Abstract
Tumour necrosis factor (TNF)-α and interferon (IFN)-γ exert detrimental effects in organ-specific autoimmune disease, while both destructive and protective roles have been demonstrated for interleukin (IL)-10, IL-4 and IL-5. We examined the production of these cytokines by peripheral blood mononuclear cells (PBMC) from patients with Hashimoto's thyroiditis (HT), Graves' disease (GD) and healthy controls, upon exposure to a thyroid self-antigen, human thyroglobulin (Tg), in the presence of autologous serum. Initially, TNF-α and IL-2 were produced in all three groups, accompanied by IL-10. Release of IFN-γ, IL-4 and, notably, IL-5 ensued. Both patient groups exhibited increased TNF-α, IL-2, IFN-γ and IL-10 responses, and PBMC from HT patients secreted lower amounts of IL-5 than male, but not female, controls. Enhanced TNF-α production by HT cells also occurred in the presence of pooled normal sera, indicating a dependency on intrinsic cellular factors. Conversely, higher production of TNF-α and IL-5 occurred in the presence of autologous sera than in the presence of pooled normal sera in both patient groups, indicating a dependency on serum constituents. Complement appeared to promote the production of IL-2 and particularly IL-5, the levels of which were reduced by neutralization of complement by heat- or zymosan treatment. The production of IFN-γ and IL-2 of the three groups together correlated directly with the serum anti-Tg activity. Moreover, TNF-α, IFN-γ, IL-5 and IL-10 responses were markedly inhibited by partial denaturation of Tg by boiling. We hypothesize that autoantibodies and complement may promote mixed Th1/Th2 cell cytokine responses by enhancing the uptake of autoantigens by antigen-presenting cells.
Keywords: autoantibodies, autoimmunity, cytokines, IL-5, IL-10
Introduction
It is well established that proinflammatory T helper cell 1 (Th1)-type cytokines, including tumour necrosis factor (TNF)-α, interferon (IFN)-γ and interleukin (IL)-2, play pivotal roles in the pathogenesis of organ-specific autoimmune diseases, including Graves' disease (GD) and Hashimoto's thyroiditis (HT) [1–5]. Concordantly, a protective effect against autoimmunity has been demonstrated for cytokines that counteract Th1 responses, namely IL-4 produced by Th2 cell subsets [6–9], and IL-10, which is produced by a T cell subset referred to as T regulatory type 1 (Tr1) cells (reviewed in reference [10]), by B cells [11] and by monocytes [12]. Recent evidence suggests that another Th2 cytokine, IL-5, plays a protective role in autoimmune processes, as transgene-mediated hyperexpression of IL-5 in systemic lupus erythematosus (SLE)-prone mice suppresses the disease [13].
The dogma that the pathogenic processes in organ-specific autoimmune diseases are driven by Th1 type cytokines only has been refuted by observations of detrimental effects of IL-4 and IL-10 (reviewed in [14]). The involvement of these cytokines has also been indicated in HT and GD [4,5,15,16] (reviewed in [17]). Their role in pathogenesis may vary depending on the developmental stage of the disease [18].
Our current knowledge about the cytokines involved in the pathogenesis of autoimmune diseases is, to a great extent, based upon in vivo models in which disease is induced in genetically susceptible animals upon immunization with self-antigen emulsified in adjuvant. However, such studies are biased by direct effects of the adjuvants on the cytokine production [19,20]. Studies of the cytokine production in vitro by single cells isolated from the affected organs of patients with autoimmune disease, on the other hand, may not reveal a comprehensive picture of cytokine responses to autoantigens, the interplay between the various cytokines involved and the factors that influence their secretion.
In the present study, we assess the production of Th1/Th2/Tr1 cytokines elicited by a thyroid self-antigen, thyroglobulin (Tg), in cultures of peripheral blood mononuclear cells (PBMC) from healthy individuals, patients with HT or patients with GD. The cultures were grown in the presence of autologous or allogenic serum, and the influence of the serum composition on the cytokine responses is assessed.
Materials and methods
Subjects
The study included 11 patients with untreated HT − characterized by elevated serum thyroid-stimulating hormone (TSH), decreased serum free T4 and presence of anti-thyroid peroxidase (TPO) antibodies − (10 females, one male, age 30–79 years), six patients with untreated GD − characterized by suppressed serum TSH, elevated serum free T4 and free T3 and presence of TSH-receptor antibodies − (five females, one male, age 24–58 years) and 10 healthy euthyroid controls without thyroid antibodies (four females, six males, age 28–57 years) who participated after informed consent. None of the included patients received pharmaceutical treatment, and none were pregnant. The controls were recruited among laboratory staff members without previous or present thyroid disease. The study was approved by the local ethics committee.
