Abstract
The role of resveratrol and curcumin is well documented in cancer, inflammation, diabetes and various other diseases. However, their immunosuppressive action on T cells, B cells and macrophages is not well documented. In the present study, we have ascertained the effect of resveratrol and curcumin on T and B cells and macrophages. The most striking findings were that both resveratrol and curcumin suppressed the activity of T and B cells and macrophages, as evidenced by significant inhibition in proliferation, antibody production and lymphokine secretion. Interestingly, curcumin imparted immunosuppression by mainly down-regulating the expression of CD28 and CD80 and up-regulating CTLA-4. Resveratrol also functioned by decreasing the expression of CD28 and CD80, as well as by augmenting the production of interleukin (IL)-10.
Keywords: CD28, CD40, CD80, CTLA-4, curcumin, IL-10, resveratrol
Introduction
A faulty immune response plays a pathogenic role in a wide spectrum of inflammatory diseases, including hypersensitivity responses to environmental antigens (allergic disorders), false recognition of self-antigen (autoimmune diseases) and immune attack against alloantigens during transplantation [1,2]. Hence, it becomes crucial to suppress the immune system to control the damage done to the self-tissues. Even though several immunosuppressive drugs are available, their mechanism of action is still not known precisely.
Change in the expression of co-stimulatory molecules, secretion of cytokines and frequency of regulatory T cells can influence the outcome of many diseases. Modulation of the expression of CD28/CTLA-4 can augment or antagonize T cell receptor signalling, and interleukin (IL)-10 is known to restrain the immune responses [3–7].
Resveratrol, present in red grapes, and curcumin in turmeric, suppress the growth of tumour cell lines, induce apoptosis and have potent anti-oxidant properties [8–13]. Because little is known about the mechanism of action of these drugs on the immune system [8,10,13], in the present study we have studied thoroughly the role of curcumin and resveratrol on lymphocyte proliferation and demonstrated for the first time that both these drugs suppress the immune system by controlling mainly the expression of CD28/CTLA-4 and CD80 co-stimulatory molecules.
Methods
Animals
Inbred female BALB/c mice 8–10 weeks old were obtained from the Institute's Animal House Facility. All experiments were performed in the Immunology Laboratory, Institute of Microbial Technology. The experimental protocols performed using animals were approved by the Institutional Animal Ethics Committee.
Drugs, reagents and antibodies
Resveratrol and curcumin were obtained from Sigma Chemicals Co. (St Louis, MO, USA). Fetal calf serum (FCS) was obtained from Sera Laboratory (Crawley Down, UK), RPMI-1640 was from Gibco (grand Island, New York, USA) and l-glutamine and streptomycin were from Serva (Heidelburg, Germany). Capture and biotinylated antibodies to mouse IL-1α, IL-4, IL-6, IL-10, tumour necrosis factor (TNF)-α and interferon (IFN)-γ and CD4-phycoerythrin (PE), CD28-cychrome (Cy) and CD25-fluorescein isothiocyanate (FITC) were purchased from BD Pharmingen (San Diego, CA, USA). Capture and biotinylated anti-mouse IgG1 and IgG2a antibodies, streptavidin–horseradish peroxidase (HRP) and ortho-phenylenediamine (OPD) were purchased from Sigma.
Medium
Cells were cultured in RPMI-1640 medium supplemented with 10% FCS, l-glutamine (2 mM), penicillin (50 μg/ml), streptomycin (50 μg/ml) and 2-mercaptoethanol (ME) (0·05 μM).
Isolation of splenocytes
BALB/c mice (four to five per group) were killed by cervical dislocation. A single-cell suspension of splenocytes was prepared by macerating the spleen in phosphate-buffered saline (PBS). The red blood cells were depleted with haemolytic Gey's solution. The cells were washed three times in PBS and adjusted to 1 × 106 cells/ml in RPMI-1640 supplemented with 10% FCS.
Lymphoproliferation
Splenocytes of BALB/c mice were cultured (1 × 105 cells/well) in U-bottomed tissue culture (TC) plates in 200 μl RPMI/FCS-10% and cultured with either concanavalin A (ConA) (1 μg/ml) or lipopolysaccharide (LPS) (5 μg/ml) for stimulation of the T and B cells, respectively, with different concentrations (1, 5, 10, 20 μM) of resveratrol and curcumin. The control cultures consisting of cells alone, cells + ConA, cells + drugs and cells + solvent (in which the drug was dissolved) were also set. After 72 h, the cultures were pulsed with 0·5 μCi of [3H]-thymidine and harvested 16 h later by automatic cell harvester (Skatron, Tranby, Norway). The radioactivity incorporated was measured using liquid scintillation counting and data expressed as mean counts per minute (cpm).
Stimulation of macrophages
Thioglycollate-elicited peritoneal macrophages (3–5 days) were harvested and washed five times with RPMI/FCS-1%. The adherent cells (1 × 105/well) were cultured with different concentrations of resveratrol and curcumin for 48 h in RPMI/FCS-10% at 37°C/7% CO2. The supernatants were removed and IL-1α, IL-6, IL-10 and TNF-α were estimated by enzyme-linked immunosorbent assay (ELISA).
