Abstract
The airway epithelium plays an active role in acute lung inflammation by producing chemotactic factors and by expressing cell adhesion molecules involved in the migration of leucocytes to extravascular spaces. We have reported previously that neutrophil migration to airways can be down-modulated by exogenously administered vitamin E (α-tocopherol). The mechanism for this effect is not well understood, however. The action of α-tocopherol was investigated in human alveolar type II and bronchial epithelial cells stimulated with tumour necrosis factor-α. Treatment of alveolar epithelial cells with α-tocopherol resulted in down-regulated cell surface expression of intercellular adhesion molecule-1 (ICAM-1). On bronchial epithelial cells, both ICAM-1 and vascular adhesion molecule-1 were decreased, leading to diminished adherence of leucocytes to the cells. The production of the neutrophil chemoattractant interleukin-8 was attenuated in both alveolar and bronchial cells. These effects were preceded by reduced activation of the mitogen-activated protein kinases (MAPK), extracellular signal-regulated kinase (ERK1/2) and p38, as well as down-regulation of nuclear factor-κB. Comparing the effects of α-tocopherol with that of specific inhibitors of MAPK and protein kinase C (PKC) revealed that effects appear to be partly independent of PKC inhibition. These results implicate the anti-inflammatory action of α-tocopherol in addition to its anti-oxidant properties.
Keywords: ARDS, asthma intervention, lung epithelial cells, MAPkinase, NF-κB, vitamin E
Introduction
The lung epithelium presents the first physical barrier to inhaled oxidants. In addition to direct oxidant–epithelial interactions, proinflammatory mediators secreted from other pulmonary cells in response to the oxidant challenge can also act upon epithelial cells indirectly. One such mediator is tumour necrosis factor (TNF)-α, which activates intracellular signal transduction pathways in bronchial epithelial cells resulting in increased cell surface expression of adhesion molecules implicated in the recruitment of leucocytes to inflammatory loci [1]. In addition, TNF-α can induce epithelial cells to secrete other cytokines such as interleukin (IL)-8, an important neutrophil chemoattractant [2].
The lung injury caused by oxidative stress and excessive inflammatory responses is counteracted by naturally occurring anti-oxidants such as vitamin E (tocopherol). Plasma levels of α-tocopherol, a member of the vitamin E family, are decreased after infections, trauma, burns and inflammatory injuries, indicating that this anti-oxidant is depleted during acute tissue injury [3]. Kelly et al. have demonstrated that lung lining fluid α-tocopherol concentrations are low in patients with asthma due to ongoing airway inflammation, and are thus potentially more sensitive to additional oxidative insults [4]. Consistent with this, dietary supplementation of asthmatics with vitamin E has been shown to protect against the effects of oxidant air pollutants such as ozone [5,6]. Further, higher vitamin E intake is associated with lower serum IgE and a decreased frequency of allergen sensitization [7]. Administration of exogenous α-tocopherol incorporated in liposomes protects against lung oedema in animal models of acute respiratory distress syndrome (ARDS) provoked by systemic inflammation [8–10] and chemical-induced lung injury caused by inhaled bleomycin [11]. We have demonstrated that systemically administered α-tocopherol reduces transendothelial migration of neutrophils and prevents lung injury in a mouse model of lipopolysaccharide (LPS)-induced airway inflammation [12], and improves respiratory function in a mouse model of septic shock induced by two consecutive intraperitoneal injections of LPS (Shwartzman reaction) [13]. These results are consistent with studies in vitro showing that α-tocopherol inhibits polymorphonuclear (PMN) leucocyte-dependent adhesion to the endothelium by suppressing the expression of cell adhesion molecules such as CD11b/CD18 on neutrophils, and/or intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells [14,15].
The pulmonary uptake of α-tocopherol is augmented in acute lung injury [16], suggesting that systemic delivery of this anti-oxidant may target the lung. Kolleck and colleagues have suggested that lipoprotein bound to α-tocopherol was delivered to alveolar type II cells with uptake mediated by lipoprotein-specific receptors [17,18]. Lipid soluble vitamin E acts in cell membranes, where it prevents the propagation of lipid peroxidation reactions [19,20].
However, it is recognized that vitamin E, particularly α-tocopherol, exerts a number of non-anti-oxidant functions, some associated with inhibition of protein kinase C (PKC) [21] such as inhibition of platelet aggregation [22], and others independent of PKC such as the expression of ICAM-1 [23], integrins [24] and CD36 [25]. These later observations may be tracked back, at least in part, to the reported inhibition of the transcription factor nuclear factor (NF)-κB [26]. Most previous studies of α-tocopherol actions have been performed on cells derived from circulation, while the intracellular effects of this drug in lung epithelial cells have been poorly investigated.
The aim of the present study was to investigate the effects of α-tocopherol on bronchial and alveolar epithelial cells stimulated with TNF-α. Both transformed cell lines and primary bronchial epithelial cells were used. We demonstrated that α-tocopherol down-regulates cell surface expression of ICAM-1 and secretion of interkeukin (IL)-8, both in bronchial cells and cells of alveolar type II origin. Because the transcription factors NF-κB and activator protein-1 (AP-1) are key regulatory molecules involved in such a response, it was decided to investigate if α-tocopherol affects the activation of these factors. The mechanisms for the inhibition were investigated further by analysis of upstream signal transduction pathways induced by TNF-α, i.e. phosphorylation of mitogen-activated protein kinases (MAPK). The inhibitory effects of α-tocopherol were also compared with those of MAPK, PKC and IκB inhibitors. Our results indicate that α-tocopherol inhibits epithelial cell function by down-regulating activation of extracellular signal-regulated kinase (ERK), p38 and NF-κB, effects that appear to be partly independent of PKC inhibition.
Materials and methods
Cell culture and treatments
A549 cells (ATCC CCL-185; American Type Culture Collection), a human type II alveolar epithelial cell line, were cultured in RPMI-1640 (Gibco brl, Paisley, UK) supplemented with 10% fetal calf serum (FCS; HyClone, Perbio Science, Aalst, Belgium) and 50 µg/ml gentamicin. Bronchial epithelial cell line (BEAS)-2B, human bronchial epithelial cells transformed by an adenovirus 12 SV40 hybrid (ATCC CRL-9609) and normal human bronchial epithelial cells (NHBE) (Clonetics, San Diego, CA, USA) were grown in serum-free bronchial epithelial cell basal medium (BEBM) with supplements (complete medium, BEGM) (Cambrex, Verviers, Belgium). BEAS-2B and NHBE were cultured on tissue culture flasks or plates precoated with fibronectin, vitrogen and bovine serum albumin. U937, a human monocytic leukaemia cell line (ATCC CRL 1593), was cultured in RPMI-1640 medium supplemented with 10% FCS and 50 µg/ml gentamicin. All cell lines were incubated at 37°C in a humidified atmosphere with 5% CO2. Vitamin E (α-tocopherol: Sigma, St Louis, MO, USA), was dissolved in dimethyl sulphoxide (DMSO) with a final concentration of solvent in the culture medium not exceeding 0·1%. Cells were exposed to various concentrations of α-tocopherol for 45 min in a concentration range of 25–100 µM prior to stimulation with 10 ng/ml TNF-α (Sigma) for time-periods indicated in the figure legends. Cells exposed only for solvent served as controls. Treatment of cells with α-tocopherol alone had no effect on any of the parameters tested. In all experiments cell cultures with 80% confluence were used.
