Abstract
Inflammation is part of the non-specific immune response that occurs in reaction to any type of bodily injury. In some disorders, the inflammatory process − which under normal conditions is self-limiting − becomes continuous and chronic inflammatory diseases might develop subsequently. Pattern recognition molecules (PRMs) represent a diverse collection of molecules responsible for sensing danger signals, and together with other immune components they are involved in the first line of defence. NALP3 and NOD2, which belong to a cytosolic subgroup of PRMs, dubbed Nod-like-receptors (NLRs), have been associated recently with inflammatory diseases, specifically Crohn's disease and Blau syndrome (NOD2) and familial cold autoinflammatory syndrome, Muckle–Wells syndrome and chronic infantile neurological cutaneous and articular syndrome (NALP3). The exact effects of the defective proteins are not fully understood, but activation of nuclear factor (NF)-κB, transcription, production and secretion of interleukin (IL)-1β and activation of the inflammasome are some of the processes that might hold clues, and the present review will provide a thorough update in this area.
Keywords: inflammasome, inflammation, interleukin-1β, NALP3, NOD2
Introduction
The word ‘inflammation’ comes from the Latin inflammare (to set on fire). Cornelius Celsus, a Roman encyclopaedist of ancient times, wrote in Book III of his only surviving work ‘De Medicina': Notae vero inflammationis sunt quattuor: rubor et tumour cum calore et dolore (Now the signs of an inflammation are four: redness and swelling with heat and pain) [1]. Celsus was the first person to record the cardinal signs of inflammation; Rudolf Virchow later added functio laesa (loss of function). Two centuries after Celsus, Galen considered inflammation as a beneficial response to injury; on the other hand, many centuries later Virchow viewed inflammation as inherently pathological. Even though Celsus's words still echo in medical schools around the world, the panorama nowadays is much more complex.
Today it is believed that inflammation is part of the non-specific immune response that occurs in reaction to any type of bodily injury and that the cardinal signs of inflammation can be explained by increased blood flow, elevated cellular metabolism, vasodilatation, release of soluble mediators, extravasation of fluids and cellular influx. In some disorders the inflammatory process, which under normal conditions is self-limiting, becomes continuous and chronic inflammatory diseases develop subsequently.
One of the most important soluble mediators of inflammation is interleukin (IL)-1β. It is one of the molecular forms of IL-1, a potent proinflammatory cytokine. It is produced mainly by blood monocytes and mediates the wide range of reactions involved in the acute phase response. Minimal levels of IL-1β evoke fever, hypotension, the release of adrenocorticotrophic hormone and the production of cytokines such as IL-6 which, in turn, induces the synthesis of hepatic acute-phase proteins such as serum amyloid A and C-reactive protein and stimulates the synthesis of adhesion molecules in endothelial cells and leucocytes, producing leukocytosis and thrombocytosis, among others. IL-1β is produced additionally by macrophages, dendritic cells and a variety of other cells in the body.
Inflammation has very specific characteristics, whether acute or chronic, and the innate immune system plays a pivotal role, as it mediates the first response. Infiltration of innate immune system cells, specifically neutrophils and macrophages, characterizes the acute inflammation, while infiltration of T lymphocytes and plasma cells are features of chronic inflammation. Monocytes/macrophages play a central role in both, contributing to the final consequence of chronic inflammation which is represented by the loss of tissue function due to fibrosis.
In the past the innate immune system was considered to be a primitive static system; nowadays we delve into its complexity. Our current definition establishes it as a system that is able to recognize and respond to danger signals represented by a limited number of highly conserved structures of microorganisms (pathogen-associated molecular patterns − PAMPs) and several cell products associated with a breach in defences. For this purpose, the innate immune system possesses a large number of soluble (e.g. pentaxins), membrane-bound (e.g. Toll-like receptors) and cytosolic (e.g. Nod-like receptors) ‘receptors’[2]. They are known collectively as pathogen recognition receptors, or pattern recognition receptors, but a more accurate term is pattern recognition molecules (PRMs), as it is not clear whether they are all actual receptors. Nod-like receptors (NLRs) are also known as NACHTs [NAIP, CIITA, HET-E and TP1, leucine rich repeats (LRR)] or CATERPILLER [caspase recruitment domain (CARD), transcription enhancer, R(purine)-binding, pyrin, many LRRs].
This review will aim to examine some of the members in one of the possible scenarios of IL-1β production, with particular emphasis on two PRMs, namely NOD2 and NALP3 (Fig. 1) and their relevance in the inflammatory process, both within normal and aberrant conditions.