Cell and serum preparations
Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation over LymphoprepTM (Nycomed Pharma AS, Oslo, Norway). Standard serum was obtained from a pool of five healthy blood group AB, rhesus D-positive donors. Anti-Tg levels were measured in all tested sera by means of an enzyme-linked immunosorbent assay (ELISA) expressing the antibody activity as a ratio to that of the AB serum pool, as described previously [21]. Complement inactivation of serum was achieved by heating to 56°C for 1 h or by incubation with zymosan (Invitrogen, San Diego, CA, USA), 5 mg/ml, for 30 min at 37°C, followed by centrifugation (1000 g, 10 min) and usage of the upper half of the supernatant.
Antigens
Thyroglobulin (Tg), purified from human thyroid, was purchased from Biogenesis (Poole, Dorset, UK). The purity was > 99%, the contaminants being IgG < 0·4%, IgA < 0·3% and IgM < 0·3%. Tetanus toxoid (TT) was a kind gift from Dr Claus Koch, State Serum Institute, Copenhagen, Denmark. In some experiments, the Tg-preparation was boiled for 10 min to achieve some denaturation of conformational Tg-epitopes, and the preparation was run through a polymixin B-agarose column (Sigma-Aldrich, Brondby, Denmark) in order to remove lipoplysaccharide (LPS).
Stimulation of PBMC with antigen
PBMC were washed in RPMI (Gibco/Invitrogen, Tåstrup, Denmark) and resuspended in 30% serum/50% RPMI/20% phosphate-buffered saline (PBS) and distributed in 96-well flat-bottomed NunclonTM MicroWellTM microtitre plates (Invitrogen), 106 PBMC per well. Unless stated otherwise, the serum present in the cultures was autologous to the PBMC. Tg or TT was added to cell suspensions to final concentrations of 90 µg/ml and 10 µg/ml, respectively; concentrations that elicited maximal CD4+ T cell proliferation in previous titration experiments [22]. The cell suspensions were incubated for 1, 4 or 7 days at 37°C, 5% CO2 in humidified air.
Measurement of cytokines in culture supernatant
The content of TNF-α, IL-2, IFN-γ, IL-4, IL-5 and IL-10 was quantified in culture supernatants at days 1, 4 and 7 by means of a Th1/Th2 cytometric bead array kit using a fluorescence activated cell sorter (FACScalibur) flow cytometer (Becton Dickinson, Copenhagen, Denmark). Data analyses were performed using cytometric bead array software (Becton-Dickinson). The levels of transforming growth factor (TGF)-β were measured by use of an ELISA kit with a detection limit of approximately 60 pg/ml (R&D Systems, Minneapolis, MN, USA).
Statistics
Differences between the patient and control groups, and differences between the effects of stimulation with Tg and TT, were tested using the Mann–Whitney U-test. The Wilcoxon matched-pairs test was used for comparison of data from identical cell populations incubated with different sera. P-values of less than 0·05 were considered significant. The software employed was JMP® (SAS Institute, Cary, NC, USA) and Prism® (GraphPad, San Diego, CA, USA).
Results
Stimulation of PBMC with Tg in medium containing autologous serum
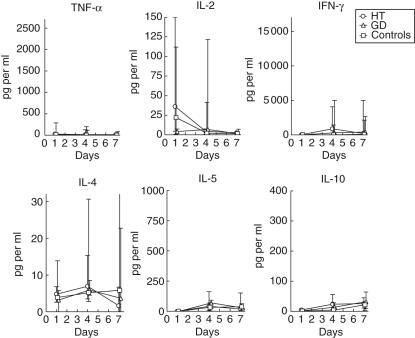
PBMC from patients with HT, GD or healthy controls were cultivated in media containing autologous serum and stimulated with human Tg. There was no significant cytokine production in the absence of added antigen (data not shown). However, as shown in Fig. 1, Tg elicited immediate release of TNF-α in all three groups of subjects. The TNF-α levels declined thereafter and were significantly higher in cell cultures from GD patients than controls from days 4 to 7. Despite small levels at day 7, also Tg-stimulated cells from HT patients exhibited an increased release of TNF-α compared to control cells (medians: 41 pg/ml versus 12 pg/ml, respectively).
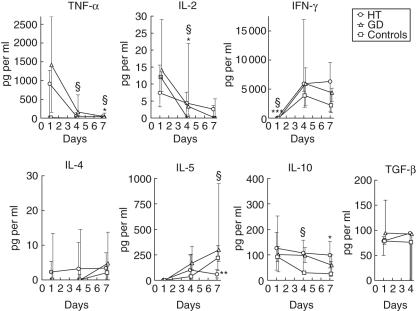
Fig. 1.
Thyroglobulin (Tg)-elicited cytokine production by peripheral blood mononuclear cells (PBMC). Cells from 10 healthy individuals (□), six patients with GD (▵) or 11 patients with HT (○) were stimulated with Tg and cultured with autologous serum (30% v/v). The resulting cytokine responses are shown as medians and interquartile ranges. Lifted symbols indicate significant differences between the groups: *HT versus controls, §GD versus controls, #HT versus GD; one symbol signifies P < 0·05, two symbols P < 0·005 and three symbols P < 0·001. In some cases the transforming growth factor (TGF)-α levels fell below the detection limit of the assay.