Analysis of IL-8 in cell supernatant by enzyme-linked immunosorbent assay (ELISA)
Lung epithelial cells were seeded in 24-well plates in complete growth medium. After 48 h, cells were stimulated with 10 ng/ml TNF-α and incubated for another 24 h. Some wells were preincubated with α-tocopherol 45 min before addition of TNF-α. In a set of experiments the MAP kinase (MEK) inhibitor PD98059 (Calbiochem, San Diego, CA, USA), the p38 inhibitor SB203580 (Calbiochem) or the PKC inhibitor Calphostin C (Sigma) were added 45 min before TNF-α stimulation. To study the effects of NF-κB inhibition, the inhibitor of kappa B (IκB) BAY 11–7082 (Calbiochem) was added 60 min before TNF-α activation. The optimal concentration of each inhibitor was determined in pilot experiments. PD98059 was used at 40 µM, SB203580 at 1 µM, Calphostin C at 0·5 µM and BAY 11–7082 at 8 µM in A549 cell cultures and at 1 µM in BEAS-2B cell cultures. The supernatants were separated by centrifugation, and the concentration of IL-8 was measured using the DuoSet ELISA Development kit (R&D Systems, Abingdon, UK), according to the manufacturer's protocol.
Flow cytometry analysis
Cells were grown in 25 cm2 tissue culture flasks and stimulated with 10 ng/ml of TNF-α (Sigma) for 20 h, or pretreated for 45 min with α-tocopherol in a concentration range of 25–100 µM prior to addition of TNF-α. In some experiments the inhibitors PD98059, SB203580 or Calphostin C were added 45 min before TNF-α stimulation. In another set of experiments the IκB inhibitor BAY 11–7082 was added 60 min before TNF-α activation. Optimal concentration of each inhibitor was determined in pilot experiments. The treated cells were rinsed with phosphate-buffered saline (PBS) and detached from tissue culture flasks using Versene or Accutase. The cells were washed by centrifugation and resuspended in 3% FCS–PBS followed by distribution of 1 × 106 cells into wells in a round-bottomed microtitre plate. Monoclonal phycoerythrin (PE)-conjugated anti-ICAM-1 (CD54; Pharmingen, San Diego, CA, USA), VCAM-1 (CD106; Pharmingen) or isotype-matched (IgG1κ; Pharmingen) antibody was then added to the wells. After incubation for 45 min in the dark (4°C), the cells were washed twice with 3% FCS–PBS, fixed with Cellfix (Becton Dickinson, San Jose, CA, USA) and transferred to fluorescence activated cell sorter (FACS) tubes (Becton Dickinson). The level of surface expression of ICAM-1 and VCAM-1 on the lung epithelial cells was determined by analysing specific median fluorescence intensity in FL1 of 10 000 cells using a FACSort flow cytometer (Becton Dickinson).
Cell adhesion assay
U937 cells (2 × 106) were labelled with 50 µl 1 mCi/ml Cr51 (Amersham Biosciences, Uppsala, Sweden) for 1 h at 37°C and washed three times with cold RPMI-1640. Thereafter, 0·5 × 105 of the labelled cells were added to the treated lung epithelial cells in a final volume of 500 µl and incubated in 37°C (5% CO2) for 45 min. Non-adherent cells were removed by gentle washing with RPMI-1640 four times. Thereafter, 300 µl 5% Triton was added to lyse the cells. After 20 min incubation at room temperature the lysates were harvested and the radioactivity in the wells was estimated in a gamma-counter. As positive controls for the inhibition of cell adhesion, saturating amounts of anti-ICAM-1 and anti-VCAM-1 monoclonal antibody (MoAb) (Genzyme Corporation, Cambridge, MA, USA) were added and incubated for 30 min at 37°C prior to addition of U937.
Preparation of nuclear extracts and electrophoretic mobility shift assay
Nuclear extract were prepared from 2 × 107 cells. Treated cells were washed with ice-cold PBS and harvested using a cell scrape. Thereafter, cells were suspended in 5 ml ice-cold PBS and centrifuged at 250 g for 10 min at 4°C. The pellet was dissolved in 100 µl hypotonic buffer [10 mM HEPES, pH 7·3, 10 mM KCl, 1·5 mM MgCl2, 2 mM Pefablock SC; Roche, Basel, Switzerland, 1 mM dithiothreitol (DTT), 0·2 mM ethylenediamine tetraacetic acid (EDTA) and complete protease inhibitors; Roche] and centrifuged at 5000 g for 1 min at 4°C. The cells were then resuspended in lysis buffer (hypotonic buffer containing 0·4% NP-40) and incubated for 10 min on ice followed by centrifugation at 8000 g for 1 min at 4°C. The nuclei pellets were mixed rapidly in 0·3 M KCl buffer (20 mM HEPES pH 7·3, 22% glycerol, 1·5 mM MgCl2, 0·2 mM EDTA, 0·3 M KCl, 1 mM DTT and complete protease inhibitors) and left on ice for 30 min. After centrifugation at 8000 g for 15 min at 4°C, aliquots of supernatant containing the nuclear proteins were collected and stored at −80°C. The nuclear protein content of the supernatant was determined by Coomassie blue reaction using a Bio-Rad kit (Richmond, CA, USA). For detection of NF-κB and AP-1 DNA binding activities in nuclear extracts, electrophoretic mobility shift assays (EMSAs) were conducted as described previously [12]. For specificity analysis, a molar excess of unlabelled probe was added to the binding reaction. The sequences of oligonucleotides used to detect the DNA-binding activity were as follows; NF-κB: 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and AP-1: 5′-CGCTTGATGAGTCAGCCGGAA-3′ (only the upper strands are indicated). The oligonucleotides were end-labelled using γ-[32P] ATP (3000 Ci/mmol; NENLife Science Products, Boston, MA, USA) and T4 polynucleotide kinase (Promega, Madison, WI, USA). The labelled probes were purified from free nucleotides on a microspin G-50 M column (Amersham Biosciences).
Western blot analysis
A549 cultures were incubated in serum-free medium and bronchial epithelial cell line (BEAS)-2B cultures in basal BEBM medium overnight before treatment with or without α-tocopherol and exposure of TNF-α for 15 min. Cells were harvested in ice-cold PBS and centrifuged at 250 g at 4°C for 10 min.
