Abstract
Interferons (IFNs) are used widely in the treatment of viral infections, tumours and neurological disorders. The aim of this study was to evaluate the endogenous expressions of various IFN-induced compounds [specifically: neopterin (NPT), beta2microglobulin (β2mg) and 2–5 oligoadenylate synthetase (2–5 OAS)] in patients with various chronic diseases requiring treatment with IFN type I. The results showed that patients with such chronic diseases as hepatitis C virus-associated type II mixed cryoglobulinaemia (MC), chronic hepatitis C (CHC) and relapsing–remitting multiple sclerosis (RRMS) are characterized by different activations of the IFN system. Furthermore, the interindividual variability in baseline levels of IFN-induced biomarkers was higher in patients with chronic diseases than in healthy individuals. When levels of the above biomarkers were measured 24 h after the first injection of IFN in patients with CHC or RRMS, significant increases in expression levels of IFN-induced compounds were recorded but, again, there is a broad range of variability in the degree of increase. Further, a significant inverse correlation between baseline levels of NPT, β2mg and 2–5 OAS activity and their relative increases after IFN administration was found in patients with CHC or RRMS. Together, the results are consistent with the observation that there is considerable interindividual heterogeneity in the clinical response to IFNs, which suggests that host factors other than disease markers must be taken into account in order to manage and optimize the IFN therapy.
Keywords: 2–5 oligoadenylate synthetase, beta2microglobulin, interferon, markers of IFN, neopterin
Introduction
Over the last 25 years, various types of interferon (IFN) have been used to treat many diseases, including chronic hepatitis caused by virus B (HBV) or C (HCV), HCV-associated type II mixed cryoglobulinaemia (MC), melanoma, renal cell carcinoma, AIDS-related Kaposis's sarcoma, hairy cell leukaemia and chronic myelogenous leukaemia [1].
An important development in treating some of these diseases was the recognition that the clinical effects of IFN-α could be greatly enhanced by combining it with ribavirin and by adding a polyethylene glycol molecule to the native IFN protein. Indeed, pegylated (Peg)–IFN-α combination therapy has produced significant improvements in the rate of sustained virological response over standard unpegylated IFN combination therapy, without any significant increase in adverse effects in chronic hepatitis C (CHC) patients as well in MC patients [2–4].
Further, several IFN-β products have become available for treatment of patients diagnosed with multiple sclerosis (MS), particularly in its relapsing–remitting (RR) phase, the most frequently seen clinical form (RRMS) [5].
However, although many efforts have been made over the past few years to improve IFN efficacy by using different schedules and combinations with other agents, a large number of patients, above all between those affected by chronic diseases, must be considered resistant to IFN therapy. Owing to the considerable number of side effects and high treatment costs, a better understanding of the factors to be taken into account to explain the heterogeneity in response to IFN therapy would aid in the optimal application of these drugs in the management of patients with chronic diseases. However, despite extensive examination of previous studies of the biological and clinical effects of IFN in such patients, baseline levels of expression of IFN-induced biomarkers in patients who require treatment with type I IFN, especially in MC and RRMS, have been reported only occasionally in the literature [6–11].
The goal of this study was to analyse the endogenous levels of various IFN-induced compounds as biomarkers of IFN system activation in patients affected by chronic diseases such as MC, CHC and RRMS. Specifically, the following biomarkers were measured: endogenous expression of the major histocompatibility complex (MHC)-associated antigen beta2microglobulin (β2mg); the metabolic factor neopterin (NPT) and the 2–5 oligoadenylate synthetase (2–5 OAS) activity. In addition, we measured the pharmacodynamics of Peg–IFN-α and IFN-β in patients with CHC or RRMS, respectively, taking into account the above markers of IFN.