A low release of IL-2 occurred within the first day of incubation in all three groups (Fig. 1). At day 4, the level had decreased in the cultures of PBMC from GD patients and controls, and was higher in both patient groups than in the control group. The initial production of TNF-α and IL-2 was succeeded by vast production of IFN-γ in all three groups (Fig. 1). Despite the relatively small levels at day 1, significantly higher production of IFN-γ was seen in the HT and GD groups than in the control group, the median levels being 47 pg/ml, 50 pg/ml and non-detectable, respectively.
The Tg-elicited production of IL-4 was low and did not differ significantly between the groups. However, a marked progressively rising production of another Th2 cytokine, IL-5, was observed (Fig. 1). At day 7, the IL-5 level was decreased significantly in the HT group compared to both the GD group and the healthy controls.
In all three groups, Tg elicited maximal release of IL-10 by PBMC within the first day of incubation. The levels of IL-10 were elevated significantly in PBMC cultures from GD patients at day 4 compared to the controls, which was not the case in the HT group due to great variation. At day 7, cultures derived from HT patients exhibited increased levels, however. The Tg-elicited release of another cytokine implicated in control of autoimmune processes, TGF-β[9], did not differ between the groups.
Influence of gender on Tg-elicited cytokine production
GD and HT predominantly affect women, as reflected by the patients included in this study. We divided the control group into males and females to examine whether the observed differences in cytokine responses were sex-related (Fig. 2).
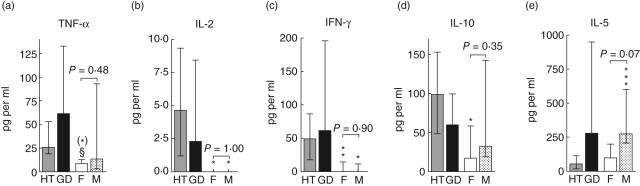
Fig. 2.
The influence of gender on cytokine responses to thyroglobulin (Tg). Differences between patients and controls, upon division of the control group into females (F, n = 4) and males (M, n = 6), are shown for (a) tumour necrosis factor (TNF)-α at day 7, (b) IL-2 at day 4, (c) interferon (IFN)-γ at day 1, (d) interleukin (IL)-10 at day 7 and (e) IL-5 at day 7. One male Hashimoto's thyroiditis (HT) patient and one male Graves' disease (GD) patient were excluded from the analysis. *HT versus controls, §HT versus GD. One symbol signifies P < 0·05, two symbols P < 0·01 and three symbols P < 0·001. (*) signifies P = 0·05.
With respect to the Tg-elicited production of TNF-α, IL-2, IFN-γ and IL-10, the differences between female HT patients and female controls remained significant, and the responses were similar in male and female controls (Fig. 2a–d). Thus, the observed differences between patients and controls could not be attributed to differences in Tg-responsiveness between the sexes. In contrast, male controls tended to produce higher amounts of IL-5 than female controls (P < 0·07) and female HT patients (P < 0·0002) (Fig. 2e).
Stimulation of PBMC with Tg in medium containing pooled sera from healthy donors
To focus on differences at the cellular level between patients and controls, we examined the Tg-elicited cytokine production by PBMC from all tested individuals in medium containing pooled serum from five healthy, blood group AB donors. The cytokine release followed similar kinetics to those observed in the presence of autologous serum; the HT group displayed elevated production of TNF-α at day 1 (Fig. 3a) and IL-4 at days 1 and 4 (P < 0·05 and P < 0·005, respectively; not shown), while an increased production of IL-10 by PBMC from GD patients was seen at day 4 (P < 0·05) (data not shown).
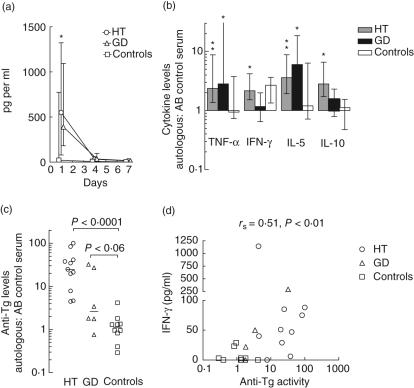
Fig. 3.
The influence of intrinsic cellular factors and serum factors on thyroglobulin (Tg)-elicited cytokine responses. (a) Peripheral blood mononuclear cells (PBMC) from all patients and controls were grown in medium containing Tg and pooled serum from healthy blood group AB donors. The kinetics of tumour necrosis factor (TNF)-α release is shown as medians and interquartile ranges. *P < 0·05 for HT versus controls. (b) The levels of TNF-α (day 4), interferon (IFN)-γ (day 7), IL-5 (day 4) and IL-10 (day 7) were compared to the corresponding levels in cultures containing autologous serum. Medians and interquartile values of the ratios (autologous serum: AB serum pool) are shown. *P < 0·05 and **P < 0·005 for the ratio equalling 1. (c) For comparison, the anti-Tg activity of each individual was measured by enzyme-linked immunosorbent assay (ELISA) and related to that of the AB serum pool (= 1 by definition). (d) Correlation between serum anti-Tg activity and the level of IFN-γ at day 1; rs indicates Spearman's correlation coefficient (for technical reasons, the level of IFN-γ was not measured for one HT patient and one GD patient at day 1).