Estimation of cytokines
IL-1α, IL-4, IL-6, IL-10, TNF-α and IFN-γ were estimated according to the manufacturer's protocols (Becton Dickinson, San Diego, CA, USA). Briefly, 50 μl/well of capture antibodies (1 μg/ml) were adsorbed overnight on polystyrene microtitre plates in binding buffer (0·1 M Na2PO4, pH 9·0) at 4°C. The supernatants were added followed by biotinylated anti-cytokine-detecting antibodies (0·5 μg/ml). Later, streptavidin–HRP (50 μl/well) followed by ortho-phenylenediamine was added and the plates were read at 492 nm. The usual steps of blocking, incubation and washing were followed at each step. Titration curves of recombinant IL-1α, IL-4, IL-6, IL-10, TNF-α and IFN-γ were used as standards for calculating cytokine concentrations in the samples tested.
Measurement of the IgG1 and IgG2a isotypes
The impact of resveratrol and curcumin on IgG1 and IgG2a isotypes was monitored in the supernatants of the LPS-stimulated splenocytes; 96-well microtitre plates (Costar, Cambridge, MA, USA) were coated overnight with anti-IgG1 and anti-IgG2a antibodies in carbonate–bicarbonate buffer (0·05 M, pH 9·6) at 4°C. After extensive washing with PBS–Tween 20 buffer, 50 μl of blocking buffer (3% skimmed milk in PBS–Tween 20) was added to the wells and incubated at 37°C for 2 h. The microplates were washed three times with PBS–Tween 20. The supernatants were added from the control and experimental wells and the plates were incubated for 2 h at 37°C. After washing, 50 μl of biotinylated goat anti-mouse IgG1 and IgG2a antibodies were added. The plates were incubated at 37°C for 1 h. After the usual washing steps, 50 μl of streptavidin–HRP was added to each well and the plates were incubated at 37°C for 1 h. The plates were washed again before adding 50 μl of ortho-phenylenediamine and were finally incubated at 37°C for 20 min. The reaction was terminated by the addition of 50 μl of 7% sulphuric acid. Absorbance was read at 492 nm with a microplate reader (Eurogenetics, Torino, Italy).
Flowcytometric analysis of the expression of CD80, CD40, CD28 and CD4+ CD25+ T cells
Different concentrations of resveratrol and curcumin were cultured with either ConA (1 μg/ml)-stimulated splenocytes (1 × 106 cells/well) or LPS (5 μg/ml)-activated peritoneal exudate cells (PEC) (1 × 105 cells/well) in flat-bottomed tissue culture plates in 200 μl RPMI/FCS-10%. The control cultures, consisting of cells alone, cells + ConA, cells + LPS, cells + drugs and cells + solvent (in which the drug was dissolved), were also set. After 48 h, the cells were harvested and three-colour staining was performed for T cells using anti-CD4-PE and CD28-Cy antibodies and for macrophages with anti-CD80-Cy, CD86-PE, CD40-FITC antibodies for 30–45 min at 4°C. Isotype-matched antibodies were used as control simultaneously. The cells were washed five times with PBS–Tween 20 and fixed with 1% paraformaldehyde.
The cells were acquired by fluorescence activated cell sorter (FACS) Caliber flowcytometer (Beckton Dickinson). Results were analysed by CellQuest software. Debris in the cell suspension was excluded from the analysis by suitable gating that allowed the collection of data from those light-scattering events (i.e. cells) only of a size consistent with either lymphocytes or macrophages. Analysis of the mean fluorescence intensity (MFI) was carried out on histograms in which the abscissa and the ordinate denote log fluorescence and relative cell count, respectively.
Cell viability assay/cytotoxicity assay
The cultures were set in a similar fashion as for lymphoproliferation, using different concentrations of the drugs. After 72 h of culture, the supernatants were removed after centrifugation of the plates and 200 μl of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl 2H-tetrazolium bromide (MTT) (0·3 mg/ml) in RPMI-1640 was added to the plates and kept for 6 h. The plates were again centrifuged after the incubation and supernatants were removed. Dimethylsulphoxide (DMSO), 100 μl, was added to lyse the cells and the plate was read at 540 nm. Percentage viability was calculated by employing cultures stimulated with mitogen as a control (100%) and calculating the percentage for each well containing different concentrations of the drug.
Statistical analysis
The data were analysed by one-way analysis of variance (anova) followed by Dunnet's t-test to assess the significance; P < 0·05 was considered to be statistically significant.