Lysates were prepared by adding 200 µl of 1× sodium dodecyl sulphate (SDS) sample buffer (62·5 mM Tris-HCl pH 6·8, 2% w/v SDS, 10% glycerol, 50 mM DTT, 0·01% w/v bromophenol blue) to the cell pellets, and the samples were then sonicated for 15 s to shear DNA. Twenty-five µl of cell lysates were loaded onto 12% precast SDS–polyacrylamide gel electrophoresis (PAGE) gels (Cambrex Bio Science, Rockland, ME, USA) and electrophoresed at 100 V for 45 min. The proteins were then electrotransferred to Immobilon-P Transfer membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% w/v non-fat dry milk in Tris-buffered saline containing 0·1% Tween-20 (TTBS), immunoblotted with rabbit anti-phospho-ERK1/2 or anti-phospho-p38 antibodies (Cell Signalling Technology, Beverly, MA, USA) overnight at 4°C, followed by incubation with a secondary horseradish peroxidase-conjugated anti-rabbit antibody (Cell Signalling Technology) for 1 h. Membranes were washed extensively with TTBS after each incubation. Detection was performed using enhanced chemiluminescence (ECL; Amersham Biosciences) followed by autoradiography. To determine equal loading of cell lysate proteins, the membranes were stripped with a buffer containing 62·5 mM pH 6·8 Tris-HCl, 2% SDS and 100 mM β-mercaptoethanol for 30 min at 52°C and reprobed with anti-total ERK1/2 or anti-total p38 antibodies (Cell Signalling Technology).
Statistical analyses
Mean values and standard deviations were calculated and shown in the figures. The tests used for analyses of statistical significance are indicated in figure legends. P < 0·05 (two-tailed) was regarded as significant (*P < 0·05, **P < 0·01 and ***P < 0·001). Calculations were performed using the computer programme SPSS (SPSS Inc., Chicago, IL, USA).
Results
α-Tocopherol down-modulates TNF-α-induced production of IL-8
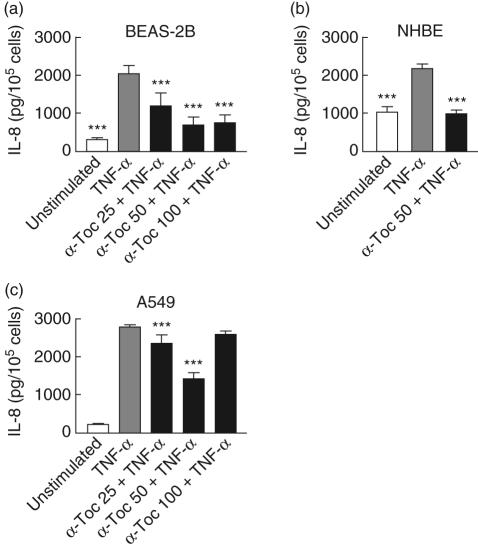
The ability of α-tocopherol to inhibit the recruitment of neutrophils by down-modulating chemotactic activity was studied in vitro. As IL-8 is considered to be an important chemotactic factor for neutrophils we investigated if α-tocopherol affects the production of this cytokine in lung epithelial cells. In both bronchial (BEAS-2B and NHBE) and alveolar (A549) cells, the secretion of IL-8 was increased after 24 h incubation with TNF-α (Fig. 1). The highest concentration of IL-8 was achieved in supernatants from A549 cell cultures. In A549 and BEAS-2B cells the most efficient inhibition of IL-8 was observed with 50 µM α-tocopherol. At this concentration, the production was reduced to 46%, in A549 cells to 45% in NHBE cells and to 33% in BEAS-2B cells of TNF-α-induced production. α-tocopherol completely attenuated the effect of TNF-α in NHBE cells and nearly reduced the IL-8 secretion to baseline level in BEAS-2B cells.
Fig. 1.
Effect of α-tocopherol on interleukin (IL)-8 secretion from lung epithelial bronchial epithelial cell line (BEAS)-2B (a), normal human bronchial epithelial cells (NHBE) (b) and A459 (c) cells. Cells were treated for 45 min with α-tocopherol in a concentration range of 25–100 µM and stimulated thereafter with 10 ng/ml tumour necrosis factor (TNF)-α. After 24-h incubation, supernatants were collected and analysed for IL-8 concentration by enzyme-linked immunosorbent assay (ELISA). Data are expressed as pg/105 cells. Statistical significance versus cells stimulated with TNF-α alone is indicated (analysis of variance and Dunnet's multiple comparison test, n = 8–12).
α-Tocopherol reduces TNF-α-induced cell surface expression of ICAM-1 and VCAM-1
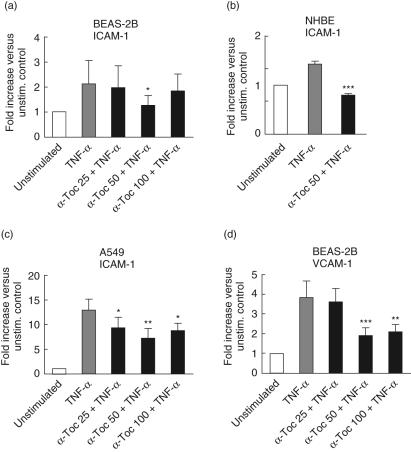
The effect of α-tocopherol on TNF-α-induced expression of adhesion molecules ICAM-1 and VCAM-1 on lung epithelial cells was analysed by flow cytometry. The median fluorescence intensity of the anti-ICAM-1 antibody on unstimulated BEAS-2B and NHBE cells was approximately three times above the fluorescence intensity of the isotype-matched IgG control antibody, indicating a significant constitutive expression of ICAM-1 on the bronchial cells. On the other hand, baseline expression of ICAM-1 on the alveolar A549 cell line was undetectable as the median fluorescence intensity of the anti-ICAM-1 antibody was similar to that of the control antibody. After 20 h incubation with TNF-α, ICAM-1 expression was up-regulated on both bronchial (BEAS-2B and NHBE) and alveolar (A549) cells (Fig. 2a–c). Expression of VCAM-1 was markedly increased on BEAS-2B cells (Fig. 2d), while this cell adhesion receptor was not induced on A549 cells and only to a minor degree on NHBE cells (data not shown). Treatment with α-tocopherol 45 min before the stimulation with TNF-α resulted in concentration-dependent inhibition of ICAM-1 expression on both A549 and BEAS-2B cells as well as VCAM-1 on BEAS-2B cells. The most pronounced inhibition of ICAM-1 was observed at 50 µM. In BEAS-2B cells the down-modulation of ICAM-1 was partially reversed at 100 µM. Treatment with 50 µM α-tocopherol resulted in decreased cell surface density of ICAM-1 to 59% on A549 cells, to 60% on BEAS-2B cells and to 59% on NHBE cells of cells activated with TNF-α alone. α-Tocopherol completely diminished the effect of TNF-α on NHBE cells and almost reduced the ICAM-1 expression to baseline level in BEAS-2B cells.