The levels of TNF-α, IFN-γ, IL-5 and IL-10 were significantly higher in the HT group when PBMC were grown in media containing autologous serum than in media containing pooled normal sera (Fig. 3b). This also applied to TNF-α and IL-5 in the GD group but in general not to the control group, indicating that factors in patient sera promoted cytokine production. Elevated anti-Tg activities were found in HT sera and, to a lesser extent, in GD sera compared to sera from healthy controls (Fig. 3c); on the whole, the anti-Tg activity correlated significantly with the culture IFN-γ levels at day 1 (Fig. 3d) and the IL-2 levels at day 7 (P < 0·02, data not shown). The TGF-β production did not depend upon the type of serum present during incubation with Tg (not shown).
Influence of complement on Tg-elicited cytokine production
Given a role for complement in the uptake and presentation of Tg by B cells [22], we compared the cytokine production elicited by Tg in PBMC grown in untreated autologous serum with the corresponding production in the presence of autologous serum in which complement had been heat inactivated prior to the incubation.
Heat inactivation of serum mediated a general decrease in IL-2 production (by median values of 33%, 50% and 45% in the HT, GD and control groups, respectively) and an increase in IL-10 production (by 33–61%), which was significant in the GD group (Fig. 4). The heat-sensitivity of IL-2 and IL-10 release was greater in the control group than in the HT group (P < 0·04 and P < 0·02, respectively) at day 4 (not shown). The most striking effect of heat inactivation concerned the IL-5 production, however. At day 4, it was reduced by median values of 90% in the HT group, 83% in the GD group and 97% in the healthy controls (Fig. 4).
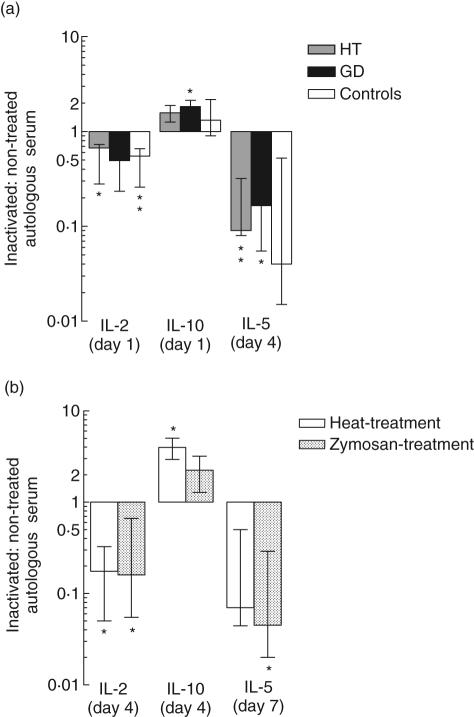
Fig. 4.
Influence of complement on the thyroglobulin (Tg)-elicited cytokine release. (a) Peripheral blood mononuclear cells (PBMC) from 11 patients with Hashimoto's thyroiditis (HT) (shaded bars), six patients with Graves' disease (GD) (filled bars) and nine healthy controls (open bars) were incubated with Tg in the presence of untreated or heat-treated autologous serum. (b) PBMC from six healthy donors were incubated with Tg in the presence of untreated autologous serum, or autologous serum in which complement had been inactivated by heating (open bars) or by incubation with zymosan (hatched bars). The ratios between the levels of cytokines under these conditions (inactivated: untreated serum) are shown as medians and interquartile ranges. The symbols indicate significance levels for the hypothesis that the ratio is 1: *P < 0·05, **P < 0·005.
Because other factors than complement proteins may be affected by heat treatment of serum, we used another approach to neutralize serum complement, namely consumption of the central complement component, C3, by preincubation of serum with zymosan. As shown in Fig. 4b, this treatment also significantly reduced Tg-elicited IL-2- and IL-5 production, which further supports a role for complement in promoting the production of these cytokines.
Tetanus toxoid-induced cytokine release
To determine whether group differences in cytokine responses were antigen-specific, we challenged PBMC from the tested donors with a control recall antigen, tetanus toxoid (TT), in the presence of autologous serum. Neither of the patient groups differed significantly from controls with respect to secretion of any of the tested cytokines in response to TT (Fig. 5). However, a tendency was seen towards increased TT-elicited production of IL-5 in both patient groups, compared to the control group (P < 0·08).
Fig. 5.
Tetanus toxoid (TT)-elicited cytokine release by mononuclear cells. Peripheral blood mononuclear cells (PBMC) from nine healthy individuals (□), six patients with Graves' disease (GD) (▵) and 11 patients with Hashimoto's thyroiditis (HT) (○) were stimulated with TT in the presence of autologous serum (30% v/v). The resulting cytokine production is shown as medians and interquartile ranges. Apart from the interleukin (IL)-2 data, the ordinate scales correspond to those in Fig. 1.