Results
Effect of resveratrol and curcumin on the proliferation of the ConA-stimulated splenocytes
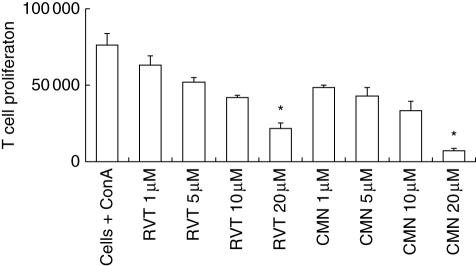
In the present study, we evaluated the role of different concentrations of curcumin and resveratrol on the in vitro proliferation of ConA-stimulated splenocytes. There was a significant decrease in the proliferation, as evidenced by less incorporation of [3H]-thymidine. Because ConA is a T cell mitogen, the data indicate that inhibition in proliferation is due to the T cell population. The decrease in proliferation with both drugs was observed in a dose-dependent manner (Fig. 1). We did not observe significant [3H]-thymidine incorporation (< 5000 cpm) in the cultures containing cells + medium, cells + curcumin (1–20 μM), cells + resveratrol (1–20 μM) and cells + DMSO.
Fig. 1.
Effect of resveratrol and curcumin on the proliferation of concanavalin A (ConA)-stimulated lymphocytes. ConA-stimulated lymphocytes were cultured with different concentrations (1, 5, 10, 20 μM) of resveratrol (RVT) and curcumin (CMN). After 72 h of incubation, [3H]-thymidine was added and the cells were harvested 16 h later and radioactivity incorporated was measured. As a control, cells + medium, cells + RVT/CMN could not generate more than 5000 counts per minute (cpm). *P < 0·05 compared to cells cultured with ConA. The data are the mean ± standard error (s.e.) of triplicate determinants.
Effect of resveratrol and curcumin on the secretion of cytokines
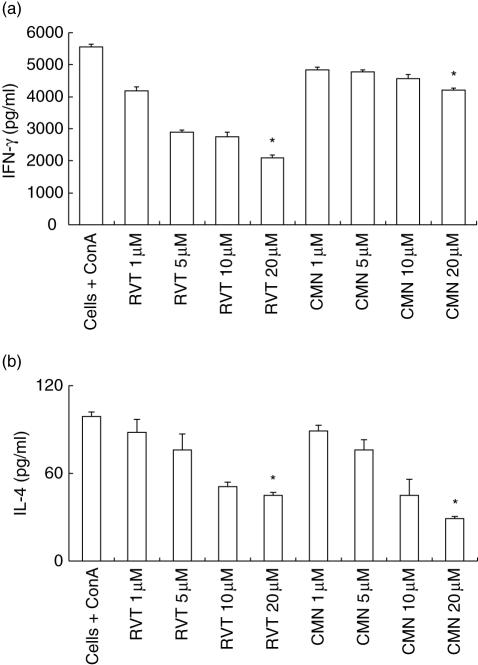
We also monitored the influence of resveratrol and curcumin on the secretion of cytokines. Compared to ConA-stimulated splenocytes, resveratrol significantly (P < 0·05) retarded production of IL-4 and IFN-γ. Similarly, curcuminalso significantly (P < 0·05) down-regulated the secretion of IL-4, but a marginal decrease in the yield of IFN-γ was observed (Fig. 2a,b). Modulation in the secretion of cytokines was observed in a dose-dependent manner, thus indicating that both resveratrol and curcumin can regulate the activity of Th1 and Th2 cells.
Fig. 2.
Effect of resveratrol (RVT) and curcumin (CMN) on interferon (IFN)-γ and interleukin (IL)-4 production. RVT and CMN were added in different concentrations (1,5, 10, 20 μM) to concanavalin A (ConA)-stimulated lymphocytes. The supernatants were collected after 48 h and lymphokines were measured by enzyme-linked immunosorbent assay (ELISA). All the data were calculated as pg/ml of IFN-γ (a) and IL-4 (b) as computed by comparison with the standard curve using recombinant lymphokines. Data expressed are the mean ± standard error (s.e.) from triplicate samples. *P < 0·05 compared to the cytokines release by ConA-treated cells.
Effect of resveratrol and curcumin on the proliferation of the LPS-stimulated splenocytes
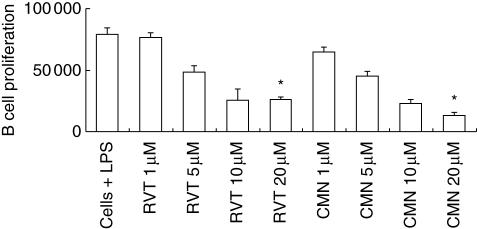
Because we observed a significant decline in the proliferation of T cells, we then conducted experiments to monitor the influence of resveratrol and curcumin on B cells. The splenocytes were stimulated with B cell mitogen LPS. Similar results to those observed in the case of ConA-stimulated lymphocytes were observed with LPS (Fig. 3). Compared to the LPS-stimulated lymphocytes, a significant (P < 0·05) level of suppression was seen in the proliferation with both curcumin and resveratrol. The inhibition in proliferation was due mainly to the B cell population, as LPS is a B cell mitogen. The results were observed in a dose-dependent fashion. In the control cultures with cells + medium, cells + curcumin (1–20 μM), cells + resveratrol (1–20 μM) and cells + DMSO, little incorporation of [3H]-thymidine (< 5000 cpm) was observed.