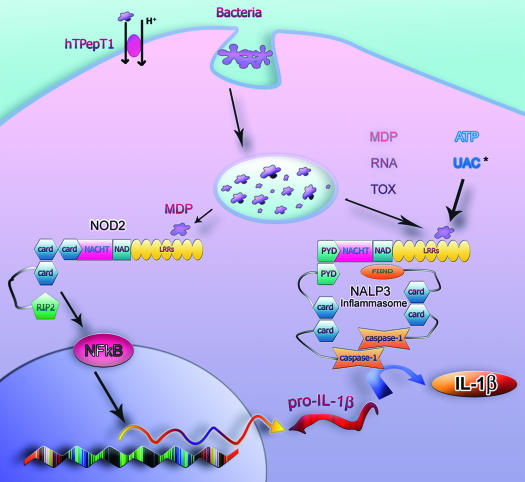
Fig. 1.
Model for possible interactions between NOD2 and NALP3. Macrophages engulf and phagocytize bacteria; the peptidoglycan (PGN) present in the bacterial wall is degraded to produce muropeptides, which are similar to muramyl dipeptide (MDP). MDP can also be translocated into the cytoplasm by the hPepT1 transporter located in the brush border of colonocytes. In the cytoplasm, MDP is then able to activate NOD2, which in turn interacts with RIP2. This sequence of events activates nuclear factor (NF)-κB, which upon translocation to the nucleus induces, among others, pro-interleukin (IL)-1β transcription. Cytoplasmic MDP might also induce assembly of the NALP3 inflammasome, but bacterial RNA, toxins, uric acid crystals (*the mechanism by which uric acid crystals or its components become available in the cytoplasm is still unknown) and adenosine triphosphate (ATP) also trigger activation of the NALP3 inflammasome. The inflammasome holds 2 caspase-1 in close proximity, allowing cross-activation. Active caspase-1 is then able to process IL-1β. Mature pro-IL-1β is then released into the extracellular environment, together with caspase-1 (not shown). UAC: uric acid crystals; RNA refers to bacterial RNA; TOX: toxins.
PRM domains
The discovery of the Drosophila Toll homologues in the human genome-encoding proteins named Toll-like receptors (TLRs) were one of the starting points on a complete overhaul of the general understanding of the innate immune system [3]. Today, there is a growing number of PRMs; NOD2 and NALP3 both belong to a newly added family, the aforementioned NLRs [4].
NLRs are characterized by a C-terminal LRR domain, an intermediary nucleotide binding domain (NBD: NACHT/NOD) and an N-terminal effector domain, which can be a pyrin domain (PYD), caspase recruitment domain (CARD) or a baculovirus inhibitor of apoptosis protein repeat (BIR) [2].
The LRR domain serves as the ligand-recognizing/binding motif that regulates oligomerization. The LRRs are generally composed of 20–29 residues and contain a conserved 11-residue segment with the consensus sequence LxxLxLxxN/CxL (x can be any amino acid and L positions can also be occupied by valine, isoleucine and phenylalanine, while N/C means asparagine/cysteine) [5].
The NACHT domain is a 300–400-residue region with predicted nucleoside triphosphatase (NTPase) activity, found in animal, fungal and bacterial proteins [6]. It is thought that NACHT domains are implicated primarily in inflammation, where they exert its effects through nuclear factor (NF)-κB) and caspase-1 activation (see below). These domains are able to form hetero-oligomers, which provides a means for various NACHT-containing proteins to interact, creating protein interaction networks that potentially modulate immune responses towards invading pathogens [7]. These characteristics are essential for the activation of the receptors and a prerequisite for transduction of the signal, mediated by the N-terminal effector domain [4].
As mentioned above, there are three types of effectors. The caspase recruitment domain (CARD) belongs to the death domain-fold superfamily, which is characterized by anti-parallel six-helix bundle of amphipathic helices; in this case, the helix structure is more parallel than any of the other members in this superfamily [8]. It is a homotypic protein interaction module which interacts typically with other CARD-containing proteins. The mechanisms by which CARDs activate caspases and NF-κB involve the assembly of multi-protein complexes [9]. These multimers can facilitate dimerization or serve as scaffolds on which proteases and kinases are assembled and activated [10]. Bouchier-Hayes et al. [11] classified CARD-containing proteins according to their domain structures as follows: NBD-CARDs, coiled-coil-CARDs, bipartite-CARDs and CARD-only proteins. Of particular interest for this review are NBD-CARDs, which contain a nucleotide binding domain (NBD/NACHT) and repeats (i.e. LRRs); NODs belong to this group. Bipartite-CARDs are also of interest. These types of CARDs have a second motif such as a kinase, in the case of RIP2, a FIIND domain in CARDINAL (CARD 8) and a PYD domain in apoptosis-associated speck-like protein containing a CARD (ASC). Generally, these domains are recruited and activated by other CARD-containing proteins or they may, by themselves, recruit other members to form a multi-protein complex [11]. The PYD domain also belongs to the death domain-fold superfamily (DD), and is described as two three-α-helix bundles [8]. It is a protein–protein homophilic interaction module [12]. It is found in over 30 human proteins associated with inflammation, apoptosis and NF-κB signalling. Finally, the BIR is a domain of tandem repeats separated by a variable-length linker that seems to confer cell death-preventing activity. It is found in proteins belonging to the IAP (inhibitor of apoptosis proteins) family. BIR domains are characterized by a number of invariant amino acids, including three conserved cysteines and one conserved histidine residue within the sequence CX2CX16HX6−8C [13].
