Abstract
The Mycobacterium tuberculosis phoP mutant strain SO2 has been shown previously to be more attenuated than Mycobacterium bovis bacillus Calmette–Guérin (BCG) and confers protective immunity against tuberculosis in mice and guinea pig models. In this study we have investigated the survival and immunological responses of Balb/c mice infected with the M. tuberculosis SO2 strain. All Balb/C mice survived intratracheal infection with M. tuberculosis SO2 strain under conditions where all the mice infected with the parental M. tuberculosis MT103 had died after 9 weeks. Infection of Balb/c mice with M. tuberculosis SO2 was associated with comparatively lower levels of interferon (IFN)-γ, interleukin (IL)-4 and tumour necrosis factor (TNF)-α and higher levels of inducible nitric oxide synthase (iNOS) during the late stage of infection, when compared with M. tuberculosis MT103 infection. The delayed-type hypersensitivity (DTH) response against M. tuberculosis culture filtrates was similar in mice infected with either the M. tuberculosis phoP SO2 strain or M. tuberculosis MT103. The protective efficacy of M. tuberculosis SO2 was compared with M. bovis BCG when delivered subcutaneously to groups of Balb/C mice. Following intratracheal challenge with M. tuberculosis H37Rv, protection was generated by 60 days post-challenge in mice vaccinated with either vaccine. At day 120 post-challenge the levels of protection were still significantly greater when compared with the non-vaccinated control group. The levels of protection conferred by vaccination with M. tuberculosis SO2 or with M. bovis BCG were similar, as measured by granuloma coalescence and pneumonia in addition to growth reduction of M. tuberculosis H37Rv.
Keywords: attenuated live vaccines, tuberculosis, two component system, virulence
Introduction
Tuberculosis is one of the leading causes of infectious disease mortality in the world. Options for dealing with the global burden of tuberculosis fall into two major categories, drug treatment and vaccination, both of which play critical roles in reducing dissemination of the disease. While these have been applied relatively successfully in the developed world, there are still serious problems in containing the transmission of infection in many under-developed countries. The directly observed treatment short course (DOTS) adopted by the WHO is highly effective at reducing prevalence of the disease in targeted regions [1]. However, it is expensive and requires a certain level of effective public health infrastructure and administration for optimal success. While effective as a therapeutic strategy, it does not address directly ongoing transmission of infection to susceptible individuals. It is in this domain that vaccination can play a key role in protecting susceptible individuals from infection. Currently, the Mycobacterium bovis bacille Calmette–Guérin (BCG) strain is the only vaccine used for the prevention of tuberculosis in humans. Developed originally following serial passage of a virulent M. bovis strain, it appears most effective against childhood forms of disease, but less so in preventing adult pulmonary tuberculosis [2]. Given the relatively poor efficacy of the BCG in a number of trials [3], major efforts are being undertaken worldwide to develop more effective vaccines preventing pulmonary tuberculosis. An attractive aspect of vaccine delivery is the potential for a high beneficial effect against relatively low costs of production and distribution to large populations. A combination of drug treatment regimens with efficacious prophylactic vaccines could therefore potentially go a long way to reduce incidence of the disease in tuberculosis endemic areas.
The use of animal models has helped to progress our understanding of the key host–pathogen responses that govern the generation of a host protective response. The immunological responses that follow infection critically determine the fate of the host. In the mouse model, as in many other hosts, the generation of immunity is predominantly mediated by a Th1 response in which the cooperative interactions of CD4/CD8 T cells producing key cytokines, such as interferon (IFN)-γ, help to activate macrophages to express anti-myocbacterial activity [4]. The desired outcome of an effective vaccine is to prime the host immune system to express enhanced T cell activity such that activated macrophages can limit multiplication of M. tuberculosis during the early stages of infection.
The development of rational attenuated mutants of M. tuberculosis offers the prospect of novel potential vaccine candidates against tuberculosis. By inactivating key genes involved in virulence, it allows for the possibility of using a live vaccine that can prime the appropriate protective immune response without generating the pathology associated with progression of disease. An advantage of the live vaccine is that many immunologically important genes are retained in the genome, unlike in M. bovis BCG substrains [5]. The two-component system phoP/R has been shown to play an essential role in M. tuberculosis virulence [6]. The M. tuberculosis phoP strain was constructed originally by a single gene disruption in the phoP gene, generating M. tuberculosis SO2 [6]. It has been shown that phoP is involved in the regulation of complex mycobacterial lipids implicated in the virulence of M. tuberculosis [7–9]. Recently we have shown that the M. tuberculosis SO2 is more attenuated than M. bovis BCG and confers equivalent protective immunity against tuberculosis in mice. In the guinea pig model it shows superior protection to M. bovis BCG against a high-dose virulent challenge [10]. In this study we have investigated the survival, the extent of tissue damage and the immunological responses of Balb/c mice infected by the intratracheal route with the attenuated M. tuberculosis SO2 and the virulent M. tuberculosis MT103 parental strain, using a well-characterized model of progressive pulmonary tuberculosis. We also compared the protective efficacy of the M. tuberculosis SO2 with M. bovis BCG-Phipps. The results demonstrated that M. tuberculosis SO2 is highly attenuated and induces strong protective immune responses without causing significant tissue damage. When used as a vaccine it confers a similar degree of protection against disease progression compared with BCG.