Fig. 3.
Effect of resveratrol (RVT) and curcumin (CMN) on the proliferation of lipopolysaccharide (LPS)-stimulated lymphocytes. RVT and CMN were added in different concentrations (1, 5, 10, 20 μM) to LPS-stimulated lymphocytes. After 72 h of incubation, [3H]-thymidine (0·5 μCi/well) was added and the cells were harvested 16 h later and radioactivity incorporated was measured. As a control, cells + medium, cells + RVT/CMN, could not generate more than 5000 counts per minute (cpm). *P < 0·05 compared to cells cultured with LPS. The data are the mean ± standard error (s.e.) of triplicate determinants.
Effect of resveratrol and curcumin on the secretion of IgG1 and IgG2a antibodies by lymphocytes stimulated with LPS
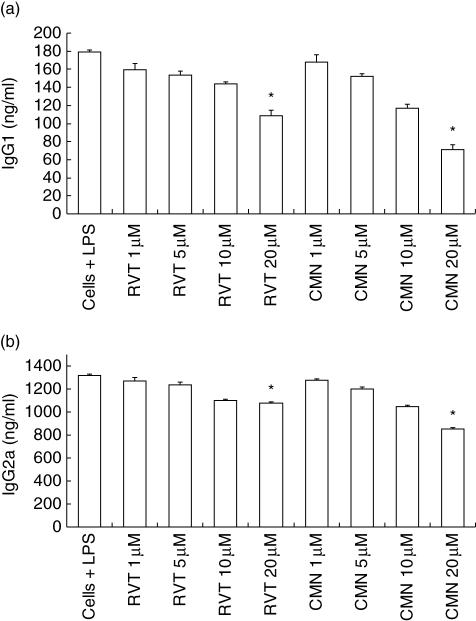
Because we observed a decrease in the proliferation of LPS-stimulated splenocytes, we next monitored the secretion of IgG1 and IgG2a-isotypes (Fig. 4a,b). As observed in the case of proliferation, a significant (P < 0·05) decrease in the yield of IgG1 and IgG2a was also noted, thus indicating that both resveratrol and curcumin can significantly retard proliferation and antibody secretion by B cells (Figs 3 and 4). It should be mentioned here that the decrease in IgG2a secretion by resveratrol was comparatively less than curcumin. Inhibition in the production of both isotypes was observed in a dose-dependent manner.
Fig. 4.
Effect of resveratrol (RVT) and curcumin (CMN) on IgG1 and IgG2a production by lymphocytes. RVT and CMN were cultured in different concentrations (1, 5, 10 and 20 μM) with lipopolysaccharide (LPS) (5 μg/ml)-stimulated lymphocytes. The supernatants were collected after 48 h and isotypes were measured by enzyme-linked immunosorbent assay (ELISA). All the data were calculated as pg/ml of IgG1 (a) and IgG2a (b) computed by comparison with the standard curve using standard IgG1 and IgG2a isotypes. Data expressed are the mean ± standard error (s.e.) from triplicate samples. *P < 0·05 compared to the release in LPS treated cells.
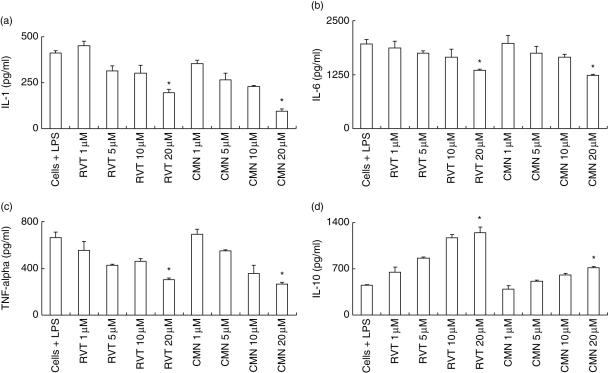
Effect of resveratrol and curcumin on the production of IL-1, IL-6, TNF-α and IL-10 by macrophages
The role of proinflammatory cytokines (IL-1, IL-6 and TNF-α) is crucial in controlling inflammation. Therefore, we also measured the secretion of IL-1, IL-6 and TNF-α by LPS-activated macrophages, the major source of these cytokines. Both resveratrol and curcumin significantly (P < 0·05) inhibited production of IL-1, IL-6 and TNF-α (Fig. 5a–c). We also monitored the release of IL-10. We noted that there was a significant (P < 0·05) enhancement in secretion by both resveratrol and curcumin (Fig. 5d). Comparatively, it was also observed that resveratrol induced more secretion of IL-10 than curcumin. Modulation in the secretion of cytokines was observed in a dose-dependent manner.
Fig. 5.