NOD2
The NOD2 gene encodes two N-terminal CARDs, a NACHT domain and multiple C-terminal LRRs (Fig. 2). It is expressed constitutively in myeloid cells, particularly macrophages, neutrophils and dendritic cells, as well as in Paneth cells in the small intestine, which are of epithelial origin [14–16]. Furthermore, NOD2 expression has also been detected in colonic epithelial cells [17,18] and recently in endothelial cells [19]. The proinflammatory mediators, interferon (IFN)-γ and tumour necrosis factor (TNF)-α (secreted by Th1 cells), up-regulate the expression of NOD2 by intestinal epithelial cells [18,20].
Fig. 2.
Structure of NOD2. NOD2 is comprised of two successive N-terminal caspase recruitment domain (CARD) domains; a central Nod-like receptor (NACHT) (NOD) domain, followed by a NACHT association domain (NAD) and C-terminal leucine-rich repeats (LRRs). The three major mutations associated with Crohn's disease affect the C-terminal portion.
The NOD2 protein is located in the cytoplasm, and through its LRRs is able to recognize a peptidoglycan component: muramyl dipeptide (MDP), the minimal bioactive peptidoglycan motif common to all bacteria [21,22].
NOD2 signalling
In 2001 Ogura et al. [15] demonstrated that NOD2 is able to induce NF-κB activation, and that this activation is dependent on I kappa B kinase (IKK)γ (NEMO). They also determined that this process was inhibited by dominant negative mutants of IκBα, IKKα, IKKβ, and IKKγ. NOD2 interacts with the serine–threonine kinase RIP2 (RICK/CARDIAK) via a homophilic CARD–CARD interaction [23]. RIP2 is then able to activate the IKK complex through IKKγ, which in turn phosphorylates IκBα, releasing and activating NF-κB.
NF-κB proteins comprise a large family of transcription factors in eukaryotes. Its members are related structurally and promote the expression of over 150 genes involved in a variety of cellular processes, including cytokines, chemokines, growth factors, immunoregulatory molecules, cell adhesion molecules, acute phase response proteins, stress response genes, cell surface receptors, regulators of apoptosis and many others. In most cell types, inactive NF-κB complexes are sequestered in the cytoplasm via their non-covalent interaction with inhibitory proteins known as inhibitor of κBs (IκB). In response to multiple stimuli − including cytokines, viral and bacterial pathogens and stress-inducing agents − the latent cytoplasmic NF-κB/IκBα complex is activated by phosphorylation (by IKKs − inhibitor of κB kinases) and IκBα is consequently ubiquitinated and tagged for proteosomal degradation [24]. This process activates NF-κB, which then translocates to the nucleus and binds to DNA in the promoter or enhancer regions of specific genes and thereby activates these genes [25].
Pro-IL-1β
Pro-IL-1β transcription is induced by NF-κB. It is synthesized as a 35-kDa precursor; this precursor is then activated by proteolytic cleavage, mediated by caspase-1 [also known as interleukin converting enzyme (ICE)], which generates a mature product of 17 kDa. Activation of caspase-1 is essential for IL-1β processing, as caspase-1 itself is generated as an inactive precursor, and a molecular machinery is necessary to put things in motion. Recently, Martinon et al. [26] identified such a ‘machine’, a multi-protein complex which they characterized as the inflammasome. After processing, IL-1β is secreted together with active caspase-1 [26–28].
The NALP3 inflammasome
The inflammasome acts as a molecular vice, holding caspases in close proximity allowing cross-activation [26]. Initially it was described as complex made of caspase-1, caspase-5, NALP1 and Pycard/ASC. However, in a more recent review, Martinon et al. [29] have presented several different caspase-activation platforms where different components constitute the various inflammasomes. The NALP3 inflammasome was one of those identified more recently. It is composed of NALP3, cardinal (CARD8) and ASC, and brings two caspase-1 into close proximity [29,30] (Fig. 3).