Materials and methods
Bacterial growth conditions
Mycobacterial cultures were grown routinely in Proskauer and Beck medium as described previously [11]. When required, kanamycin (20 µg/ml), was added to the growth medium. After 1 month of culture mycobacteria were harvested, adjusted to different bacterial counts in phosphate-buffered saline (PBS), aliquoted and maintained at −70°C until used. Before use, bacteria were recounted and their viability checked.
Infection of Balb/c mice
Animal work was performed with approval from the local Ethical Committee for Experimentation in Animals in Mexico. 8-week-old Balb/c mice (50 per group) were anaesthetized with 56 mg/kg intraperitoneal pentothal. The trachea was exposed via a small midline incision and 2·5 × 105 of viable bacteria of the wild-type parental M. tuberculosis MT103 or M. tuberculosis SO2 were injected after suspension in 100 µl PBS. The incision was then sutured with sterile silk and the mice were maintained in groups of five in cages fitted with microisolators [11]. In the follow-up study, a total 200 Balb/c mice were recruited for the experiment. The mice were euthanased by exsanguination at time-points of 1, 3, 7, 14, 21, 28, 60 and 120 days after intratracheal infection. Twenty infected animals per group were left untouched and during the experiment their deaths were recorded to construct survival curves. For delayed-type sensitivity (DTH) measurement, mice received an injection of 20 µg of culture filtrate antigen in 40 µl of PBS into the hind foot-pad. The footpad was measured before and 24 h after the antigen injection, as described previously [12].
Real time polymerase chain reaction (PCR) analysis of cytokines in lungs
Three mice were used for each time-point in the experimental groups. Each lung was placed in 2 ml of RPMI medium containing 0·5 mg/ml collagenase type 2 (Worthington, Lakewood, NJ, USA), and incubated for 1 h at 37°C. They were then passed through a 70-µm cell sieve, crushed with a syringe plunger and rinsed with the medium. Cells were centrifuged at 1500 r.p.m. (300 g) for 5 min and the supernatant and the residual red blood cells were removed following a wash with a lysis buffer. After washing the cells with the medium, they were counted; 350 µl of RLT buffer (Quiagen Inc., Valencia, USA) was added to 5 × 106 cells and treated with the RNeasy mini kit (Qiagen), according to the manufacturer's instructions. Reverse transcription of the mRNA was performed according to standard procedures using 5 µg RNA and 4 units omniscript reverse transcriptase (Qiagen, Inc.). Real-time PCR was performed using the 7500 real-time PCR system (Applied Biosystems, Branchburg, NJ, USA). Primers were designed for the following targets: glyceraldehyde-3-phosphate dehydrogenase: (G3PDH): 5′-CATTGTGGAAGGGCTCATGA-3′, 5′-GGAAGGCCATGCCAGTGAGC-3′, inducible nitric oxide synthase (iNOS): 5′-AGCGAGGAGCAGGTGGAAG-3′, 5′-CATTTCGCTGTCTCCCCAA-3′; tumour necrosis factor (TNF)-α: 5′-TGTGGCTTCGACCTCTACCTC-3′, 5′-GCCGAGAAAGGCTGCTTG-3′; interferon (IFN)-γ: 5′-GGTGACATGAAAATCCTGCAG-3′, 5′-CCTCAAACTTGGCAATACTCATGA-3′; and interleukin (IL)-4: 5′-CGTCCTCACAGCAACGGAGA-3′, 5′-GCAGCTTATCGATGAATCCAGG-3′. Cycling used conditions were: initial denaturation at 95°C for 15 min, followed by 40 cycles at 95°C for 20 s, 60°C for 20 s, 72°C for 34 section values are represented as ratios of mRNA copies cytokine per 106 copies of G3PDH).
Preparation of lung tissue for histology
Groups of eight mice in two different experiments were killed by exsanguination at 14, 21, 28, 60 and 120 days after intratracheal infection. For the histological studies, the lungs were perfused with 10% formaldehyde dissolved in PBS via the trachea, immersed for 24 h and embedded in paraffin. Sections 5 µm thick taken through the hilus were stained with haematoxylin and eosin. In these slides, the area (µm2) occupied by granuloma and the percentage of lung surface affected by pneumonia was determined using an automated image analyser (Q Win Leica, Milton Keynes, UK) [11].