Somewhat surprisingly, the self-antigen Tg (0·15 μM) was a stronger stimulus than the foreign, secondary antigen TT (0·07 μM) for the production of most cytokines. Even in the control group (compare Figs 1 and 5), Tg induced higher levels than did TT of IFN-γ, IL-10 and IL-4 at day 1 (P < 0·007–0·04), and of TNF-α at days 4–7 (P < 0·008). In the HT group, Tg induced production of TNF-α more efficiently than TT at day 1 (P < 0·03) and of IL-10 over the entire observation period (P < 0·02–0·03), and the same applied to IL-10 at days 1 and 4 in the GD group (P < 0·008). Conversely, TT elicited a higher IL-4 response than Tg within the first day of incubation in the HT group (P < 0·03). No difference was observed in either group at any time-point in the capability of the two antigens to elicit IL-2 or IL-5.
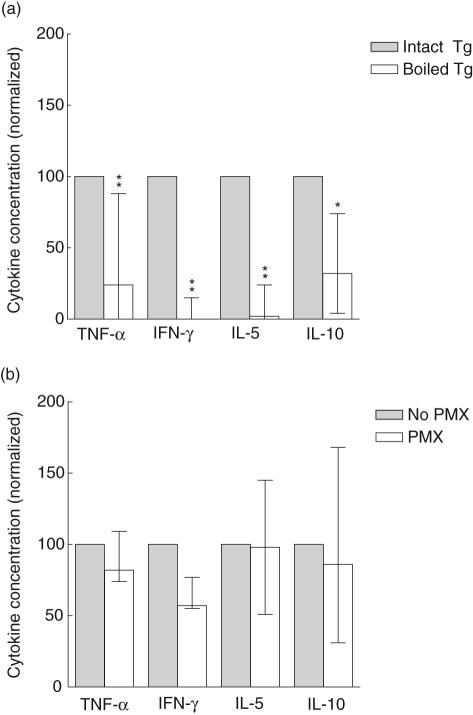
Effect of boiling of Tg on Tg-elicited cytokine production
We next examined whether an intact tertiary structure of Tg was required for induction of cytokine responses by PBMC. To this end Tg was denatured by boiling. As shown in Fig. 6a, boiling of Tg markedly reduced the induction of TNF-α, IFN-γ, IL-5 and IL-10. IL-4 and IL-2 were undetectable in four of six and three of six experiments, respectively (data not shown). These data show that nonlinear epitopes on Tg were critical for elicitation of the above-mentioned cytokine responses. Moreover, the responses could not have been induced by LPS contaminating the Tg-preparation, as LPS is resistant to the boiling procedure. This was confirmed by the fact that pretreatment of Tg with polymyxin (PMX) only slightly affected TNF-α, IL-5 and IL-10 responses (Fig. 6b), as well as IL-2 and IL-4 responses (not shown). However, the production of IFN-γ appeared to be influenced by PMX-treatment, so an adjuvant effect of LPS with respect to production of this cytokine cannot be ruled out.
Fig. 6.
The influence of boiling and polymyxin B treatment of thyroglobulin (Tg) on cytokine responses. Normal peripheral blood mononuclear cells (PBMC) were stimulated, in the presence of autologous serum, with (a) Tg boiled for 5 min, in order to disrupt the tertiary structure or (b) Tg preincubated with polymyxin B (PMX) to remove any lipopolysaccharide. The cytokine levels in day 7 cultures were normalized to those found upon stimulation with untreated, intact Tg (= 100), and are shown as median (range) of six experiments in (a) and three experiments in (b). *P < 0·05, and **P < 0·008.
Discussion
We have examined the release of cytokines by cultivated PBMC from patients with GD and HT as well as healthy controls after challenge with a thyroid self-antigen, Tg, and a foreign, secondary control antigen, TT. Tg induced initial TNF-α, IL-2 and IL-10 responses in all three groups, followed by the production of IFN-γ, IL-5 and small amounts of IL-4. The significantly increased levels of TNF-α, IFN-γ and IL-2 in both patient groups (Fig. 1) support previous demonstrations of Th1 cytokine production in association with autoimmune thyroid disease HT [1–5], and show that Tg alone can elicit the release of these cytokines from patient PBMC but also, to some extent, from PBMC isolated from healthy donors.