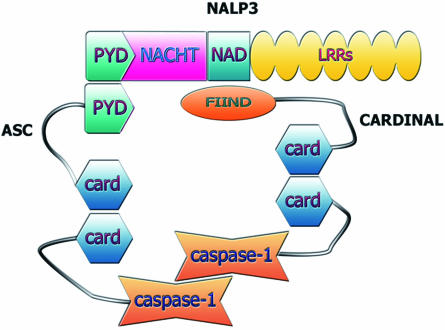
Fig. 3.
Structure of the NALP3 inflammasome. NALP3 assembles with apoptosis-associated speck-like protein containing a CARD (ASC) and CARDINAL. CARD domains in the two adaptor molecules are able to interact with caspase-1 CARD domains and hold 2 caspases in close proximity to allow cross-activation.
NALP3
NALP3 (also known as cryopyrin, PYPAF1 and CIAS1) is composed of a central NACHT domain, a PYD domain and the LRR receptor domain. It associates with ASC through PYD–PYD interactions and to CARDINAL through NACHT–FIIND interactions [30] (Fig. 3). NALP3 expression is restricted to immune cells and chondrocytes [31]; it binds ASC and activates caspase-1. O'Connor et al. [32] reported in 2003 that NALP3 gene expression is induced by TNF-α as well as ligands recognized by multiple TLRs in primary human monocytes. There is evidence suggesting that NALP3 is able to respond to a variety of signals: adenosine triphosphate (ATP), nigericin, maitotoxin, Staphylococcus aureus and Listeria monocytogenes[33], RNA [34] and uric acid crystals (monosodium urate and calcium pyrophosphate dehydrate) released from dying cells [35].
In 2004 the Tschopp group showed that MDP was an activator of the NALP3 inflammasome [28], which suggested a very interesting connection between NOD2 and NALP3; however, this observation has not been confirmed by others. Kanneganti et al. [34] were unable to generate any significant difference in IL-1β production between NALP3–/– and wild-type mice peritoneal macrophages stimulated with MDP and other lipid mediators [including lipopolysaccharide (LPS)], as well as when the macrophages were stimulated with bacterial ligands and then treated briefly with ATP. This group suggested that bacterial RNA and small anti-viral compounds are the specific ligands of NALP3 rather than MDP. Also, Mariathasan et al. [33] showed that MDP enhanced LPS-induced secretion of TNF-α and IL-12 p40 in both wild-type and NALP3–/– peritoneal mouse macrophages, but are unable to determine whether there is an enhancement of IL-1β secretion, as NALP3 is essential for this process; furthermore, stimulation of wild-type macrophages with MDP alone and pulsed with ATP did not generate detectable levels of IL-1β.
Cross-interactions
O'Connor et al. [32] characterized NALP3 as a dampening factor of NF-κB-dependent proinflammatory signals. In 2004, Dowds et al. [36] performed studies on THP-1 cells, where they found that three common NALP3 mutants (R260W, D303N and E637G) were constitutively active and were able to potently induce NF-κB activation and IL-1β secretion, contrary to the wild-type. On the other hand, Yu et al. [37] demonstrated that neither wild-type nor mutant NALP3 induced a significant activation of NF-κB, but induced ASC oligomerization and caspase-1 activation. Sutterwala et al. [38] did not observe consistent differences in the production of NF-κB-dependent cytokines in NALP3–/– macrophages, suggesting that NALP3 has no influence over NF-κB activity but it is, however, necessary for caspase-1 activation.
Lamfanki et al. [39] have reported that caspase-1 is able to activate NF-κB independently of its enzymatic activity. His group performed caspase-1 overexpression studies on 293T cells, and observed that when the cells were protected from caspase-1-induced apoptosis by CrmA (cytokine response modifier A), a significant activation of NF-κB occurred. None the less, they also observed that NF-κB activation and pro-IL-1β maturation might occur simultaneously.
However, not only caspases are able to exert an effect over NF-κB; cardinal, a component of the NALP3 inflammasome, potently suppresses NF-κB activation through its interaction with IKKγ[40–42]. This would act as a positive feedback loop, when cardinal is sequestered (assembled in the inflammasome), and unable to exert its negative regulatory effects on IKKγ. In this aspect, when the inflammasome is assembled and caspase-1 is actively processed, wild-type NOD2, cardinal and capase-1 [39] serve similar roles in NF-κB activation.