Materials and methods
Patients and study design
The study included 37 patients with MC [age, years, mean ± standard deviation (s.d.), 58·0 ± 9·3; sex, male/female, 11/26; criocrit level 10%; rheumatoid factor, 100%; mean HCV RNA Log international unit (IU)/ml, 6·6]; 38 patients with CHC [age, years, mean ± s.d., 46·0 ± 6·3; sex, male/female 25/13; mean HCV RNA Log IU/ml 5·9; mean alanine aminotransferase 109 IU/l] and 30 patients with RRMS [age, years, mean ± s.d., 34·0 ± 8·1; sex, male/female 12/18; disease duration, years, mean ± s.d., 5·6 ± 4·2; expanded disability status scale score, mean ± s.d., 1·2 ± 0·8]. None of the patients had been treated previously with IFNs or other immunosuppressive therapy (treatment-naive patients). Ten healthy controls were also examined. Written informed consent was obtained from each patient, and the study was approved by the Ethics Committees and/or Institutional Review Boards of the participating institutions.
From each patient venous peripheral blood was drawn into anti-coagulant-free tubes just before the start of IFN treatment. Endogenous activation of the IFN system was assessed by evaluation of serum levels of β2mg and NPT as well as 2–5 OAS activity.
In addition, the pharmacodynamics of IFN-β and Peg–IFN-α were analysed in patients with RRMS and CHC, respectively, taking into account the above-mentioned markers. In these patients blood samples were also obtained 24 h after the first injection of type I IFN. In particular, RRMS patients were treated with IFN-β-1a (Avonex, Biogen, Cambridge, MA, USA) at a dose of 6 MIU (30 µg) intramuscularly; HCV patients were treated by subcutaneous injection with either 180 µg Peg–IFN-α-2a (Pegasys; Hoffmann-LaRoche, Basel, Switzerland) (n = 19) or 1·5 µg/kg Peg–IFN-α-2b (PegIntron; Schering-Plough, Kenilworth, NJ, USA) (n = 19).
Blood sampling
Venous peripheral blood samples from healthy controls and patients with different chronic diseases were drawn into anticoagulant-free tubes. Serum samples, obtained after centrifugation, were stored at −80°C until used.
Detection of NPT, β2mg and 2–5 OAS
Serum concentrations of β2mg (Spa Italiana Laboratori Bouty, Milan, Italy) and NPT (DRG Instruments GmbH, Marburg, Germany) were immunoassayed following the manufacturers' instructions, using sera stored at −80 C° until tested.
The activity of 2–5 OAS in serum was measured using an assay kit from Eiken Immunochemical Laboratory (Medical System Spa, Genova, Italy) that measures the amount of adenosine triphosphate (ATP) converted into oligoadenylate by radioimmunoassay. Enzyme activity was expressed as pmol/dl. Sera samples were tested in duplicate.
Statistical analysis
All results are expressed as mean ± s.d. Where necessary, Log transformation was used to stabilize variance.
The coefficient of variation (CV) was used to measure the interpatient variability in blood concentrations of biomarkers of IFN. The CV is the ratio of the s.d. of the mean of each variable to the mean, expressed as a percentage [CV = (100) (s.d./mean)]. Relative increases in levels of β2mg, NPT and 2–5 OAS activity from pre- to post-dose were given as the post- to pre-dose levels ratio. Differences between patient groups, in terms of blood concentrations and relative increases in surrogate biological markers for IFN action, were compared using Student's t-test. Pearson's r coefficient was calculated in order to assess the correlation between pre-dose and relative increase of NPT, β2mg and 2–5 OAS activity in both CHC and RRMS patients. Significance was fixed at the 5% level. Analysis was performed with spss version 13·0 for Windows.
Results
Endogenous levels of IFN-associated biomarkers in patients with MC, CHC or RRMS
The levels of expression of β2mg and NPT, together with 2–5 OAS activity, have been measured in 37 patients with MC, 38 with CHC and 30 with RRMS. All blood samples from patients who were given IFN therapy were obtained within 1–7 days of starting treatment. Samples from 10 healthy individuals were also examined as controls.