Effect of resveratrol (RVT) and curcumin (CMN) on interleukin (IL)-1, IL-6, IL-10 and tumour necrosis factor (TNF)-α production. RVT and CMN were added in different concentrations (1, 5, 10, 20 μM) to lipopolysaccharide (LPS)-stimulated macrophages. The supernatants were collected after 48 h and cytokines were measured. All the data were calculated as pg/ml of IL-1 (a), IL-6 (b), TNF-α (c) and IL-10 (d), as computed by comparison with the standard curve using recombinant cytokines. Data expressed are the mean ± standard error (s.e.) from triplicate determinants. *P < 0·05 compared to the cytokines release by LPS-treated cells.
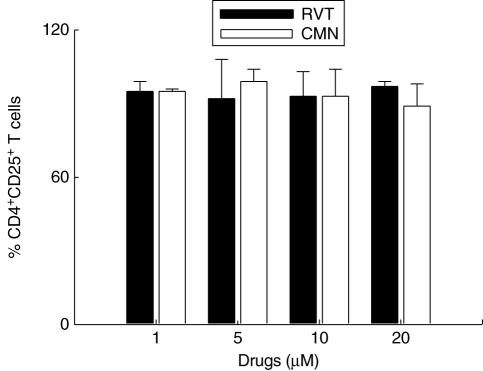
Effect of resveratrol and curcumin on the CD4+ CD25+ T cells
To determine whether the inhibitory activity of resveratrol and curcumin is due to CD4+ CD25+ T regulatory (Treg) cells, we measured the frequency of CD4+ CD25+ Treg cells (Fig. 6). Compared to ConA-stimulated cells, there was no difference in the percentage of the CD4+ CD25+ Treg cells in cultures stimulated with resveratrol and curcumin (1–20 μM).
Fig. 6.
Effect of resveratrol (RVT) and curcumin (CMN) on the CD4+CD25+ T regulatory (Treg) cells. RVT and CMN were added in different concentrations (1, 5, 10, 20 μM) to concanavalin A (ConA)-stimulated lymphocytes and CD4+ and CD25+ were enumerated by phycoerythrin (PE)-labelled anti-CD4 and fluorescein isothiocyanate (FITC)-labelled anti-CD25 antibodies. The CD4+ CD25+ Treg cells were analysed by flowcytometer and the data representing the bar diagrams are expressed as percentage positive CD4+ CD25+ cells calculated, taking into consideration the ConA-treated cells as 100%. The data shown are from duplicate determinations.
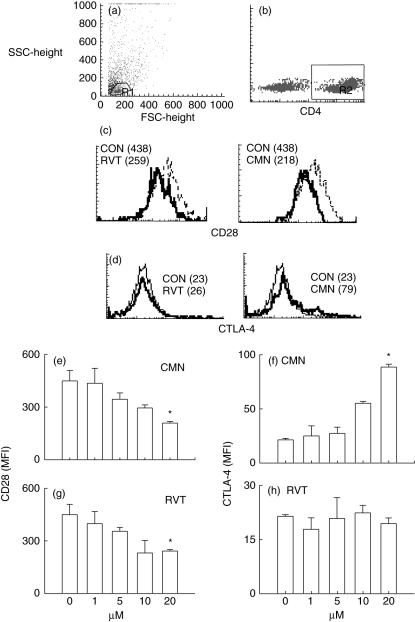
Effect of resveratrol and curcumin on the expression CD28 and CTLA-4 molecules
Because there was no difference in the CD4+ CD25+ T cells, we next measured the expression of CD28 and CTLA-4 on CD4+ T cells using different concentrations (1–20 μM) of resveratrol and curcumin (Fig. 7). Expression was monitored by flowcytometer on the CD4+ T cell population (R2 gate) (Fig. 7b) selected on lymphocyte zone (R1 gate) (Fig. 7a). Compared to ConA (MFI: CD28: 438, CTLA-4: 23), curcumin (20 μM) significantly (P < 0·05) down-regulated CD28 (MFI: 218) (Fig. 7c) and up-regulated CTLA-4 (MFI: 79) (Fig. 7d) expression. However, resveratrol (20 μM) could also significantly (P < 0·05) decrease the expression of CD28 (MFI: 259) (Fig. 7c) but failed to show any change in CTLA-4 (MFI:26) (Fig. 7d). Modulation in the expression of CD28 and CTLA-4 by curcumin (Fig. 7e,f) and resveratrol (Fig. 7g,h) was noted in a dose-dependent manner.
Fig. 7.