Interleukin-1β secretion
Little is known about IL-1β secretion, but evidence strongly supports that production and secretion are two independent processes. A current model, proposed by Andrei et al. [43], explains that caspase-1 activation and pro-IL-1β occurs inside the endosome, where because of ATP's co-stimulatory role during bacterial challenges there is a depletion of extracellular potassium and an increase in intracellular calcium, which triggers exocytosis after the activation of different phospholipases. This model suggests answers to the reasons why it is so difficult to demonstrate the presence of active caspase-1 in the cytosol of activated monocytes, as well as how the apoptotic effect of active caspase-1 is avoided.
Wewers et al. [44] demonstrated that endotoxin alone generated large intracellular stores of pro-IL-1β, but it was extremely inefficient at inducing release of the mature product from alveolar macrophages. Several other groups have determined that exogenous ATP induces rapid processing and massive release of the endotoxin-induced IL-1β[45–47], indicating that in this scenario an additional, separate stimulus is necessary to generate release. Li et al. [48] comment on their inability to induce secretion of IL-1β in purified monocytes from homozygous patients with the 3020insC NOD2 (see discussion below); although there was no IL-1β transcriptional response to MDP stimulation, there was a response to TNF-α stimulation; nevertheless, no protein could be detected in the media. Interestingly, Martinon et al. [28] also encountered similar problems stimulating release of IL-1β in THP-1 cells. If we consider these two incidents, it might be inferred that they support the theory proposing pro-IL-1β processing and subsequent secretion are two independent processes, but intriguingly, in the same paper by Martinon et al. [28], these problems were not encountered in purified human monocytes stimulated with MDP. Li's group [48], on the other hand, ascribe their problem to a defect in caspase-1 activation mediated by the 3020insC NOD2 variant. They argue that RIP2 (downstream of NOD2) is responsible for engaging pro-caspase-1 and activating its autocatalytic properties and because NOD2 is defective this process cannot take place. They fail to consider that caspase-1 is activated through several pathways and that in order to conclude this they would have to ascertain levels of pro-IL-1β/IL-1β in the cytoplasm. In any case, details are important; in Martinon's [28] study it is unsure what role is played by the conditions of the purified monocytes, as they were obtained from only a single patient who carried the R260W mutation, had been treated with anakinra (IL-1 receptor antagonist) and was asymptomatic at the time of the sample collection. One might also ponder on whether the lack of secretion in these two cases has something to do with lack of co-stimulation.
When things go wrong
NOD2 has been linked to Crohn's disease (CD) and Blau syndrome (BS). However, CD-associated polymorphisms occur in the LRR domain, whereas BS mutations occur in the central NATCH domain. In CD, NOD2 mutations act as a risk factor, being more common among CD patients than the background population; on the other hand, in BS NOD2 mutations are linked directly to this syndrome, as it is an autosomal-dominant disease. Even though inflammation is a common theme in both diseases, CD is associated with chronic granulomatous inflammation of various intestinal segments, often the distal part of ileum including extra-intestinal manifestations such as arthritis, uveitis and skin lesions (erythema nodosum and pyoderma gangraenosum), whereas BS is characterized by more diffuse chronic inflammation: arthralgia, uveitis and exanthema [49]. Other organs are seldom affected in BS [50].
Crohn's disease
The first susceptibility locus for CD involved NOD2 and was mapped to chromosome 16. In 2001 Hugot et al. [51] identified three independent variants related to CD: a frame shift (c3020insC) and two missense mutations (R702W, G908R) of NOD2. The frame shift mutation was also reported independently by Ogura et al. [52]; they presumed that this defect generated a truncated NOD2 protein. Subsequently, this NOD2 mutation (c3020insC) has been identified as the variant with the clearest association to CD and it fully abrogates NOD2-dependent detection of MDP [21,22].
The three CD-associated NOD2 variants, R702W, G908R and c3020insC, also named single nucleotide polymorphisms (SNP) SNP8, SNP12 and SNP13, are associated with an early age at onset (< 25 years of age), ileal affection and fibrostenosis. Even though CD is characterized by a strong induction of NF-κB [53–55] and high levels of its products NOD2's role(s) in relation to CD is still unclear, and a highly controversial area revolves around the mutant NOD2 protein's abilities to activate or inhibit NF-κB. In 2001, Ogura et al. [52], reported that NOD2 mutants seem to induce only a weak activation of NF-κB. Since then several authors have confirmed Ogura's findings [21,22,48,56,57]. The controversy gained momentum when in 2005 Maeda et al. [58] performed a study on mutant mice expressing the homologue of the c3020insC human mutation and determined that NF-κB activity and IL-1β processing were potentiated. Prompted by this controversy, Netea et al. [59] designed and performed studies in three variants of CD patients: heterozygous and homozygous for the c3020insC mutation, and wild-type allele carriers. Their results support the loss of function theory (confirming previous results from the same laboratory), as the peripheral blood mononuclear cells (PBMCs) of homozygous patients for the c3020insC NOD2 mutation showed a strongly decreased production of IL-1β upon stimulation with either MDP or peptidoglycan.