The finding that the production of TNF-α, IFN-γ, IL-4 and IL-10 by patient cells was greater in the presence of autologous sera containing high anti-Tg activities than in low-titred sera from healthy individuals suggests that autoantibodies play a role in the stimulation of T cells and/or monocytes for cytokine production in autoimmune thyroid disease (AITD), as they facilitate CD4+ T cell proliferation as shown previously [21,22]. Although receptors for thyrotropin (TSH) have been reported on lymphocytes [23], and TSH may enhance the production of TNF-α by bone marrow cells [24], we regard a direct effect of TSH or thyroid hormones on PBMC as unlikely to account for the promotion of cytokine production by patient sera, as (i) it was observed for hypothyroid HT patients, as well as hyperthyroid GD patients, and (ii) patient cells did not exhibit an increased cytokine production in response to stimulation with a control antigen, TT. We cannot exclude the possibility that TSH in HT sera, and factors other than antibodies in patient sera, in general, promote cytokine release from PBMC. However, a significant correlation between serum anti-Tg activities and the culture levels of IFN-γ at day 1, and of IL-2 at day 7, supports strongly the contention of antibody involvement in promotion of Tg-elicited cytokine responses, as did the pronounced reduction in cytokine production that occurs upon boiling (and thereby the loss in integrity of nonlinear antigenic epitopes) of Tg. This reduction also showed that the cytokine production observed could not be attributed to contamination of the Tg-preparation with LPS, the structure of which remains intact upon boiling.
The increased Tg-induced production of IL-10 observed here in both patient groups is in keeping with a reported IL-10 mRNA expression in samples of autoimmune thyroid glands [4,25]. A role for IL-10 in GD has been indicated by recent reports of increased levels of serum IL-10 and IgG3-secreting cells in patients with intractable GD, but not in patients with GD in remission or in HT patients [15,16]. IL-10 stimulates synthesis of antibodies of the complement-activating isotypes IgG1 and IgG3 [26], suggesting that it promotes the formation of complement-opsonized complexes between self-antigens and autoantibodies, and the subsequent uptake and presentation of self-antigen by B cells [21,22]. IL-10 plays a protective role in several animal models of autoimmune disease [27–29], presumably by inhibiting Th1 cytokine responses in concert with IL-4 [7,8,27,28]. Tr1 cells are believed to be the major source of IL-10 for suppression of autoimmune processes [10], but also B cells provide IL-10-mediated protection against autoimmunity in mice [11]. It remains unclear whether the IL-10 production elicited by Tg in the present study represents a response of Tr1 cells and/or B cells to control autoimmune processes, or whether it promotes pathogenic processes.
In this study, Tg elicited a pronounced IL-5 production in PBMC from healthy individuals as well as from both patient groups. In humans, the biological effects of IL-5 are best characterized for eosinophils, the maturation, survival, chemotactic activity and adhesion of which it promotes [30,31]. Mice expressing IL-5 transgenes have markedly increased numbers of B-1 cells and a concomitant hypergammaglobulinaemia, including polyreactive anti-DNA autoantibodies of IgM class [32]. Accordingly, IL-5 responsive B-1 cells are increased in the systemic lupus erythematosus-prone (NZB × NZW) F1 mice [13,33]. Although these findings indicate a potentially harmful role of IL-5, Wen et al. recently demonstrated that hyperexpression of IL-5 suppresses autoimmunity [13]. A protective effect of IL-5 would be in keeping with an inverse relationship between IL-5 production and disease activity associated with Th1 cytokines [34,35].
Interestingly, IL-5 was the only cytokine the production of which appeared to be sex-dependent, as the Tg-elicited production by healthy males was considerably higher than that of female HT patients, and tended to be higher than that of female controls. It remains to be investigated whether IL-5 production is dependent on sex hormones, and whether decreased IL-5 production is linked to the increased susceptibility of women to develop most autoimmune diseases.
The production of IL-5 and, to a lesser extent, IL-2 were inhibited by heat treatment of serum, which inactivates all three pathways of complement activation. Although other serum proteins may be affected by heat treatment, a similar inhibition pattern by preincubation of serum with zymosan, leading to consumption of C3, indicated strongly that the inhibited cytokine production was caused by neutralization of complement. Complement may enhance the presentation of Tg-derived peptides to IL-5- or IL-2-producing T cells by opsonizing either Tg-containing immune complexes (IC) [22] or Tg itself [36], thereby promoting and/or prolonging antigen presentation by B cells [or other antigen-presenting cells (APC)]. Alternatively, complement may influence T cells directly through the interaction of C1q-bearing IC with T cells [37] and/or interaction of IC- or Tg-bound iC3b with CR3 (CD11b/CD18) on a T cell subset [38]. Jiang et al. showed that C1q-opsonized IC stimulated T cells for TNF-α and IFN-γ production [37], but we found no convincing effect of complement inactivation on the production of these cytokines.
In addition to reducing the production of IL-5 and IL-2, i.e. both Th2 and Th1 responses, heat treatment of serum also resulted in enhanced production of IL-10, indicating that complement may inhibit Tr1 responses. This finding was not fully confirmed by treatment of serum with zymosan, however, and we cannot exclude the possibility that inactivation of other heat-labile factors than complement have contributed to the effects caused by heat treatment of serum.