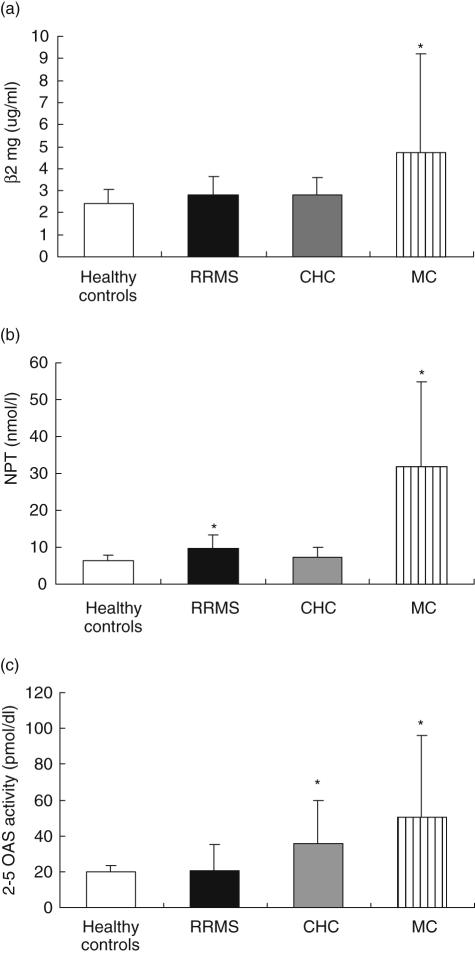
The results are shown in Fig. 1. It can be seen that, before IFN administration, patients with MC display greater expressions of all IFN-associated biomarkers compared with healthy individuals and with patients with RRMS and CHC (in this case with the exception of 2–5 OAS activity, which is higher but not at significant levels). This was not true for the other patients examined. Indeed, before IFN administration, patients with RRMS or CHC express similar levels of IFN-associated markers compared with healthy individuals, with the exception of NPT and 2–5 OAS, which are expressed more highly in RRMS and CHC, respectively. Further, Fig. 1 shows that the level of expression of NPT is higher in RRMS patients than in CHC patients and that expression of 2–5 OAS is greater in CHC than in RRMS patients.
Fig. 1.
Serum level of beta2microglobulin (β2mg) (a), neopterin (NPT) (b) and 2–5 oligoadenylate synthetase activity (2–5 OAS) (c) in patients with relapsing-remitting multiple sclerosis (RRMS, n = 30), chronic hepatitis C (CHC, n = 38), hepatitis C virus (HCV)-associated type II mixed cryoglobulinaemia (MC, n = 37). Ten healthy individuals were also examined. All blood samples were obtained from patients who were included for interferon (IFN) therapy within 1–7 days of the starting date. *β2mg (a) MC versus CHC, RRMS and healthy controls, P < 0·05; NPT (b) MC versus CHC, RRMS and healthy controls, P < 0·05; RRMS versus CHC and healthy controls, P < 0·05; 2–5 OAS (c) MC versus RRMS and healthy controls, P < 0·05; CHC versus RRMS and healthy controls P < 0·05.
The data show that a disease-specific pattern of IFN system activation may exist before the initiation of IFN therapy.
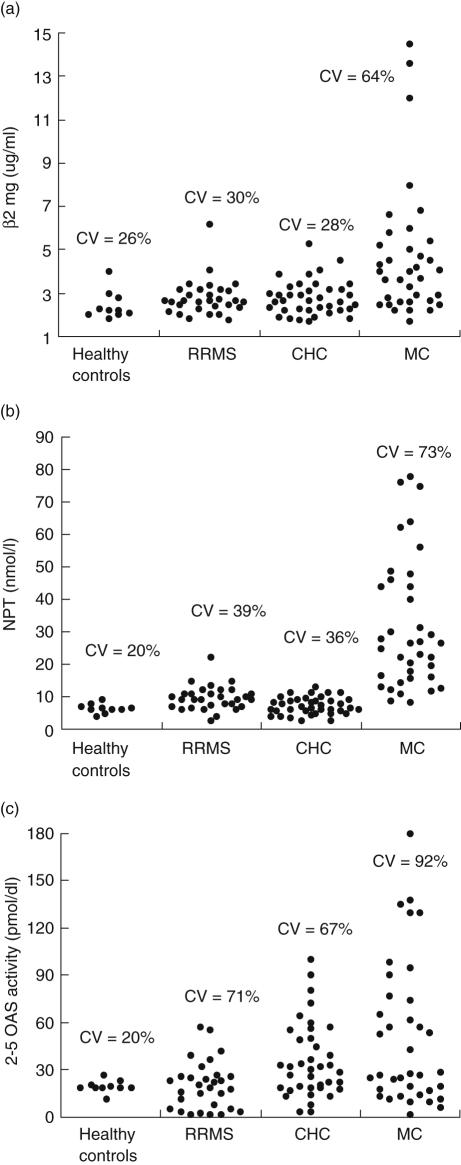
We next measured the variability of expression of IFN-associated biomarkers in the same patients. As shown in Fig. 2, 2–5 OAS (CV range: 20–92%) and β2mg (CV range: 26–64%) are IFN markers with higher and lower interpatient variability, respectively. Moreover, MC has a greater variability of the IFN-system endogenous activation than CHC and RRMS.