More recently, an increasingly complicated picture presents itself; Netea et al. [60] investigated the response of human peripheral blood mononuclear cells from CD patients to several muramyl peptides and determined that NOD2 c3020insC homozygous carriers were unresponsive to the muramyl peptide agonist of NOD1; suggesting some sort of interaction between the two pathways. They managed to establish that this effect was not due to a dominant-negative effect of the NOD2 variant, and after demonstrating that these patients express high levels of the peptidoglycan recognition protein S (which is able to interact with muramyl peptides) they suggested that this protein acts as a scavenger and is thus able to dampen NOD1-associated responses in these patients.
Moving a little further away from the NOD2/NF-κB controversy is the role of NOD2 in defensin expression. Although defensins are not the subject of this review, there have been several publications recently [61–63] suggesting their crucial role in CD, and more importantly establishing a link between NOD2 CD-associated variants and defensin expression. Worth mentioning is Voss et al.'s [64] study on human embryonic kidney 239 cells overexpressing NOD2. The group concluded that when these cells are stimulated with MDP, expression of human beta-defensin-2 is induced; and when the 3020insC variant is overexpressed, the production of this defensin is defective.
Blau syndrome
In 1996, Tromp et al. [65], identified the susceptibility locus of BS at 16p12-q21 by linkage analysis of a large pedigree. In 2001, Miceli-Richard et al. [66] identified three mutations: R334Q, R334W and L469F, which are located in the region encoding the central NACHT domain of NOD2. In 2005, van Duist et al. [67] identified a new mutation: E382K, which also affects the NACHT domain. All these mutations lie close to the ATP-binding site in the NACHT domain; it is thought that they might interfere directly with ATP hydrolysis or interfere indirectly with the conformational switch; van Duist et al. [67] suggest that this would allow excessive signalling due to a reduced disassembly of NOD2 signalling complexes, and result in constitutively increased NF-κB activation.
NALP-3 and rare autosomal dominant diseases
There are more than 20 NALP-3 mutations described in the literature, all missense mutations which result in an increased processing of pro-IL-1β and the subsequent release of the active cytokine [68]. Missense mutations of the NALP3 gene are associated with three different autosomal dominant diseases: Muckle–Wells syndrome (MWS) [30,69], characterized by rashes, fever and arthralgia; chronic infantile neurological cutaneous and articular (CINCA) syndrome [70], characterized by chronic meningitis, rashes, arthritis and fever; and familial cold autoinflammatory syndrome (FCAS) [69], with recurrent rashes, arthralgia and fever as main clinical symptoms. These disorders might be treated by anakinra, an IL-1 antagonist. A curious characteristic of NALP-3 mutations is that the same genetic defect might cause a wide variety of phenotypes [71], suggesting that additional factors are involved.
What do all these above-mentioned diseases have in common? The answer is: chronic inflammation (see Box 1 for brief facts about those disorders).
Box 1. Brief facts
Crohn's disease (CD)
CD is a chronic inflammatory disease of the bowel, characterized by periods of remission and relapse. Any part of the gastrointestinal tract can be affected, but most commonly, the terminal ileum and caecum are affected. It is characterized by the presence of segments of normal bowel between affected regions, known as ‘skip’ lesions. It presents with recurrent episodes of abdominal pain, diarrhoea, malnutrition and anaemia. In children it might present with growth failure. Narrowing the gut lumen due to oedema, leading to strictures and bowel obstruction, abcess formation and fistulization (especially in the perianal region) to skin and internal organs are some of the complications.
Blau syndrome (BS)
BS is a rare autosomal dominant disorder characterized by granulomatous polyarthritis, panuveitis, cranial neuropathies and exanthema.
Familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS) and chronic infantile neurological cutaneous and articular syndrome (CINCA)
Rare autosomal, dominant, autoinflammatory syndromes that usually develop during childhood.
FCAS and MWS are characterized by intermittent episodes of rash, arthralgia, fever, the frequent development of systemic AA amyloidosis. As determinants are sensitivity to cold in FCAS and sensorineural hearing loss in MWS.
CINCA is characterized by fever, chronic meningitis, uveitis, sensorineural hearing loss, urticarial skin rash and a characteristic deforming arthropathy.