Effect of resveratrol (RVT) and curcumin (CMN) on the expression of CD28 and CTLA-4. RVT and CMN were added in different concentrations (1,5, 10, 20 μM) to concanavalin A (ConA)-stimulated lymphocytes and the expression of CD28 and CTLA-4 was monitored on CD4+ T cells using phycoerythrin (PE)-labelled anti-CD4+ antibodies, cychrome (Cy)-labelled anti-CD28 and fluorescein isothiocyanate (FITC)-labelled anti-CTLA-4 antibodies. The expression was analysed by flowcytometer. The histograms indicate gated (R1) population of lymphocytes (a), gated (R2) population of CD4 cells (b), expression of CD28 (c) and CTLA-4 (d) on CD4+ T cells cultured with ConA (dotted line) and 20 μM of RVT and CMN (solid lines). The data shown in parentheses depict mean fluorescence intensity (MFI). The CD28 and CTLA-4 data shown as bar diagrams are expressed as the MFI (mean ± standard error (s. e.) of the cells cultured with different doses (1–20 μM) of RVT and CMN (e–h). The data shown are from triplicate determinants. *P < 0·05 compared to ConA-treated cells.
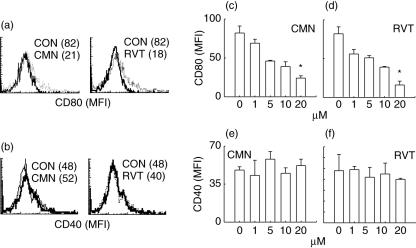
Effect of resveratrol and curcumin on the expression of CD80 and CD40 molecules
Compared to LPS (MFI: 82), macrophages cultured with 20 μM of either resveratrol (MFI: 18) or curcumin (MFI: 21) significantly (P < 0·05) down-regulated the expression of CD80 (Fig. 8a). The decrease in the expression of CD80 was observed in a dose-dependent manner (1–20 μM) with both drugs (Fig. 8c,d). Similar results were observed with CD86 (data not shown). No change was noted in the expression of CD40 with either of the drugs (Fig. 8b,e,f). CD80 and CD86 are considered to be important co-stimulatory molecules for the activation of T cells and their down-regulation has been shown to have relevance to immune suppression.
Fig. 8.
Effect of resveratrol (RVT) and curcumin (CMN) on the expression of CD80 and CD40. RVT and CMN were added in different concentrations (1, 5, 10, 20 μM) to lipopolysaccharide (LPS)-stimulated macrophages and the expression of CD80 and CD40 was monitored using cychrome (Cy)-labelled anti-CD80 and fluorescein isothiocyanate (FITC)-labelled anti-CD40 antibodies. The expression was analysed by flowcytometer and the histograms indicate expression of CD80 (a) and CD40 (b) on the cells cultured with LPS (dotted line) and 20 μM of RVT and CMN (solid lines). The data shown in parentheses depict mean fluorescence intensity (MFI). The CD80 and CD28 data shown as bar diagrams are expressed as the MFI (mean ± standard error (s. e.) of the cells cultured with different doses (1–20 μM) of RVT and CMN (c–f). *P < 0·05 compared to the LPS-treated cells.
Cumulative effect of resveratrol and curcumin on the proliferation and cytokines secretion by T cells
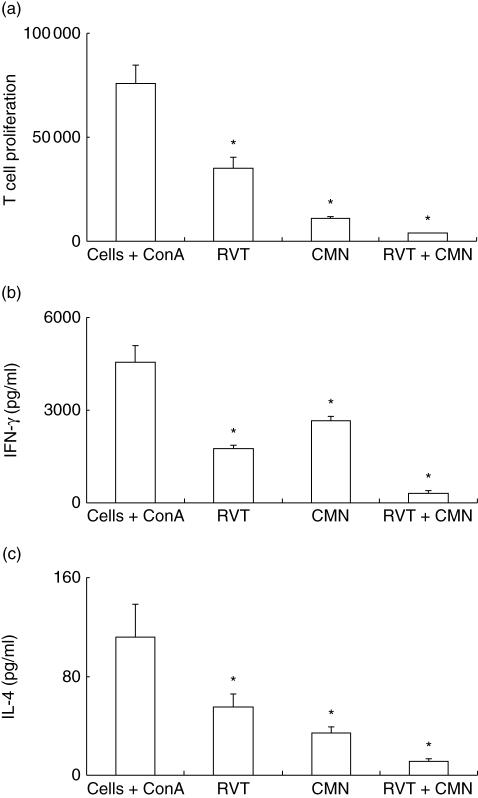
Because our data indicate that both these drugs have similar properties, we therefore evaluated the cumulative role of curcumin (20 μM) and resveratrol (20 μM) on the proliferation of ConA-stimulated splenocytes (Fig. 9a). Addition of both drugs showed synergistic effect and inhibited the proliferation and secretion of IFN-γ and IL-4 (Fig. 9a–c). This indicates that both drugs can be used together for suppressing the T cell response effectively.
Fig. 9.
Cumulative effect of resveratrol (RVT) and curcumin (CMN) on proliferation and cytokine secretion by T cells. Equal concentrations (20 μM) of RVT and CMN were cultured separately or in combination with concanavalin A (ConA)-stimulated lymphocytes. The culture conditions for proliferation and lymphokines secretion were the same as stated in the legends to Figs 1 and 2. The data are the mean ± standard error (s.e.) of triplicate determinants for proliferation (a), interferon (IFN)-γ (b) and IL-4 (c) secretion. *P < 0·05 compared to cells cultured with ConA.