Fig. 2.
Variability of expression of beta2microglobulin (β2mg) (a), neopterin (NPT) (b) and 2–5 oligoadenylate synthetase activity (2–5 OAS) (c) in patients with relapsing-remitting multiple sclerosis (RRMS, n = 30), chronic hepatitis C (CHC, n = 38) and hepatitis C virus (HCV)-associated type II mixed cryoglobulinemia (MC, n = 37) prior to commencement of interferon (IFN) therapy. The coefficient of variation (CV) was used to measure interpatient variability in blood concentrations of IFN-induced biomarkers.
Levels of IFN-induced biomarkers in patients with CHC or RRMS after the first injection of IFN type I
We next examined the expression of the IFN-induced biomarkers β2mg and NPT, together with 2–5 OAS activity, in patients with both CHC and RRMS after the first injection of type I IFNs.
In order to identify the best time-table for blood sample collections we tested preliminarily the expression of these biomarkers of IFN in five patients with CHC and five patients with RRMS; blood samples were taken before and at 24, 48 and 72 h after IFN administration. On the basis of the results, we decided to collect samples before and 24 h after the first IFN administration (data not shown).
We analysed the expression of β2mg and NPT, as well as 2–5 OAS activity, in 38 patients with CHC and in 30 with RRMS.
The results are summarized in Table 1. It can be seen that 24 h after IFN-α or -β administration, in the majority of patients we could record a significant increase in IFN biomarkers independent of the examined disease. The exceptions were the values of 2–5 OAS activity at post-dose, which were higher than pre-dose but not at a significant level (P = 0·06) in patients with RRMS. Moreover, we observed that the increase varied within the IFN-induced biomarkers. In particular, in patients with CHC or RRMS the increase was greater for 2–5 OAS (from 0·2 to 19 times), whereas it was smaller for NPT (from 0·4 to nine times) and for β2mg (from 0·6 to two times). Further, the value of the IFN biomarkers relative increase was comparable within patients with RRMS and those with CHC. However, there was a trend towards greater IFN-induced 2–5 OAS activity in patients with CHC compared with RRMS. Interestingly, Table 1 also shows a marked interpatient variability in terms of the increase in IFN-induced levels of biomarkers that exists in both patients with CHC or RRMS. Specifically, the CVs were 22% for β2mg, 42–55% for NPT and 67–81% for 2–5 OAS activity.
Table 1.
Change of serum levels of beta2microglobulin (β2mg), neopterin (NPT) as well as 2–5 oligoadenylate synthetase (2–5 OAS) activity after the first injection of interferons (IFNs) in patients with chronic hepatitis C (CHC) and relapsing-remitting multiple sclerosis (RRMS).
