeficiency on the control of P. gingivalis infection have not been assessed in these mouse strains [45]. All these lymphocyte subsets are supposed to participate in the control of periodontopathogens, but their specific contributions to host protection in PD remain unclear.
Our results clarify the molecular mechanisms by which the TNF-α p55 receptor determines the severity of experimental PD, sustaining the inflammatory reaction through the up-regulation of chemokine expression, and increasing the levels of tissue destructive mediators such as MMPs and RANKL. TNF-α, however, was found to present an important role in immune protection against A. actinomycetemcomitans, which is supposed to be due a reduced chemotaxis and activation of leucocytes, resulting in lower levels of anti-microbial factors such as MPO and iNOS. However, because C57BL/6 mice are known to develop a predominant Th1 type response, it would be interesting to evaluate the role of TNF-α p55 in other mouse strains. In addition, knocking out a gene may fail to reproduce the characteristics observed when their human counterpart is inactivated, and this limitation must be considered when comparing KO mice data with human disease. Therefore, a dual role for TNF-α p55 in the pathogenesis of experimental PD is demonstrated, and this knowledge may allow us to direct the development of strategies to prevent and treat PD, aimed to attenuate tissue destruction without impairing host response against infection.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (01/09998–7, 04/10102–6, 04/06731–8). J. S. S. is a research fellow of the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
References
- 1.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2:1181–92. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- 2.Kinane DF, Lappin DF. Clinical, pathological and immunological aspects of periodontal disease. Acta Odontol Scand. 2001;59:154–60. doi: 10.1080/000163501750266747. [DOI] [PubMed] [Google Scholar]
- 3.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 4.Pfizenmaier K, Wajant H, Grell M. Tumor necrosis factors in 1996. Cytokine Growth Factor Rev. 1996;7:271–7. doi: 10.1016/s1359-6101(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello C. Proinflammatory cytokines. Chest. 2000;188:503–8. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 6.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–52. [PubMed] [Google Scholar]
- 7.Tervahartiala T, Koski H, Xu JW, et al. Tumor necrosis factor-alpha and its receptors, p55 and p75, in gingiva of adult periodontitis. J Dent Res. 2001;80:1535–9. doi: 10.1177/00220345010800061101. [DOI] [PubMed] [Google Scholar]
- 8.Engebretson SP, Lamster IB, Herrera-Abreu M, et al. The influence of interleukin gene polymorphism on expression of interleukin-1beta and tumor necrosis factor-alpha in periodontal tissue and gingival crevicular fluid. J Periodontol. 1999;70:567–73. doi: 10.1902/jop.1999.70.6.567. [DOI] [PubMed] [Google Scholar]
- 9.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–9. [PubMed] [Google Scholar]
- 10.Oates TW, Graves DT, Cochran DL. Clinical, radiographic and biochemical assessment of IL-1/TNF-alpha antagonist inhibition of bone loss in experimental periodontitis. J Clin Periodontol. 2002;29:137–43. doi: 10.1034/j.1600-051x.2002.290208.x. [DOI] [PubMed] [Google Scholar]
- 11.Garlet GP, Avila-Campos MJ, Silva JS. Actinobacillus actinomycetencomitans-induced periodontal disease in mice: pattern of cytokines, chemokines, chemokine receptors expression and leukocyte migration. Microbes Infect. 2005;7:738–47. doi: 10.1016/j.micinf.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Garlet GP, Cardoso CR, Silva TA, et al. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;2:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 13.Souza MH, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791–6. doi: 10.1136/gut.2002.012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilkuna-Rautiainen T, Pussinen PJ, Mattila K, et al. Antigenically diverse reference strains and autologous strains of Actinobacillus actinomycetemcomitans are equally efficient antigens in enzyme-linked immunosorbent assay analysis. J Clin Microbiol. 2002;40:4640–5. doi: 10.1128/JCM.40.12.4640-4645.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J Clin Invest. 1997;100:1557–65. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemmell E, Carter CL, Seymour GJ. Chemokines in human periodontal disease tissues. Clin Exp Immunol. 2001;125:134–41. doi: 10.1046/j.1365-2249.2001.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu RK, Cao CF, Meng HX, Gao Y. Polymorphonuclear neutrophils and their mediators in gingival tissues from generalized aggressive periodontitis. J Periodontol. 2001;72:1545–53. doi: 10.1902/jop.2001.72.11.1545. [DOI] [PubMed] [Google Scholar]