Conclusions
Chronic inflammation culminates in devastating events that can lead ultimately to major organ dysfunction due to abnormalities in tissue architecture after regeneration and replacement by non-functional fibrous tissue. Once this stage is reached, little can be done. Some of the chronic diseases mentioned above present a fairly simple aetiology of Mendelian inheritance, while others present with a much more complex framework influenced by a number of known and unknown factors. Therapies developed against these illnesses are not foolproof, as when more than one factor comes into play the number of interactions also raises exponentially; we might well be standing before a family of very closely related diseases that differ only due to a very small change at some point along the inflammatory cascade.
Understanding the cell-signalling networks involved in chronic inflammation may, however, give rise to insight into new pharmacologically relevant targets to control troublesome diseases, where ‘old’ therapies such as glucocorticoid and immunosuppressants seem to have only a limited clinical efficacy.
Acknowledgments
This paper was supported by grants from the Augustinus Foundation, Aase and Ejnar Danielsen's Foundation and the Director Emil C. Hertz and spouse Inger Hertz Foundation.
References
- 1.Spencer WG, editor. Celsus. De medicina. London: Heinemann; 1935. and trans. [Google Scholar]
- 2.Fritz JH, Girardin SE. How Toll-like receptors and Nod-like receptors contribute to innate immunity in mammals. J Endotoxin Res. 2005;11:390–4. doi: 10.1179/096805105X76850. [DOI] [PubMed] [Google Scholar]
- 3.Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. doi: 10.1007/978-3-642-59430-4_5. [DOI] [PubMed] [Google Scholar]
- 4.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–54. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–32. doi: 10.1016/s0959-440x(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 6.Koonin EV, Aravind L. The NACHT family − a new group of predicted NTPases implicated in apoptosis and MHC transcription activation. Trends Biochem Sci. 2000;25:223–4. doi: 10.1016/s0968-0004(00)01577-2. [DOI] [PubMed] [Google Scholar]
- 7.Damiano JS, Oliveira V, Welsh K, Reed JC. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem J. 2004;381:213–9. doi: 10.1042/BJ20031506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohl A, Grutter MG. Fire and death: the pyrin domain joins the death-domain superfamily. C R Biol. 2004;327:1077–86. doi: 10.1016/j.crvi.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Lamkanfi M, Declercq W, Vanden BT, Vandenabeele P. Caspases leave the beaten track: caspase-mediated activation of NF-kappaB. J Cell Biol. 2006;173:165–71. doi: 10.1083/jcb.200509092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stehlik C, Reed JC. The PYRIN connection: novel players in innate immunity and inflammation. J Exp Med. 2004;200:551–8. doi: 10.1084/jem.20032234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchier-Hayes L, Martin SJ. CARDINAL roles in apoptosis and NFkappaB activation. Vitam Horm. 2004;67:133–47. doi: 10.1016/S0083-6729(04)67008-7. [DOI] [PubMed] [Google Scholar]
- 12.Masumoto J, Taniguchi S, Sagara J. Pyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASC. Biochem Biophys Res Commun. 2001;280:652–5. doi: 10.1006/bbrc.2000.4190. [DOI] [PubMed] [Google Scholar]
- 13.Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol. 2001;2:3009. doi: 10.1186/gb-2001-2-7-reviews3009. [Reviews] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lala S, Ogura Y, Osborne C, et al. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 15.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–18. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 16.Ogura Y, Lala S, Xin W, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut. 2003;52:1591–7. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrebi D, Maudinas R, Hugot JP, et al. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn's disease colon. Gut. 2003;52:840–6. doi: 10.1136/gut.52.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 19.Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT. Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc Res. 2006;71:103–7. doi: 10.1016/j.mvr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstiel P, Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–9. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 21.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 22.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 23.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–27. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 27.Mariathasan S, Newton K, Monack DM, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–8. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 28.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–34. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 29.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–74. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity. 2004;20:319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 31.Feldmann J, Prieur AM, Quartier P, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol. 2003;171:6329–33. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 33.Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 34.Kanneganti TD, Ozoren N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–6. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 35.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 36.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem. 2004;279:21924–8. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- 37.Yu JW, Wu J, Zhang Z, et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Diff. 13:236–49. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 38.Sutterwala FS, Ogura Y, Szczepanik M, et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–27. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Lamkanfi M, Kalai M, Saelens X, Declercq W, Vandenabeele P. Caspase-1 activates nuclear factor of the kappa-enhancer in B cells independently of its enzymatic activity. J Biol Chem. 2004;279:24785–93. doi: 10.1074/jbc.M400985200. [DOI] [PubMed] [Google Scholar]