| Chronic diseases | β2mg (µg/ml) | NPT (nmol/l) | 2–5 OAS (pmol/dl) |
|---|---|---|---|
| CHC | |||
| Pre-dose (T0) | 2·8 ± 0·8 (2·7)* | 7·2 ± 2·6 (6·6)* | 35·8 ± 24·2 (32)* |
| Post-dose (T24) | 3·6 ± 0·8 (3·5) | 20·1 ± 11·1 (18·2) | 91·6 ± 74·8 (78) |
| Increase (T24/T0) | 1·3 ± 0·2 (1·3) | 2·9 ± 1·8 (2·9) | 3·9 ± 4·7 (2·2) |
| T0versus T24/T0 correlation | r = −0·54 (P < 0·05) | r = −0·44 (P < 0·05) | r = −0·44 (P < 0·05) |
| RRMS | |||
| Pre-dose (T0) | 2·7 ± 0·8 (2·6)* | 9·2 ± 3·6 (9·6)* | 20·3 ± 14·6 (21·5) |
| Post-dose (T24) | 3·7 ± 0·8 (3·7) | 21·5 ± 9·2 (19·7) | 29·4 ± 19·8 (26·5) |
| Relative increase (T24/T0) | 1·3 ± 0·3 (1·3) | 2·5 ± 0·9 (2·3) | 2·2 ± 2·3 (1·5) |
| T0versus T24/T0 correlation | r = −0·57 (P < 0·05) | r = −0·47 (P < 0·05) | r = −0·53 (P < 0·05) |
Values are expressed in mean ± s.d. (median).
T0versus T24, P < 0·05.
Furthermore, we studied the relationship between pre-dose levels of IFN-inducible biomarkers and their relative increase after IFN therapy in patients with CHC or RRMS. The data, summarized in Table 1, revealed that there was a weak but significant inverse correlation between the pre-dose levels of IFN-inducible biomarkers and their relative increase after IFN therapy in patients with CHC. Similar results were also obtained for patients with RRMS.
Together, these findings indicate that when the baseline levels of expression of IFN-induced markers are high, the probability of this level increasing after IFN administration is low and vice versa. This appears to be independent of the type of the administered IFN (-α or -β) and the disease (CHC or RRMS).
Discussion
The study focused on the evaluation of some IFN-associated products (namely: β2mg, NPT and 2–5 OAS) as markers of biological activity in patients with different chronic diseases and whose treatments included IFN type I as a first-choice therapy [2,3,5,12].
To our knowledge, the results show for the first time that patients with different chronic diseases, such as MC, CHC and RRMS, were characterized by different activations of the endogenous IFN system. Specifically, patients with MC basically had greater levels of IFN biomarkers expression than healthy controls or CHC and RRMS patients; patients with CHC showed greater basal levels of 2–5 OAS activity than healthy controls or RRMS patients; and patients with RRMS displayed greater levels of NPT than healthy controls or CHC patients.
It is noticeable and reasonable that during the course of a disease such as MC, which is both a viral and an autoimmune disease, we can observe a high endogenous activation of the IFN system. Indeed, it is tempting to speculate that during the course of MC disease, the viral component and the immune activation might act synergistically in activating IFN-induced product expression. However, during HCV infection the viral component alone, although able to induce the synthesis of some IFN anti-viral proteins such as mixovirus-resistant protein A, protein kinase R and 2–5 OAS [13–16], is not able to stimulate the activation of the IFN system in a way that is comparable to that obtained in MC patients. Directly related to this, our results for patients with CHC show that while higher basal levels of 2–5 OAS activity were found in these patients than in healthy controls, no differences were seen in the levels of NPT and β2mg, which are associated more closely with the activation of the cellular immune system than 2–5 OAS [17].
Furthermore, it is known that patients with MC produce high levels of Th1 cytokines such as interleukin (IL)-2, IL-12 and IFN-γ, whereas the expression of Th2 cytokines such as IL-4, IL-10 is reduced [18]. The pathological levels of IFN-γ and cytokines in the serum of patients with MC, together with IFN type I, could lead to significant alterations in the levels of NPT and β2mg, as well as in 2–5 OAS activity, as reported in many in vitro studies [1,19,20].
For patients with MS, our results reveal that RRMS patients show endogenous activity of some IFN markers that are comparable to those in healthy individuals, as reported by other studies on autoimmune diseases with unknown aetiology [8,21–23]. These data could mean that MS patients possess lower endogenous IFN levels than those with viral infections. It is possible that the absence of any active viral replication during the course of the disease could justify the very low basal levels of IFN-related product expression when compared with MC or CHC. In fact, although several viruses have been considered in the aetiology of MS, at present no definitive association has been found between MS development and a specific virus infection [24,25].