Fig. 2.
Effect of α-tocopherol on cell surface expression of adhesion molecules intercellular adhesion molecule-1 (ICAM-1) on bronchial epithelial cell line (BEAS)-2B (a), normal human bronchial epithelial cells (NHBE) (b), A549 (c) and vascular cell adhesion molecule (VCAM)-1 on BEAS-2B (d) cells. Cells were treated for 45 min with α-tocopherol in a concentration range of 25–100 µM and thereafter stimulated for 20 h with 10 ng/ml tumour necrosis factor (TNF)-α. The surface expression of ICAM-1 and VCAM-1 was measured by flow cytometry. Data are presented as fold increase of median fluorescence of ICAM-1 or VCAM-1 expression versus unstimulated cells. Statistical significance versus cells stimulated with TNF-α alone is indicated (analysis of variance and Dunnet's multiple comparison test). n = 4 except for cells stimulated with TNF-α alone and α-tocopherol 50 in (a) and (c) (n = 8).
α-Tocopherol diminishes the adhesion of monocytes to lung epithelial cells
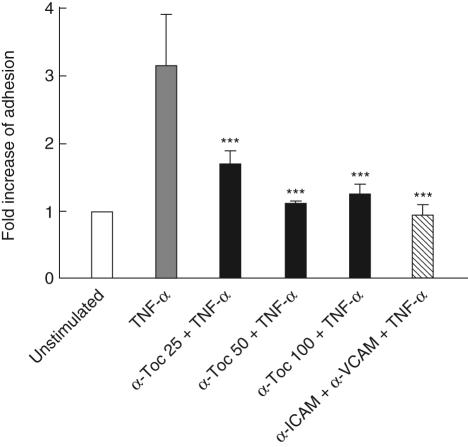
To determine the biological role of the down-modulation of cell adhesion molecules, we performed a cell adhesion assay using a Cr51 labelled monocyte cell line U937 and a monolayer of TNF-α-induced BEAS-2B cells. Stimulation with TNF-α 20 h prior to the assay clearly enhanced adhesion of the monocytes to the bronchial epithelial cells (Fig. 3). Incubation with α-tocopherol for 45 min before TNF-α stimulation reduced the adhesion of U937, especially at the 50 µM concentration. Similar results were obtained with A549 cells (data not shown). Pretreatment of BEAS-2B cells with anti-ICAM-1 and anti-VCAM-1 antibodies completely abolished the TNF-α-induced adhesion of U937 cells, confirming that the cell adherence in our in vitro system is dependent on these surface receptors.
Fig. 3.
Effect of α-tocopherol on monocyte adherence to tumour necrosis factor (TNF)-α-activated bronchial epithelial cell line (BEAS)-2B cells. 51Cr-labelled U937 cells were added to BEAS-2B monolayer treated with α-tocopherol in a concentration range of 25–100 µM followed by stimulation with 10 ng/ml TNF-α for 20 h. After 45 min non-adherent cells were removed by washing and remaining cells were lysed with 5% Triton. The lysates were assayed for 51Cr activity. Data are presented as fold increase of adhesion versus unstimulated cells. For comparison, the effect of pretreatment with antibodies against intercellular adhesion molecule-1 (α-ICAM) and vascular cell adhesion molecule-1 (α-VCAM) is also shown. Statistical significance versus cells stimulated with TNF-α alone is indicated (analysis of variance and Dunnet's multiple comparison test, n = 6).
α-Tocopherol inhibits TNF-α-induced NF-κB but not AP-1 activation
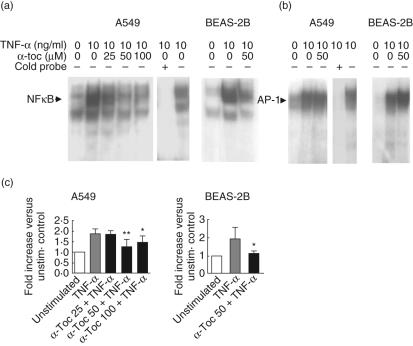
Because α-tocopherol down-regulated both cell surface expression of adhesion molecules and IL-8 production in lung epithelial cells, we investigated whether these effects could be explained by inhibition of key inflammatory transcription factors. TNF-α induced strong activation of NF-κB and AP-1 in both A549 and BEAS-2B cells 30 min after stimulation (Fig. 4). Treatment with α-tocopherol reduced the DNA binding of nuclear NF-κB within the nucleus in A549 cells, with the most pronounced effect observed with 50 µM (Fig. 4a). At this concentration, a similar effect was seen in BEAS-2B cells. Both 50 µM and 100 µM α-tocopherol significantly reduced DNA binding of NF-κB in A549 cells. The 50 µM concentration of α-tocopherol also reduced NF-κB binding in BEAS-2B cells (Fig. 4c). The down-modulation of nuclear NF-κB was followed by decreased levels of IL-8 and ICAM-1 mRNA 1·5 h later, supporting that the observed suppression of the transcription factor resulted in reduced gene transcription, although altered mRNA levels may also be reflected by changes in mRNA stability (data not shown). In contrast, 50 µM α-tocopherol had no effect on AP-1 binding activity in either A549 or BEAS-2B cells compared with cells stimulated with only TNF-α (Fig. 4b).
Fig. 4.
Effect of α-tocopherol on nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1) activity in A549 and bronchial epithelial cell line (BEAS)-2B cells. Cells were treated for 45 min with or without α-tocopherol and stimulated thereafter for 30 min with 10 ng/ml tumour necrosis factor (TNF)-α. Nuclear extracts were analysed using electrophoretic mobility shift assay (EMSA). Five µg nuclear extract/lane was loaded. Representative autoradiograms of NF-κB (a) and AP-1 (b) activity are shown. The results from unstimulated cells are shown in the left lane of each gel. Control experiment using excess of unlabelled NF-κB or AP-1 verifying that the binding of [32P]-labelled oligonucleotides was specific are shown to the right in EMSA analysis of A549 cells. Intensity of bands, corresponding to NF-κB, was quantified using an image analysis system (c). Data are expressed as fold increase in intensity versus unstimulated cells. Statistical significance versus cells stimulated with TNF-α alone is indicated (analysis of variance and Dunnet's multiple comparison test n = 5).