We compared the cytokine responses to Tg with those elicited by TT at a comparable molar concentration. No significant differences were found between the controls and patient groups with regard to the latter, indicating that the differences observed between PBMC from the groups upon stimulation with Tg were antigen-specific. TT did, in general, elicit stronger IL-4 responses than Tg. However, far more IL-10 was released in response to Tg than to TT, indicating a special role for IL-10 in modulating the response to self-antigens.
It is noteworthy that the differences seen here between patients and controls are quantitative rather than qualitative. All the cytokines tested were produced by PBMC from all three groups, but the Tg-elicited production of TNF-α, IFN-γ, IL-4 and IL-10 was increased in the two patient groups. The cytokine profile observed in patients with GD and patients with HT might thus represent an exaggeration of the normal response to Tg. Our data indicate that this overreaction to Tg is based partly on intrinsic cellular factors, as exemplified by TNF-α production in HT-patients, and partly on serum factors. Such serum factors may include autoantibodies and complement, possibly acting together in complement-opsonized IC that may facilitate uptake and presentation of self-antigens by APC [21,22] and contribute to inflammatory processes by activation of macrophages and neutrophils [39].
Acknowledgments
The excellent technical assistance by Ms Bettina Jepsen, Ms Winnie Hansen, and Ms Nanna Bøgesvang is greatly appreciated. We thank Vagn Andersen MD for helpful discussions. This work was supported by the Danish Rheumatism Association, the Danish Biotechnology Program, the Novo Nordisk Foundation and the Agnes and Knut Mørk Foundation.
References
- 1.Del Prete GF, Tiri A, De Carli M, et al. High potential to tumor necrosis factor (TNF-) production of thyroid infiltrating lymphocytes in Hashimoto's thyroiditis: a peculiar feature of destructive thyroid autoimmunity. Autoimmunity. 1989;4:267–76. doi: 10.3109/08916938909014703. [DOI] [PubMed] [Google Scholar]
- 2.Del Prete GF, Tiri A, Mariotti S, Pinchera A, Ricci M, Romagnani S. Enhanced production of gamma-interferon by thyroid-derived T cell clones from patients with Hashimoto's thyroiditis. Clin Exp Immunol. 1987;69:323–31. [PMC free article] [PubMed] [Google Scholar]
- 3.Guo J, Rapoport B, McLachlan SM. Balance of Th1/Th2 cytokines in thyroid autoantibody synthesis in vitro. Autoimmunity. 1999;30:1–9. doi: 10.3109/08916939908994754. [DOI] [PubMed] [Google Scholar]
- 4.Ajjan RA, Watson PF, McIntosh RS, Weetman AP. Intrathyroidal cytokine gene expression in Hashimoto's thyroiditis. Clin Exp Immunol. 1996;105:523–8. doi: 10.1046/j.1365-2249.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson PF, Pickerill AP, Davies R, Weetman AP. Analysis of cytokine gene-expression in Graves-disease and multinodular goiter. J Clin Endocrinol Metab. 1994;79:355–60. doi: 10.1210/jcem.79.2.8045947. [DOI] [PubMed] [Google Scholar]
- 6.Nagayama YJ, Mizuguchi H, Hayakawa T, Niwa M, McLachlan SM, Rapoport B. Prevention of autoantibody-mediated Graves'-like hyperthyroidism in mice with IL-4, a Th2 cytokine. J Immunol. 2003;170:3522–7. doi: 10.4049/jimmunol.170.7.3522. [DOI] [PubMed] [Google Scholar]
- 7.Fox CJ, Danska JS. IL-4 expression at the onset of islet inflammation predicts nondestructive insulitis in nonobese diabetic mice. J Immunol. 1997;158:2414–24. [PubMed] [Google Scholar]
- 8.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–9. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4(+) CD45RC(−) cells and CD4(+) CD8(−) thymocytes. J Exp Med. 1999;189:279–88. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 11.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 12.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J Immunol. 2002;168:1968–77. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 13.Wen XS, Zhang DQ, Kikuchi Y, et al. Transgene-mediated hyper-expression of IL-5 inhibits autoimmune disease but increases the risk of B cell chronic lymphocytic leukemia in a model of murine lupus. Eur J Immunol. 2004;34:2740–9. doi: 10.1002/eji.200425267. [DOI] [PubMed] [Google Scholar]
- 14.Moore KW, Malefyt RD, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 15.Takeoka K, Watanabe M, Matsuzuka F, Miyauchi A, Iwatani Y. Increase of serum interleukin-10 in intractable Graves' disease. Thyroid. 2004;14:201–5. doi: 10.1089/105072504773297876. [DOI] [PubMed] [Google Scholar]
- 16.Nakamoto Y, Niki M, Watanabe M, Iwatani Y. Increase in immunoglobulin G3-secreting cells in intractable Graves' disease. Thyroid. 2003;13:325–31. doi: 10.1089/105072503321669794. [DOI] [PubMed] [Google Scholar]