The low levels of IFN-induced proteins in MS patients could also be the result of genetic defects in the IFN transduction signal found specifically in patients with MS [23]. Further studies are needed to verify these and other hypotheses, as other authors have reported different findings; at the moment there remain deep discrepancies in the published results on IFN-induced proteins expression and on serum IFN levels in patients with MS [26–29].
Whatever the interpretation of the reported observations, it is important to mention that they may be important in terms of clinical practice. In fact, all three of the chronic diseases mentioned are treated with IFN type I, and the IFN markers examined are often used as pharmacodynamic parameters in monitoring the patients' therapy.
In this context, the most interesting feature of our data derives from the observation that all the examined markers are characterized by high interpatient variability, either in basal levels or in those obtained after a single IFN administration, independently of the disease. In the majority of cases, an increase in IFN markers has been detected 24 h after IFN-α or -β administration but the increase varies from patient to patient, being negligible in some. This variability seems to apply particularly to patients with MC in whom the values of basal expression of IFN biomarkers varied considerably, and it is possible that the highest values, especially in β2mg expression, could be due to a specific hyper-responsiveness status.
The above findings make more evident the complexity of the analysed phenomenon and the difficulty in interpretation of the data. Nevertheless, several general points can be derived from the present study. Patients with MC, CHC or RRMS, although undergoing the same therapeutic approach after filling stringent inclusion criteria, represent heterogeneous populations. The different levels of markers reported, whether baseline- or IFN-α/-β-induced expression, varying from patient to patient, could indicate that patients with apparently similar clinical features may display ‘different activations’ of the immune system. Hence, the different responses to IFN-α/-β treatment reported are not surprising, as different conditions in the immune system activation may produce different clinical effects. If this is correct, we should acknowledge that, although in many patients the administered exogenous IFN can produce effects by stimulating the functional pathway of IFN, other patients with high basal levels of IFN will not benefit from such treatment because their IFN transduction pathways are already saturated.
This seems to apply both to CHC and RRMS patients. Indeed, to our knowledge, our results indicate for the first time that there is an inverse correlation between the relative increase in IFN-induced biomarkers and their baseline level. Specifically, a lower baseline expression level correlates with a greater level of increase in expression after IFN type I injection (and vice versa) independent of both the disease and the type of IFN (-α or -β). As clinical data were not included in our study, any correlation between the level of IFN-induced biomarkers and clinical outcome could not be evaluated. However, a direct correlation has been reported between IFN biomarker induction and clinical response in both CHC and RRMS patients treated with IFN [6,14,16,30–33]. Further evaluations combining clinical and biological investigations in the same individuals, together with large studies focused specifically on the relationship between clinical outcome and expression of IFN biomarkers, are certainly warranted in order to reach more definite conclusions.
In conclusion, our data demonstrate that baseline and post-dose level of IFN biomarkers are highly variable in patients with different chronic diseases treated with IFNs. The results prompt us to consider whether host factors, other than disease markers, should be taken into account in order to understand fully why IFN therapy fails. At present the reasons for the high level of interindividual variability remain unclear, but the data indicate that there is an inverse correlation between the relative increase in IFN-induced biomarkers and their baseline levels. To our knowledge this study represents the first attempt to address this issue, which we consider to be crucial in attempting to optimize IFN therapy in patients with chronic diseases.
Acknowledgments
This work was supported by FISM-Fondazione Italiana Sclerosi Multipla-Cod. 2004/R/1 and by MIUR PRIN 2005.