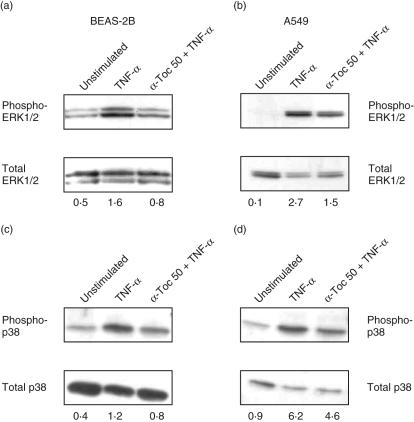
α-Tocopherol inhibits TNF-α-induced ERK1/2 and p38 activation
To investigate the effect of α-tocopherol on MAPK signalling pathways activated by TNF-α, cell lysates were analysed by immunoblot analysis using antibodies recognizing the phosphorylated active state of ERK1/2 and p38. The phosphorylated form of these MAPK were expressed only slightly in resting A549 and BEAS-2B cells, while stimulation with TNF-α for 15 min induced a clearly detectable increase of both phosphorylated-ERK1/2 and phospho-p38 in both cell lines (Fig. 5). Treatment with α-tocopherol reduced the phosphorylation of ERK1/2 55% in A549 and to 50% in BEAS-2B cells of cells treated with TNF-α alone. Similarly, the phosphorylation of p38 was reduced in A549 by 25% and by 33% in BEAS-2B cells. α-Tocopherol almost completely attenuated the activation of ERK1/2 and p38 in BEAS-2B cells.
Fig. 5.
Effect of α-tocopherol on tumour necrosis factor (TNF)-α-induced extracellular signal-regulated kinase (ERK)1/2 (a and b) and p38 (c and d) activation in bronchial epithelial cell line (BEAS)-2B (a and c), A549 cells (b and d). Cells were treated for 45 min with 50 µM α-tocopherol or solvent before stimulation with 10 ng/ml TNF-α for 15 min. Cells were lysed and 25 µl of cell lysates were loaded onto 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by electrotransfer to Immobilon-P membranes. Immunoblotting was performed with anti-phospho-specific mitogen-activated protein kinases (MAPK) antibody or an antibody recognizing total MAPK, followed by incubation with horseradish peroxidase-conjugated secondary antibody. The bands were visualized with the enhanced chemiluminescence (ECL) Western blot detection system. The double bands in (a) and (b) indicate the ERK1 (p42) and ERK2 (p44) isoforms, respectively. Numbers at the bottom of the lanes are densitometry ratio of phosphorylated MAPK versus total MAPK corrected for variations in protein loading. The blots are representative of three independent experiments.
Effects of Iκb inhibitor on TNF-α-induced IL-8 and ICAM-1 expression
We used the specific IκB inhibitor BAY 11–7082 to investigate if secretion of IL-8 and expression of ICAM-1 was dependent on the NF-κB activation pathway in lung epithelial cells. Addition of this inhibitor diminishes the activation of NF-κB by preventing phosphorylation of the inhibitory protein IκBα bound to NF-κB. Cell cultures of A549 cells and BEAS-2B were incubated with BAY 11–7082 (8 µM and 1 µM, respectively) 60 min before exposed to 10 ng/ml TNF-α for 20–24 h. The production of IL-8 was measured in cell supernatants by ELISA and the cell surface expression of adhesion molecules were analysed by flow cytometry. The Iκb inhibitor almost completely inhibited TNF-α-induced secretion of IL-8 and expression of ICAM-1 in both cell lines, indicating that the induction of these inflammatory mediators is dependent on the NF-κB activation pathway (Table 1).
Table 1.
Effect the of the inhibitor of kappa B (IκB) (BAY 11–7082) on tumour necrosis factor (TNF)-α- induced interleukin (IL)-8 secretion and intercellular adhesion molecule-1 (ICAM-1) expression.
| Cells | Treatment | IL-8 Fold increase versus unstimulated control | ICAM-1 Fold increase versus unstimulated control |
|---|---|---|---|
| A549 | Unstimulated | 1·0 | 1·0 |
| TNF-α | 4·6 ± 0·4 | 5·5 ± 0·1 | |
| TNF-α + BAY 11–7082 | 1·1 ± 0·1*** | 1·4 ± 0·1*** | |
| BEAS-2B | Unstimulated | 1·0 | 1·0 |
| TNF-α | 35·1 ± 3·5 | 3·2 ± 0·1 | |
| TNF-α + BAY 11–7082 | 6·0 ± 0·9*** | 1·3 ± 0·1*** |
P < 0·001 (P-values are indicated when the IκB inhibitor BAY 11–7082 + TNF-α treatment was significantly different from treatment with TNF-α alone). Mean values ± s.d. are indicated, n = 4. BEAS: bronchial epithelial cell line.
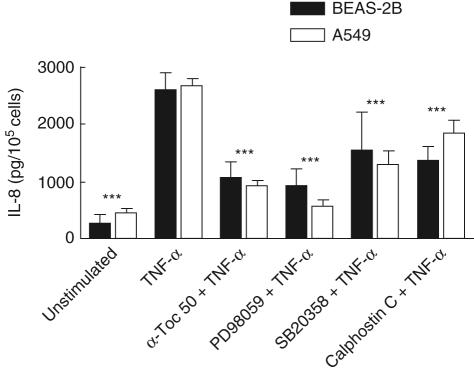
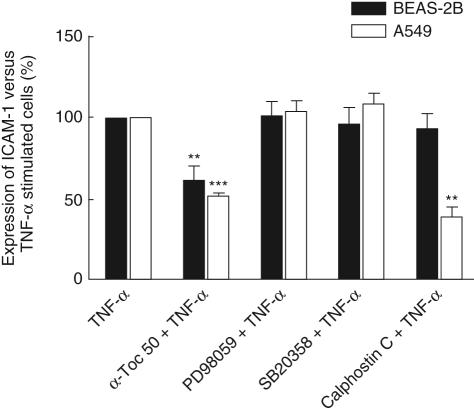
Effects of MAPK and PKC inhibitors on TNF-α-induced IL-8 and ICAM-1 expression
To examine the intracellular signalling pathways involved in TNF-α-induced ICAM-1 expression and IL-8 secretion, the p38 inhibitor SB203580, the MEK inhibitor PD98059 and the PKC inhibitor Calphostin C were used. SB203580 acts by competitively inhibiting ATP binding to p38 [27]. PD98059 is a selective inhibitor of the MAPK kinases MEK1/2 which phosphorylates and activates ERK1/2 [23]. Calphostin C interacts with the PKC regulatory domain by competing at the binding site of diacylglycerol [28]. Cell cultures of A549 and BEAS-2B cells were incubated with either 1 µM SB2035190, 40 µM PD98059 or 0·5 µM Calphostin C for 45 min before stimulation with 10 ng/ml TNF-α for 24 h. The production of IL-8 was measured in cell supernatants by ELISA (Fig. 6) and the cell surface expression of adhesion molecules were analysed by flow cytometry (Fig. 7). All three inhibitors decreased IL-8 secreted from BEAS-2B cell cultures to 40–60% of cells stimulated by TNF-α only (Fig. 6). In A549 cells, the ERK1/2 inhibitor PD98059 almost completely abolished the TNF-α-induced IL-8 production while the p38 inhibitor reduced the cytokine concentration to 50%, and the PKC inhibitor Calphostin C reduced the concentration to 70%. The ICAM-1 cell surface expression was not affected by the MAPK inhibitors neither in A549 or BEAS-2B cells, compared with cells incubated with TNF-α only (Fig. 7). Calphostin C showed no significant effect on ICAM-1 expression on BEAS-2B while this inhibitor down-modulated the expression of ICAM-1 to 60% on the A549 cells. For comparison, samples treated with 50 µM α-tocopherol were included in the experiment and the effects are shown in Figs 6 and 7.