- 17.Weetman AP. Cellular immune responses in autoimmune thyroid disease. Clin Endocrinol. 2004;61:405–13. doi: 10.1111/j.1365-2265.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 18.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin-10 by islet cells accelerates immune-mediated destruction of beta-cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–84. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaccone P, Fehervari Z, Blanchard L, Nicoletti F, Edwards CK, Cooke A. Autoimmune thyroid disease induced by thyroglobulin and lipopolysaccharide is inhibited by soluble TNF receptor type 1. Eur J Immunol. 2002;32:1021–8. doi: 10.1002/1521-4141(200204)32:4<1021::AID-IMMU1021>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Yip HC, Karulin AY, Tary-Lehmann M, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–9. [PubMed] [Google Scholar]
- 21.Nielsen CH, Hegedus L, Leslie RG. Autoantibodies in autoimmune thyroid disease promote immune complex formation with self antigens and increase B cell and CD4+ T cell proliferation in response to self antigens. Eur J Immunol. 2004;34:263–72. doi: 10.1002/eji.200324413. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen CH, Leslie RG, Jepsen BS, Kazatchkine MD, Kaveri SV, Fischer E. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur J Immunol. 2001;31:2660–8. doi: 10.1002/1521-4141(200109)31:9<2660::aid-immu2660>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Francis T, Burch HB, Cai WY, et al. Lymphocytes express thyrotropin receptor-specific mRNA as detected by the PCR technique. Thyroid. 1991;1:223–8. doi: 10.1089/thy.1991.1.223. [DOI] [PubMed] [Google Scholar]
- 24.Wang HC, Dragoo J, Zhou Q, Klein JR. An intrinsic thyrotropin-mediated pathway of TNF-alpha production by bone marrow cells. Blood. 2003;101:119–23. doi: 10.1182/blood-2002-02-0544. [DOI] [PubMed] [Google Scholar]
- 25.De la Vega JR, Vilaplana JC, Biro A, Hammond L, Bottazzo GF, Mirakian R. IL-10 expression in thyroid glands: protective or harmful role against thyroid autoimmunity? Clin Exp Immunol. 1998;113:126–35. doi: 10.1046/j.1365-2249.1998.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 27.Skapenko A, Niedobitek GU, Kalden JR, Lipsky PE, Schulze-Koops H. Generation and regulation of human Th1-biased immune responses in vivo: a critical role for IL-4 and IL-10. J Immunol. 2004;172:6427–34. doi: 10.4049/jimmunol.172.10.6427. [DOI] [PubMed] [Google Scholar]
- 28.Joosten LAB, Lubberts E, Durez P, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis − protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–60. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 29.Kasama T, Strieter RM, Lukacs NW, Lincoln PM, Burdick MD, Kunkel SL. Interleukin-10 expression and chemokine regulation during the evolution of murine type-II collagen-induced arthritis. J Clin Invest. 1995;95:2868–76. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–9. [PubMed] [Google Scholar]
- 31.Hitoshi Y, Yamaguchi N, Mita S, et al. Distribution of IL-5 receptor-positive B cells − expression of IL-5 receptor on Ly-1 (CD5)+ B cells. J Immunol. 1990;144:4218–25. [PubMed] [Google Scholar]
- 32.Tominaga A, Takaki S, Koyama N, et al. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin-5) develop eosinophilia and autoantibody production. J Exp Med. 1991;173:429–37. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirai T, Hirose S, Okada T, Nishimura H. CD5+ B cells in autoimmune disease and lymphoid malignancy. Clin Immunol Immunopathol. 1991;59:173–86. doi: 10.1016/0090-1229(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 34.Tian JD, Atkinson MA, ClareSalzler M, et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med. 1996;183:1561–7. doi: 10.1084/jem.183.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, Gran B, Costello K, Johnson K, Martin R, Dhib-Jalbut S. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Multiple Sclerosis. 2001;7:209–19. doi: 10.1177/135245850100700401. [DOI] [PubMed] [Google Scholar]
- 36.Jacquier-Sarlin MR, Gabert FM, Villiers MB, Colomb MG. Modulation of antigen processing and presentation by covalently linked complement C3b fragment. Immunology. 1995;84:164–70. [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang K, Chen Y, Xu CS, Jarvis JN. T cell activation by soluble C1q-bearing immune complexes: implications for the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 2003;131:61–7. doi: 10.1046/j.1365-2249.2003.02046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner C, Hansch GM, Stegmaier S, Denefleh B, Hug F, Schoels M. The complement receptor 3, CR3 (CD11b/CD18), on T lymphocytes: activation-dependent up-regulation and regulatory function. Eur J Immunol. 2001;31:1173–80. doi: 10.1002/1521-4141(200104)31:4<1173::aid-immu1173>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen CH, Antonsen S, Matthiesen SH, Leslie RGQ. The roles of complement receptors type 1 (CR1, CD35) and type 3 (CR3, CD11b/CD18) in the regulation of the immune complex-elicited respiratory burst of polymorphonuclear leukocytes in whole blood. Eur J Immunol. 1997;27:2914–9. doi: 10.1002/eji.1830271125. [DOI] [PubMed] [Google Scholar]