References
- 1.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes CA, Shafran SD. Chronic hepatitis C virus management: 2000–05 update. Ann Pharmacother. 2006;40:74–82. doi: 10.1345/aph.1G263. [DOI] [PubMed] [Google Scholar]
- 3.Ward RP, Kugelmas M. Using pegylated interferon and ribavirin to treat patients with chronic hepatitis C. Am Fam Physician. 2005;72:655–62. [PubMed] [Google Scholar]
- 4.Cacoub P, Saadoun D, Limal N, Sene D, Lidove O, Piette JC. PEGylated interferon alpha-2b and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum. 2005;52:911–5. doi: 10.1002/art.20958. [DOI] [PubMed] [Google Scholar]
- 5.Goodin DS. Treatment of multiple sclerosis with human beta interferon. Int MS J. 2005;12:96–108. [PubMed] [Google Scholar]
- 6.Kracke A, von Wussow P, Al-Masri AN, Dalley G, Windhagen A, Heidenreich F. Mx proteins in blood leukocytes for monitoring interferon beta-1b therapy in patients with MS. Neurology. 2000;54:193–9. doi: 10.1212/wnl.54.1.193. [DOI] [PubMed] [Google Scholar]
- 7.Chieux V, Chehadeh W, Hautecoeur P, Harvey J, Wattre P, Hober D. Increased levels of antiviral MxA protein in peripheral blood of patients with a chronic disease of unknown etiology. J Med Virol. 2001;65:301–8. doi: 10.1002/jmv.2034. [DOI] [PubMed] [Google Scholar]
- 8.Bagnato F, Pozzilli C, Scagnolari C, et al. A one-year study on the pharmacodynamic profile of interferon-beta1a in MS. Neurology. 2002;58:1409–11. doi: 10.1212/wnl.58.9.1409. [DOI] [PubMed] [Google Scholar]
- 9.Scagnolari C, Casato M, Bellomi F, et al. Serum interferon (IFN)-neutralizing antibodies and bioactivities of IFNs in patients with severe type II essential mixed cryoglobulinemia. Clin Diagn Lab Immunol. 2003;10:70–7. doi: 10.1128/CDLI.10.1.70-77.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pachner AR, Bertolotto A, Deisenhammer F. Measurement of MxA mRNA or protein as a biomarker of IFN beta bioactivity: detection of antibody-mediated decreased bioactivity (ADB) Neurology. 2003;61(Suppl 5):S24–6. doi: 10.1212/01.wnl.0000092361.04511.d0. [DOI] [PubMed] [Google Scholar]
- 11.Gilli F, Marnetto F, Caldano M, Sala A, Malucchi S, Capobianco M, Bertolotto A. Biological markers of interferon-beta therapy: comparison among interferon-stimulated genes MxA, TRAIL and XAF-1. Mult Scler. 2006;12:47–57. doi: 10.1191/135248506ms1245oa. [DOI] [PubMed] [Google Scholar]
- 12.Della Rossa A, Tavoni A, Baldini C, Bombardieri S. Treatment of chronic hepatitis C infection with cryoglobulinemia. Curr Opin Rheumatol. 2002;14:231–7. doi: 10.1097/00002281-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Chieux V, Hober D, Harvey J, et al. The MxA protein levels in whole blood lysates of patients with various viral infections. J Virol Meth. 1998;70:183–91. doi: 10.1016/s0166-0934(97)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.MacQuillan GC, de Boer WB, Platten MA, et al. Intrahepatic MxA and PKR protein expression in chronic hepatitis C virus infection. J Med Virol. 2002;68:197–205. doi: 10.1002/jmv.10182. [DOI] [PubMed] [Google Scholar]
- 15.MacQuillan GC, Mamotte C, Reed WD, Jeffrey GP, Allan JE. Upregulation of endogenous intrahepatic interferon stimulated genes during chronic hepatitis C virus infection. J Med Virol. 2003;70:219–27. doi: 10.1002/jmv.10381. [DOI] [PubMed] [Google Scholar]
- 16.Gerotto M, Dal Pero F, Bortoletto G, et al. PKR gene expression and response to pegylated interferon plus ribavirin therapy in chronic hepatitis C. Antivir Ther. 2004;9:763–70. [PubMed] [Google Scholar]
- 17.Bagnato F, Durastanti V, Finamore L, Volante G, Millefiorini E. Beta-2 microglobulin and neopterin as markers of disease activity in multiple sclerosis. Neurol Sci. 2003;24:S301–4. doi: 10.1007/s10072-003-0180-5. [DOI] [PubMed] [Google Scholar]