- 18.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity. essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol. 2001;166:4042–8. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- 19.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodont Res. 2001;36:194–203. doi: 10.1034/j.1600-0765.2001.360309.x. [DOI] [PubMed] [Google Scholar]
- 20.Garlet GP, Martins W, Jr, Ferreira BR, Milanezi CM, Silva JS. Patterns of chemokines and chemokine receptors expression in different forms of human periodontal disease. J Periodont Res. 2003;38:210–17. doi: 10.1034/j.1600-0765.2003.02012.x. [DOI] [PubMed] [Google Scholar]
- 21.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 22.Sedgwick JD, Riminton DS, Cyster JG, Korner H. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol Today. 2000;21:110–13. doi: 10.1016/s0167-5699(99)01573-x. [DOI] [PubMed] [Google Scholar]
- 23.Tessier PA, Naccache PH, Clark-Lewis I, Gladue RP, Neote KS, McColl SR. Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–602. [PubMed] [Google Scholar]
- 24.Vaday GG, Franitza S, Schor H, et al. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J Leukoc Biol. 2001;69:885–92. [PubMed] [Google Scholar]
- 25.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 115:282–90. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 27.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 28.Pender SL, Fell JM, Chamow SM, Ashkenazi A, MacDonald TT. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998;160:4098–103. [PubMed] [Google Scholar]
- 29.Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–64. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 30.Garlet GP, Martins W, Jr, Fonseca BA, Ferreira BR, Silva JS. Matrix metalloproteinases, their physiological inhibitors and osteoclast factors are differentially regulated by the cytokine profile in human periodontal disease. J Clin Periodontol. 2004;31:671–9. doi: 10.1111/j.1600-051X.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 31.Pfeffer K, Matsuyama T, Kundig TM, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–67. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 32.Aliberti JC, Souto JT, Marino AP, et al. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am J Pathol. 2001;158:1433–40. doi: 10.1016/s0002-9440(10)64094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 34.Leon LR, Kozak W, Peschon J, Kluger MJ. Exacerbated febrile responses to LPS, but not turpentine, in TNF double receptor-knockout mice. Am J Physiol. 1997;272:563–9. doi: 10.1152/ajpregu.1997.272.2.R563. [DOI] [PubMed] [Google Scholar]
- 35.Backes JM, Howard PA, Moriarty PM. Role of C-reactive protein in cardiovascular disease. Ann Pharmacother. 2004;38:110–8. doi: 10.1345/aph.1D203. [DOI] [PubMed] [Google Scholar]
- 36.Offenbacher S, Beck JD. A perspective on the potential cardioprotective benefits of periodontal therapy. Am Heart J. 2005;149:950–4. doi: 10.1016/j.ahj.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Chakravortty D, Hensel M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003;5:621–7. doi: 10.1016/s1286-4579(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 38.Carreras MC, Pargament GA, Catz SD, Poderoso JJ, Boveris A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 1994;341:65–8. doi: 10.1016/0014-5793(94)80241-6. [DOI] [PubMed] [Google Scholar]
- 39.Shiloh MU, MacMicking JD, Nicholson S, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 40.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 42.McArthur WP, Clark WB. Specific antibodies and their potential role in periodontal diseases. J Periodontol. 1993;64:807–18. doi: 10.1902/jop.1993.64.8s.807. [DOI] [PubMed] [Google Scholar]
- 43.Schaible UE, Collins HL, Kaufmann SH. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–377. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 44.Baker PJ, Howe L, Garneau J, Roopenian DC. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2002;34:45–50. doi: 10.1111/j.1574-695X.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 45.Baker PJ, Roopenian DC. Genetic susceptibility to chronic periodontal disease. Microbes Infect. 2002;4:1157–67. doi: 10.1016/s1286-4579(02)01642-8. [DOI] [PubMed] [Google Scholar]