Fig. 6.
Effects of extracellular signal-regulated kinase (ERK)1/2 (PD98059), p38 SD203580) or protein kinase C (PKC) (Calphostin C) inhibitors on tumour necrosis factor (TNF)-α-induced interleukin (IL)-8 production. Bronchial epithelial cell line (BEAS)-2B or A549 cells were incubated with 40 µM PD98059, 1 µM SB203580 or 0·5 µM Calphostin C. For comparison, treatment 50 µM α-tocopherol was included in the experiment. After 24 h, supernatants were collected and the concentration of IL-8 was measured by enzyme-linked immunosorbent assay (ELISA). Statistical significance of both cell lines versus cells stimulated with TNF-α alone is indicated (analysis of variance and Dunnet's multiple comparison test, n = 12).
Fig. 7.
Effects of extracellular signal-regulated kinase (ERK)1/2 (PD98059), p38 (SP203580) and protein kinase C (PKC) (Calphostin C) inhibitors on tumour necrosis factor (TNF)-α-induced cell surface expression of intercellular adhesion molecule-1 (ICAM-1) on bronchial epithelial cell line (BEAS)-2B and A549 cells. Cells were preincubated with 40 µM PD98059, 1 µM SB203580 or 0·5 µM Calphostin C. For comparison, treatment with 50 µM α-tocopherol was included in the experiment. The surface expression of ICAM-1 was measured by flow cytometry. Results are expressed as percentage of remaining cell surface expression of ICAM-1 versus TNF-α-stimulated cells. Expression on cells stimulated with TNF-α alone was given the value 100%. Statistical comparisons were performed using one-sample t-test versus 100% (n = 4).
Discussion
In the present study, we investigated the effects of α-tocopherol on the responsiveness of human lung epithelial cells to a proinflammatory signal, TNF-α. We demonstrated that exogenously administered α-tocopherol inhibited production of the neutrophil chemoattractant IL-8, as well as down-regulating the expression of cell adhesion molecules in both A549 and BEAS-2B cell lines and untransformed primary cells. The inhibition was concentration-dependent, with the most pronounced effect at 50 µM. Furthermore, we demonstrated that the down-modulation of cell adhesion molecules were reflected by a reduced ability of BEAS-2B and A549 cells to bind leucocytes in vitro. Further increase of the concentration to 100 µM did not, however, result in enhanced inhibition. Instead, a tendency towards reversed effect was observed. Such a response might be explained by disturbance of the cellular balance of water-soluble and lipophilic anti-oxidants at very high α-tocopherol concentrations, leading to action as an intracellular oxidant. Our observation is in agreement with a previous study by Shang et al., reporting that > 80 µM vitamin E enhances the toxicity of hydrogen peroxide on epithelial cells [29]. However, it should be noted that from our data it is evident that responsiveness to high concentrations of α-tocopherol was not equal in A549 and BEAS-2B cells, and also that we observed a tendency towards differential effects on IL-8 and ICAM-1 expression.
In our experimental system cells were stimulated with TNF-α, a proinflammatory cytokine derived from many inflammatory cells, such as monocytes and macrophages. This activation of lung epithelial cells is compatible with two major scenarios of acute airway inflammation in vivo: (1) macrophage ingestion of inhaled compounds leading to a vigorous cytokine secretion from these cells to airspaces and (2) fulminant inflammatory response in lung interstitium following a systemic trauma, i.e. the setting of indirect ARDS. Such responses can be provoked in laboratory animals by exposure to compounds that activate innate immune mechanisms. We have previously utilized C57BL/6 mice to study early inflammatory events in airways following inhalation of aerosolized E. coli LPS [30]. In these mice, engagement of Toll-like receptor 4 by LPS [31] induces onset of proinflammatory cytokines and chemokines in lung tissue within 2 h leading to accumulation of neutrophils in airspaces [30]. These sequestered neutrophils exhibit an enhanced ability to produce reactive oxygen metabolites [32], and concurrent with the airway inflammation, lung oedema and signs of epithelial cell injury were observed [12]. Using this model, we have demonstrated that exogenously administered α-tocopherol reduces neutrophilia and prevents lung injury [12]. Chow and coworkers have suggested three possible mechanisms for the action of α-tocopherol in vivo: (i) prevention of neutrophil adhesion by abrogating the expression of adhesion molecules on the surface of endothelial cells and/or neutrophils; (ii) impaired neutrophil binding to the endothelium due to changes in the topographic distribution or binding affinities of the surface adhesion molecules; and (iii) impaired neutrophil transmigration due to changes in the membrane stability of the migrating neutrophils, the endothelial and/or the epithelial cells [33].
In addition to the direct effects on cell membrane morphology, it is evident from our data that α-tocopherol interacts with intracellular activation pathways controlling inflammatory responses. Our results, together with previously reported data, indicate clearly that IL-8, as well as the cell adhesion receptor ICAM-1, are transcriptionally regulated by NF-κB [34,35]. Thus, it can be assumed that the observed down-modulation of this transcription factor accounts at least partially for the decreased responses observed in the α-tocopherol-treated cells. Consistent with this, α-tocopherol has been shown to reduce NF-κB transcriptional activity in the THP-1 monocyte cell line [26]. In bronchial epithelial cells, engagement of TNF-α with the 55-kDa TNF-α receptor (TNF-RI) activates protein kinase C (PKC) through a pathway involving phospholipase C-mediated hydrolysis of phospatidylcholine to diacylglycerol [1]. Krunkosky et al. suggested that activation of PKC is an upstream signalling event controlling nuclear translocation of NF-κB, although this linkage was not demonstrated conclusively [1]. If this putative pathway is present in the epithelial cells, our data on DNA binding of NF-κB could be assigned as a downstream effect of the previously described dephosphorylation of PKC [36]. In addition, Sabat et al. have demonstrated that depletion of vitamin E increased ICAM-1 expression on type II cells. This effect was assumed to be mediated by PKC activation, as the redox status was not affected [37]. However, other investigators have suggested that down-regulation of ICAM-1 and integrins by α-tocopherol can occur in a PKC-independent manner [23,24]. In our study, we used the specific PKC inhibitor Calphostin C to investigate the PKC dependency of the TNF-α-induced inflammatory response. This inhibitor clearly diminished ICAM-1 expression on the type II alveolar A549 cells, while the expression on bronchial BEAS-2B cells was unaffected. The differential inhibition of ICAM-1 could be due hypothetically to expression of different PKC isoforms in A549 and BEAS-2B cells. Notably, this observation contrasts with the reduced IL-8 production in both A549 and BEAS-2B cells after treatment with Calphostin C. Treatment with α-tocopherol did not result in such differential effects, indicating that the actions of this vitamin do not mimic those of PKC inhibitors, at least with regard to intervention of ICAM-1 expression on BEAS-2B cells.