- 18.Saadoun D, Boyer O, Trebeden-Negre H, et al. Predominance of type 1 (Th1) cytokine production in the liver of patients with HCV-associated mixed cryoglobulinemia vasculitis. J Hepatol. 2004;41:1031–7. doi: 10.1016/j.jhep.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Hamerlinck FF. Neopterin: a review. Exp Dermatol. 1999;8:167–76. doi: 10.1111/j.1600-0625.1999.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa T, Nakao K, Nakata K, et al. Involvement of IL-1beta and IL-10 in IFN-alpha-mediated antiviral gene induction in human hepatoma cells. Biochem Biophys Res Commun. 2002;294:414–22. doi: 10.1016/S0006-291X(02)00502-8. [DOI] [PubMed] [Google Scholar]
- 21.Haahr S, Moller-Larsen A, Justesen J, Pedersen E. Interferon induction, 2′−5′ oligo A synthetase and lymphocyte subpopulations in out-patients with multiple sclerosis in a longitudinal study. Acta Neurol Scand. 1986;73:345–51. doi: 10.1111/j.1600-0404.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- 22.Schattner A, Merlin G, Bregman V, et al. (2′-5′) Oligo A synthetase in human polymorphonuclear cells increased activity in interferon treatment and in viral infections. Clin Exp Immunol. 1984;57:265–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Feng X, Petraglia AL, Chen M, Byskosh PV, Boos MD, Reder AT. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. J Neuroimmunol. 2002;129:205–15. doi: 10.1016/s0165-5728(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 24.Cook SD. Does Epstein–Barr virus cause multiple sclerosis? Rev Neurol Dis. 2004;1:115–23. [PubMed] [Google Scholar]
- 25.Lutton JD, Winston R, Rodman TC. Multiple sclerosis: etiological mechanisms and future directions. Exp Biol Med. 2004;229:12–20. doi: 10.1177/153537020422900102. [DOI] [PubMed] [Google Scholar]
- 26.Kamin-Lewis RM, Panitch HS, Merigan TC, Johnson KP. Decreased interferon synthesis and responsiveness to interferon by leukocytes from multiple sclerosis patients given natural alpha interferon. J Interferon Res. 1984;4:423–32. doi: 10.1089/jir.1984.4.423. [DOI] [PubMed] [Google Scholar]
- 27.Dettke M, Scheidt P, Prange H, Kirchner H. Correlation between interferon production and clinical disease activity in patients with multiple sclerosis. J Clin Immunol. 1997;17:293–300. doi: 10.1023/a:1027374615106. [DOI] [PubMed] [Google Scholar]
- 28.Hertzog PJ, Wright A, Harris G, Linnane AW, Mackay IR. Intermittent interferonemia and interferon responses in multiple sclerosis. Clin Immunol Immunopathol. 1991;58:18–32. doi: 10.1016/0090-1229(91)90145-z. [DOI] [PubMed] [Google Scholar]
- 29.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988;78:318–23. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 30.Antonelli G, Simeoni E, Turriziani O, et al. Correlation of interferon-induced expression of MxA mRNA in peripheral blood mononuclear cells with the response of patients with chronic active hepatitis C to IFN-alpha therapy. J Interferon Cytokine Res. 1999;19:243–51. doi: 10.1089/107999099314171. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez M, Quiroga JA, Martin J, et al. In vivo and in vitro induction of MxA protein in peripheral blood mononuclear cells from patients chronically infected with hepatitis C virus. J Infect Dis. 1999;180:262–7. doi: 10.1086/314859. [DOI] [PubMed] [Google Scholar]
- 32.Bagnato F, Durastanti V, Finamore L, Volante G, Millefiorini E. Beta-2 microglobulin and neopterin as markers of disease activity in multiple sclerosis. Neurol Sci. 2003;24(Suppl. 5):S301–4. doi: 10.1007/s10072-003-0180-5. [DOI] [PubMed] [Google Scholar]
- 33.Giannelli G, Guadagnino G, Dentico P, Antonelli G, Antonaci S. MxA and PKR expression in chronic hepatitis C. J Interferon Cytokine Res. 2004;24:659–63. doi: 10.1089/jir.2004.24.659. [DOI] [PubMed] [Google Scholar]