Effect of various doses of resveratrol and curcumin on cell viability
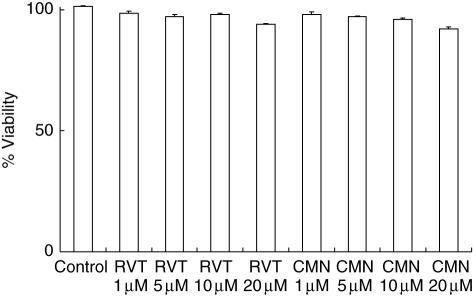
To determine whether inhibition in proliferation may be due either to over-proliferation and ultimately the death of the cells or toxicity of the drugs, we also monitored the viability of the cells cultured with resveratrol and curcumin. Compared to the cells cultured with ConA, cells treated with various concentrations of resveratrol and curcumin showed no difference in viability (Fig. 10), indicating that the results observed are due to inhibition in proliferation and not because of either over-proliferation or death of the cells, or toxicity caused by resveratrol and curcumin.
Fig. 10.
Effect of resveratrol (RVT) and curcumin (CMN) on cell viability. The cells were cultured with different concentrations (1, 5, 10, 20 μM) of RVT and CMN. Viability was measured after 6 h of culture by MTT. Values are expressed as mean ± standard error (s.e.) from triplicate samples. Percentage viability of the cells is taken as 100% in control and calculated for viability in the drug-treated cultures.
Discussion
To date, studies related to curcumin and resveratrol have largely been performed using cancer cells. Few attempts have been made to analyse their ability to regulate the activity of T cells, B cells and macrophages, etc. [8–13]. Hence, in the present study, we investigated the role of curcumin and resveratrol on the lymphocytes and addressed their mechanism of action. Six major findings have emerged from the study. Both resveratrol and curcumin suppress: (i) the proliferation of T cells and secretion of IFN-γ and IL-4; (ii) the proliferation of B cells and production of IgG1 and IgG2a isotypes; (iii) proinflammatory cytokines (IL-1, IL-6, TNF-α) but enhances the levels of anti-inflammatory cytokine IL-10; (iv) both resveratrol and curcumin down-regulates the expression of CD80 and CD28; (v) curcumin but not resveratrol up-regulates the expression of CTLA-4; and (vi) both curcumin and resveratrol fail to modulate CD4+ CD25+ regulatory T cells and expression of CD40.
The decrease in proliferation and secretion of IFN-γ and IL-4 indicated that curcumin and resveratrol suppresses the activity of Th1 and Th2 cells. It also indicated that these drugs can inhibit both cell-mediated immunity (CMI) and humoral immunity (HI). Th1 cells secrete mainly IL-2 and IFN-γ and are responsible for CMI. In contrast, Th2 cells produce chiefly IL-4 and are responsible for HI [14,15]. Further, we also conducted experiments to measure the secretion of IgG1 and IgG2a isotypes. It is known that when B cells come into contact with Th1 cells they secrete IgG2a, but on interaction with Th2 cells they produce IgG1 isotypes [16]. We observed that curcumin and resveratrol inhibit the secretion of both IgG1 and IgG2a, indicating that these drugs act on both T and B cells, further substantiating our results that both drugs can inhibit CMI and HI. Furthermore, we wanted to monitor the influence of curcumin and resveratrol on macrophages, considered to be important cells in controlling CMI [17,18]. Interestingly, both drugs inhibit production of proinflammatory cytokines IL-1, IL-6 and TNF-α but augmented the secretion of anti-inflammatory cytokine IL-10. The role of IL-10 is very well established as a suppressor cytokine that plays a regulatory role in controlling the activity of T and B cells [6,7]. It may be inferred from these results that curcumin and resveratrol may be suppressing the immune response by acting on macrophages to elicit the production of IL-10, thereby inhibiting T and B cells activation.
Finally, we performed further experiments to determine whether other immunoregulatory mechanisms, such as involvement of CD4+ CD25+ regulatory T cells and co-stimulatory molecules CTLA-4, CD28, CD80 and CD40 [1–5], are also responsible for suppressing the immune response mediated by curcumin and resveratrol. Both drugs down-regulated the expression of CD28 on CD4+ T cells and CD80 on macrophages. Further, curcumin also augmented the expression of CTLA-4. None of the molecules showed any influence on CD4+ CD25+ Treg cells, that are known to play a key role in controlling autoimmunity and inflammation [19].