- 40.Bouchier-Hayes L, Conroy H, Egan H, et al. CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-kappa B activation pathways. J Biol Chem. 2001;276:44069–77. doi: 10.1074/jbc.M107373200. [DOI] [PubMed] [Google Scholar]
- 41.Pathan N, Marusawa H, Krajewska M, et al. TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J Biol Chem. 2001;276:32220–9. doi: 10.1074/jbc.M100433200. [DOI] [PubMed] [Google Scholar]
- 42.Razmara M, Srinivasula SM, Wang L, et al. CARD-8 protein, a new CARD family member that regulates caspase-1 activation and apoptosis. J Biol Chem. 2002;277:13952–8. doi: 10.1074/jbc.M107811200. [DOI] [PubMed] [Google Scholar]
- 43.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–50. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wewers MD, Rennard SI, Hance AJ, Bitterman PB, Crystal RG. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest. 1984;74:2208–18. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–9. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–203. [PubMed] [Google Scholar]
- 47.Griffiths RJ, Stam EJ, Downs JT, Otterness IG. ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol. 1995;154:2821–8. [PubMed] [Google Scholar]
- 48.Li J, Moran T, Swanson E, et al. Regulation of IL-8 and IL-1beta expression in Crohn's disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–25. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 49.Blau EB. Familial granulomatous arthritis, iritis, and rash. J Pediatr. 1985;107:689–93. doi: 10.1016/s0022-3476(85)80394-2. [DOI] [PubMed] [Google Scholar]
- 50.Pastores GM, Michels VV, Stickler GB, Su WP, Nelson AM, Bovenmyer DA. Autosomal dominant granulomatous arthritis, uveitis, skin rash, and synovial cysts. J Pediatr. 1990;117:403–8. doi: 10.1016/s0022-3476(05)81080-7. [DOI] [PubMed] [Google Scholar]
- 51.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 52.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 53.Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981–8. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- 54.Neurath MF, Fuss I, Schurmann G, et al. Cytokine gene transcription by NF-kappa B family members in patients with inflammatory bowel disease. Ann NY Acad Sci. 1998;859:149–59. doi: 10.1111/j.1749-6632.1998.tb11119.x. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–84. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamaillard M, Girardin SE, Viala J, Philpott DJ. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–92. doi: 10.1046/j.1462-5822.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 57.Bonen DK, Ogura Y, Nicolae DL, et al. Crohn's disease-associated NOD2 variants share a signaling defect in response to lipopolysaccharide and peptidoglycan. Gastroenterology. 2003;124:140–6. doi: 10.1053/gast.2003.50019. [DOI] [PubMed] [Google Scholar]
- 58.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 59.Netea MG, Ferwerda G, de Jong DJ, Girardin SE, Kullberg BJ, van der Meer JW. NOD2 3020insC mutation and the pathogenesis of Crohn's disease: impaired IL-1 and beta; production points to a loss-of-function phenotype. Neth J Med. 2005;63:305–8. [PubMed] [Google Scholar]
- 60.Netea MG, Ferwerda G, de Jong DJ, et al. The frameshift mutation in Nod2 results in unresponsiveness not only to Nod2- but also Nod1-activating peptidoglycan agonists. J Biol Chem. 280:35859–67. doi: 10.1074/jbc.M504924200. [DOI] [PubMed] [Google Scholar]
- 61.Wehkamp J, Harder J, Weichenthal M, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–64. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schauber J, Rieger D, Weiler F, et al. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18:615–21. doi: 10.1097/00042737-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–11. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 65.Tromp G, Kuivaniemi H, Raphael S, et al. Genetic linkage of familial granulomatous inflammatory arthritis, skin rash, and uveitis to chromosome 16. Am J Hum Genet. 1996;59:1097–107. [PMC free article] [PubMed] [Google Scholar]
- 66.Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 67.van Duist MM, Albrecht M, Podswiadek M, et al. A new CARD15 mutation in Blau syndrome. Eur J Hum Genet. 2005;13:742–7. doi: 10.1038/sj.ejhg.5201404. [DOI] [PubMed] [Google Scholar]
- 68.Dinarello CA. Unraveling the NALP-3/IL-1beta inflammasome: a big lesson from a small mutation. Immunity. 2004;20:243–4. doi: 10.1016/s1074-7613(04)00055-x. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nat Genet. 2001;29:301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bihl T, Vassina E, Boettger MK, et al. The T348M mutated form of cryopyrin is associated with defective lipopolysaccharide-induced interleukin 10 production in CINCA syndrome. Ann Rheum Dis. 2005;64:1380–1. doi: 10.1136/ard.2004.031179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aganna E, Martinon F, Hawkins PN, et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 2002;46:2445–52. doi: 10.1002/art.10509. [DOI] [PubMed] [Google Scholar]