In this study, we also demonstrated that α-tocopherol down-modulates phosphorylation of ERK1/2 and p38. These protein kinases belong to a family of MAPK used by human cells to transduce extracellular signals into a cellular response. ERK1/2 differs from the two other major members, p38 and JNK, by the lack of strong linkage with induction of proinflammatory responses [37] but, rather, is thought to play a role regulating proliferation, transformation and differentiation [38,39]. Both p38 and JNK phosphorylation has been related clearly to NF-κB and AP-1 activation partly by converging pathways, while the linkage between ERK1/2 and these transcription factors has been elusive [40–46]. Other studies have shown that inhibition of the ERK pathway diminishes TNF-α production in monocytes and macrophages [47,48] and IL-8 secretion in respiratory epithelial cells [49,50]. We demonstrated that the ERK inhibitor PD98059 blocks IL-8 production both in the A459 and BEAS-2B cell lines, further supporting a role of ERK1/2 activation in the expression of proinflammatory cytokines by lung epithelial cells. The effect on ERK1/2 phosphorylation by α-tocopherol can be due hypothetically to down-regulation of upstream signalling events. Two such major pathways leading to ERK phosphorylation have been described: (1) the PKC signalling pathway and (2) activation of RAS through receptor tyrosine kinase with subsequent phosphorylation of RAF and MEK1/2 [51]. Although it appears that these ERK activation pathways are independent, it should be emphasized that signal transduction mechanisms are generally cross-regulated in an intricate network [51,52]. Thus, it is not possible to delineate exactly the intracellular mechanisms leading to activation of this MAPK. Our observation that both Calphostin C and PD98059 inhibit IL-8 expression in A459 cells indicates that the IL-8 production is dependent on both ERK and PKC activation. In contrast to IL-8, the induction of ICAM-1 was independent of both ERK and p38 activation. Comparing the effects of α-tocopherol with that of the different inhibitors revealed that α-tocopherol, differently to the specific MAPK and PKC inhibitors, consistently down-modulated ICAM-1 expression in the two cell lines. This implicates a broader action of α-tocopherol, probably targeting more than one signal transduction mechanism.
In conclusion, the action of α-tocopherol on activated epithelium can protect cells from oxidative stress and excessive inflammation by inhibition of signal transduction pathways. We have demonstrated that α-tocopherol reduces ERK1/2 and p38 phosphorylation, as well as DNA binding of NF-κB in bronchial and alveolar epithelial cells. This was followed by diminished secretion of IL-8 and down-regulation of leucocyte adhesion by the epithelial cells. These results add new insights to the mechanisms of protection against inflammatory responses in the lung epithelium by α-tocopherol.
Acknowledgments
We thank Dr David Rocksén and Dr Thomas Sandström for critical reading of the manuscript. The study was supported financially by the Swedish Ministry of Defence.
References
- 1.Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNFα on expression of ICAM-1 in human airway epithelial cells in vitro. Am J Respir Cell Mol Biol. 2000;22:685–92. doi: 10.1165/ajrcmb.22.6.3925. [DOI] [PubMed] [Google Scholar]
- 2.Cromwell O, Hamid Q, Corrigan CJ, et al. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Richard C, Lemonnier F, Thibault M, Couturier M, Auzepy P. Vitamin E deficiency and lipoperoxidation during adult respiratory distress syndrome. Crit Care Med. 1990;18:4–9. doi: 10.1097/00003246-199001000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandström T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–3. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 5.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–9. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 6.Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 2001;56:242–9. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 7.Fogarty A, Lewis S, Weiss S, Britton J. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 2000;356:1573–4. doi: 10.1016/S0140-6736(00)03132-9. [DOI] [PubMed] [Google Scholar]
- 8.Suntres ZE, Shek PN. Treatment of LPS-induced tissue injury: role of liposomal antioxidants. Shock. 1996;6:57–64. [PubMed] [Google Scholar]
- 9.Suntres ZE, Shek PN. Prophylaxis against lipopolysaccharide-induced acute lung injury by α-tocopherol liposomes. Crit Care Med. 1998;26:723–9. doi: 10.1097/00003246-199804000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Fan JF, Shek PN, Suntres ZE, Li YH, Orepoulos GD, Rotstein OD. Liposomal antioxidants provide prolonged protection against acute respiratory distress syndrome. Surgery. 2000;128:332–8. doi: 10.1067/msy.2000.108060. [DOI] [PubMed] [Google Scholar]
- 11.Suntres ZE, Shek PN. Protective effect of liposomal alpha-tocopherol against bleomycin-induced lung injury. Biomed Envron Sci. 1997;10:47–59. [PubMed] [Google Scholar]
- 12.Rocksén D, Ekstrand-Hammarström B, Johansson L, Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol. 2003;28:199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- 13.Rocksén D, Koch B, Sandström T, Bucht A. Lung effects during a generalized Shwartzman reaction and therapeutic intervention with dexamethasone or vitamin E. Shock. 2004;22:482–90. doi: 10.1097/01.shk.0000142254.38630.36. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T, Yoshida N, Manabe H, Terasawa Y, Takemura T, Kondo M. α-Tocopherol protects against expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 1998;7:15–9. doi: 10.1002/biof.5520070103. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida N, Yoshikawa T, Manabe H, et al. Vitamin E protects against polymorphonuclear leukocyte-dependent adhesion to endothelial cells. J Leukoc Biol. 1999;65:757–63. doi: 10.1002/jlb.65.6.757. [DOI] [PubMed] [Google Scholar]
- 16.Suntres ZE, Shek PN. The pulmonary uptake of intravenously administered liposomal alpha-tocopherol is augmented in acute lung injury. J Drug Target. 1996;4:151–9. doi: 10.3109/10611869609015972. [DOI] [PubMed] [Google Scholar]
- 17.Kolleck I, Wissel H, Guthmann F, Schlame M, Sinha P, Rüstow B. HDL-holoparticle uptake by alveolar type II cells. Am J Respir Cell Mol Biol. 2002;27:57–63. doi: 10.1165/ajrcmb.27.1.4774. [DOI] [PubMed] [Google Scholar]
- 18.Kolleck I, Sinha P, Rüstow B. Vitamin E as an antioxidant of the lung. Am J Respir Crit Care Med. 2002;166:S62–6. doi: 10.1164/rccm.2206019. [DOI] [PubMed] [Google Scholar]