The down-regulation of CD28 attenuates the immune response and renders T cells tolerant. In contrast, up-regulation of CTLA-4 is well documented as suppressing the immune system. Further, it is also a well-established fact that the absence of co-stimulatory molecules such as CD80, CD86 and CD40 can also annul the T cell function [20–23]. Hence, this study reports categorically that curcumin and resveratrol suppress activity of immune cells by affecting the expression of co-stimulatory molecules that are necessary for the activation/inhibition of T cells. Furthermore, they also augment production of IL-10, a suppressor cytokine that plays a regulatory role in controlling the activity of T and B cells [6,7]. Specific immune suppression and induction of anergy are essential processes in the regulation and circumvention of the immune defence. Down-regulating the expression of co-stimulatory molecules is a promising therapeutic target to prevent autoimmune diseases, hypersensitivity reactions and allograft rejection. Therefore, the present study suggests the possibility of immunotherapy for immunosuppression, using curcumin and resveratrol by selective regulation of CTLA-4, CD28, CD80 and IL-10.
Acknowledgments
The Senior Research Fellowship (S. Sharma) of the Defense Research Development Organization (DRDO), New Delhi, is gratefully acknowledged. The author would also like to acknowledge Manzoor A. Mir for helping in the cytokines assay and Ranbaxy Research Laboratories, Gurgaon for granting study leave for carrying out the doctorate programme.
References
- 1.Bohm M, Luger TA, Schneider M, Schwarz T, Kuhn A. New insight into immunosuppression and treatment of autoimmune diseases. Clin Exp Rheumatol. 2006;24:S67–71. [PubMed] [Google Scholar]
- 2.Regazzi MB, Alessiani M, Rinaldi M. New strategies in immunosuppression. Transplant Proc. 2005;37:2675–8. doi: 10.1016/j.transproceed.2005.06.104. [DOI] [PubMed] [Google Scholar]
- 3.Ansari MJ, Sayegh MH. Co-stimulation couture: a designer approach to regulating autoimmunity. J Clin Invest. 2006;116:2080–3. doi: 10.1172/JCI29455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayegh MH, Turka LA. T cell co-stimulatory pathways: promising novel targets for immunosuppression and tolerance induction. J Am Soc Nephrol. 1995;6:1143–50. doi: 10.1681/ASN.V641143. [DOI] [PubMed] [Google Scholar]
- 5.Thomson AW, Forrester JV. Therapeutic advances in immunosuppression. Clin Exp Immunol. 1994;98:351–7. doi: 10.1111/j.1365-2249.1994.tb05496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–6. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KW, de Waal Malefyt R, Coffman RL. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 8.Anto RJ, Mukhopadadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome C release: its suppression by ectopic expression of Bcl-2 and Bcl-xL. Carcinogenesis. 2002;23:143–50. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 9.Bielak-Zmijewska A, Koronkiewicz M, Skierski J, Piwocka K, Radziszewska E, Sikora E. Effect of curcumin on the apoptosis of rodent and human nonproliferating and proliferating lymphoid cells. Nutr Cancer. 2000;38:131–8. doi: 10.1207/S15327914NC381_18. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Gautam SC, Xu YX, Pindolia KR, Janakiraman N, Chapman RA. Nonselective inhibition of proliferation of transformed and transformed cells by anticancer agent curcumin (diferuloylmethane) Biochem Pharmacol. 1998;55:1333–7. doi: 10.1016/s0006-2952(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Xu YX, Janakiraman N, Chapman RA, Gautam SC. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem Pharmacol. 2001;62:1299–308. doi: 10.1016/s0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 13.Savouret JF, Quesne M. Resveratrol and cancer: a review. Biomed Pharmacother. 2002;56:84–7. doi: 10.1016/s0753-3322(01)00158-5. [DOI] [PubMed] [Google Scholar]
- 14.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 16.Stevens TL, Bossie A, Sanders VM, et al. Regulation of antibody isotype secretion by subsets of antigen specific helper T cells. Nature. 1998;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 17.Tang C, Inman MD, van Rooijen N, et al. Th type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J Immunol. 2001;166:1471–81. doi: 10.4049/jimmunol.166.3.1471. [DOI] [PubMed] [Google Scholar]
- 18.Zou W, Borvak J, Marches F, et al. Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12. J Immunol. 2000;165:4388–96. doi: 10.4049/jimmunol.165.8.4388. [DOI] [PubMed] [Google Scholar]
- 19.Beyersdorf N, Gaupp S, Balbach K, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–55. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson DE, Sharpe AH, Hafler DA. The B7-CD28/CTLA-4 co-stimulatory pathways in autoimmune disease of the central nervous system. Curr Opin Immunol. 1999;11:677–83. doi: 10.1016/s0952-7915(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds J, Tam FW, Chandraker A, et al. CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest. 2000;105:643–51. doi: 10.1172/JCI6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Ljunggren H, Mix E, et al. CD28-B7 co-stimulation: a critical role for initiation and development of experimental autoimmune neuritis in C57BL/6 mice. J Neuroimmunol. 2001;114:114–21. doi: 10.1016/s0165-5728(01)00241-7. [DOI] [PubMed] [Google Scholar]
- 23.Keir ME, Sharpe AH. The B7/CD28 co-stimulatory family in autoimmunity. Immunol Rev. 2005;204:128–43. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]