- 19.Herrera E, Barbas C. Vitamin E action, metabolism and perspectives. J Physiol Biochem. 2001;7:43–56. [PubMed] [Google Scholar]
- 20.Doelman CJA, Bast A. Oxygen radicals in lung pathology. Free Radic Biol Med. 1990;9:381–400. doi: 10.1016/0891-5849(90)90015-b. [DOI] [PubMed] [Google Scholar]
- 21.Azzi A, Ricciarrelli R, Zingg JM. Non-antioxidant molecular functions of α-tocopherol. FEBS Lett. 2002;519:8–10. doi: 10.1016/s0014-5793(02)02706-0. [DOI] [PubMed] [Google Scholar]
- 22.Freedman JE, Farhat JH, Loscalzo J, Keanney JF. α-Tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation. 1996;94:2434–40. doi: 10.1161/01.cir.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, Koga T, Martin KR, Meydani M. Effect of vitamin E on human aortic endothelial cell production of chemokines and adhesion to monocytes. Atherosclerosis. 1999;147:297–307. doi: 10.1016/s0021-9150(99)00199-9. [DOI] [PubMed] [Google Scholar]
- 24.Breyer I, Azzi A. Differential inhibition by α- and β-tocopherol of human erythroleukemia cell adhesion: role of integrins. Free Radic Biol Med. 2001;30:1381–9. doi: 10.1016/s0891-5849(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 25.Ricciarelli R, Zingg JM, Azzi A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–7. doi: 10.1161/01.cir.102.1.82. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Goto M, Matsamuto A, Tanaka I. Inhibition of NF-κB transcriptional activity by α-tocopherol succinate. Biofactors. 1998;7:21–30. doi: 10.1002/biof.5520070104. [DOI] [PubMed] [Google Scholar]
- 27.Young PR, McLaughlin MM, Kurmar S, et al. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–21. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 28.Gopalakrishna R, Chen ZH, Gundimeda U. Irreversible oxidative interaction of protein kinase C by photosensitive inhibitor Calphostin C. FEBS Lett. 1992;314:149–54. doi: 10.1016/0014-5793(92)80962-g. [DOI] [PubMed] [Google Scholar]
- 29.Shang F, Lu M, Dudek E, Reddan J, Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic Biol Med. 2003;1:521–30. doi: 10.1016/s0891-5849(02)01304-7. [DOI] [PubMed] [Google Scholar]
- 30.Larsson R, Rocksén D, Lilliehöök B, Jonsson Å, Bucht A. Dose-dependent activation of lymphocytes in endotoxin-induced airway inflammation. Infect Immun. 2000;68:6962–9. doi: 10.1128/iai.68.12.6962-6969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poltorak A, He X, Smirnova I, Liu MY, et al. Defective LPS signalling in C3H/HeJ and C57BL/10ScCr mice mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 32.Rocksén D, Lilliehöök B, Larsson R, Johansson T, Bucht A. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000;122:249–56. doi: 10.1046/j.1365-2249.2000.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow CW, Abreu MTH, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–31. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 34.Blackwell TS, Christman JW. The role of nuclear factor-κB in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 35.Rahman I, Macnee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–12. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricciarrelli R, Tasinato A, Clement S, Özer NK, Boscoboinik D, Azzi A. α-Tocopherol specifically inactivates cellular protein kinase C α by changing its phosphorylation state. Biochem J. 1998;334:243–9. doi: 10.1042/bj3340243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabat R, Kolleck I, Witt W, Volk H-D, Sinha P, Rustow B. Immunological dysregulation of lung cells in response to vitamin E deficiency. Free Radic Biol Med. 2001;30:1145–53. doi: 10.1016/s0891-5849(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 38.Kolch W. Meaningful relationships. the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 39.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–589. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganiatsas S, Kwee LK, Fujiwara Y, et al. SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc Natl Acad Sci USA. 1998;95:6881–6. doi: 10.1073/pnas.95.12.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derijard B, Raingeaud J, Barrett T, et al. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–5. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 42.Beyaert R, Cuenda A, Vanden Berghe W, et al. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumour necrosis factor. EMBO J. 1996;15:1914–23. [PMC free article] [PubMed] [Google Scholar]
- 43.Wesselborg S, Bauer MK, Vogt M, Schmitz ML, Schulze-Osthoff K. Activation of transcription factor NF-kB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J Biol Chem. 1997;272:12422–9. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 44.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression: the role of the TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–63. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 45.Zechner D, Craig R, Hanford DS, McDonough PM, Sabbadini RA, Glembotski CC. MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J Biol Chem. 1998;273:8232–9. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]
- 46.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 47.Bauer GJ, Arbadi S, Garcia IA, deHingh I, Rosengart MR, Maier RV. Adherence regulates macrophage signal transduction and primes tumour necrosis factor production. Shock. 2000;14:435–40. doi: 10.1097/00024382-200014040-00003. [DOI] [PubMed] [Google Scholar]
- 48.Rosengart MR, Arbadi S, Garcia SI, Maier RV. Interactions of calcium/calmodulin-dependent protein kinase (CaMK) and extracellular-regulated kinase (ERK) in monocyte adherence and TNFa production. Shock. 2000;13:183–9. doi: 10.1097/00024382-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Monick MM, Carter AB, Hunninghake GW. Activation of ERK2 by respiratory syncytial vitus in A549 cells is linked to the production of interleukin 8. Exp Lung Res. 2000;26:13–26. doi: 10.1080/019021400269934. [DOI] [PubMed] [Google Scholar]
- 50.Graness A, Chwieralski CE, Reinhold D, Thim L, Hoffman W. Protein kinase C and ERK activation are required for TFF-peptide-stimulated bronchial epithelial cell migration and tumor necrosis factor-alpha-induced interleukin-6 (IL-6) and IL-8 secretion. J Biol Chem. 2002;277:18440–6. doi: 10.1074/jbc.M200468200. [DOI] [PubMed] [Google Scholar]
- 51.Lowes VL, Ip NY, Wong YH. Integration of signals from receptor tyrosine kinases and G protein-coupled receptors. Neurosignals. 2002;11:5–19. doi: 10.1159/000057317. [DOI] [PubMed] [Google Scholar]
- 52.Arbadi S, Maier RV. Mitogen-activated protein kinases. Crit Care Med. 2002;30:S74–S78. [PubMed] [Google Scholar]