Mycobacterial DNA detection by in situ PCR in lungs from M. tuberculosis SO2-infected mice
Lungs removed from mice after 4 months of infection with M. tuberculosis SO2 showed almost normal histology but relatively few colony-forming units (CFU), so we investigated the cellular localization of mutant bacteria using in situ PCR for detecting the insertion sequence IS6110 [4]. The same paraffin blocks used for the histopathological study were used in this experiment. Sections were mounted on silane-coated slides, deparaffinized for 18 h at 60°C and immersed sequentially in xylene (30 min at 37°C), absolute ethanol, 75%, 50%, 25% ethanol and water. Cells were rendered permeable by incubation for 10 min at room temperature in 0·02 M HCl. Proteins were then depleted by incubation with proteinase K 1 µg/ml (Gibco, Gaithersburg, MD, USA) for 30 min at 37°C. The proteinase was then inactivated by boiling in a microwave for 15 s and the section plunged immediately into 20% acetic acid for 15 s to inactivate endogenous alkaline phosphatase. The PCR was carried out by incubating the sections with 50 µl of 1 × reaction buffer (Gibco/BRL), 1·5 U Taq polymerase, 2 µM MgCl2, 40 µM dNTP and 0·2 M deoxyuridine triphosphate (dUTP) labelled with digoxigenin (Boehringer Mannheim, Mannheim, Germany) and 60 pg each of IS 6110 M. tuberculosis insertion sequence primers. The sequence of the primers was: forward 5′CCCT GCG AGC GTA GGC GTC GG3′ and reverse 5′CTC GTC CAG CGC CGC TTC GG-3′. The slides were sealed using the assembly tool (Applied Biosystems, Branchburg, NJ, USA) and placed in the thermocycler (Touch Down, Hybaid, Middlesex, UK). The M. tuberculosis DNA amplification started with denaturation at 94°C for 1 min, annealing at 70°C for 1 min and extension at 72°C for 1 min, for 35 cycles. PCR products were detected with sheep anti-digoxigenin antibodies coupled to alkaline phosphatase (Boehringer Mannheim) diluted 1/500. The chromogen was 5 bromo-4-chloro-3-3 indolyl phosphate toluidine salt (BCIP) and tetrazolium nitroblue (Boehringer Mannheim), diluted 1/50. Sections were counterstained with nuclear fast red to avoid any interference with the blue signal generated by mycobacterial DNA in the in situ PCR. Lung sections from Balb/c mice infected with M. tuberculosis MT103 wild-type mycobacteria were used as positive controls. The negative control consisted of performing the complete procedure with non-infected mouse lungs. A further control used the same slide as the test section of a duplicate section to which PCR mix was added without Taq, and another section added without primers.
Vaccination of Balb/c mice with M. tuberculosis SO2 or M. bovis BCG and intratracheal challenge with M. tuberculosis H37Rv
Twenty Balb/c mice per group in two different experiments were vaccinated subcutaneously in the base of the tail with 2·5 × 103 CFUs of either the attenuated M. tuberculosis SO2 or M. bovis BCG-Phipps [13]. At 60 days post-vaccination, all mice were challenged by the intratracheal route with 2·5 × 105 cfu of M. tuberculosis H37Rv. Ten mice were then killed at day 60 and another 10 animals at day 120 post-challenge. The levels of protection were determined by evaluating the numbers of viable M. tuberculosis H37Rv CFU recovered from the lungs at both time-points, the presence of histological lesions in the lung, the size of granulomas and the percentage of lung surface affected by pneumonia.
Statistical analysis
Statistical analysis was performed for survival curves using Kaplan–Meier plots and log-rank tests. A difference of P < 0·05 was considered significant. The CFU, cytokines and lesion consolidation were analysed by Student's t-test and analysis of variance (anova), using Fisher's pairwise comparisons to compare mean values of the groups.
Results
Survival and bacillary burden of Balb/c mice infected with M. tuberculosis SO2 and M. tuberculosis MT103
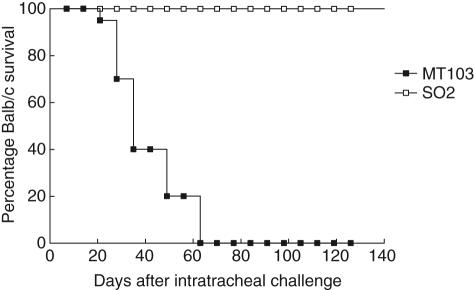
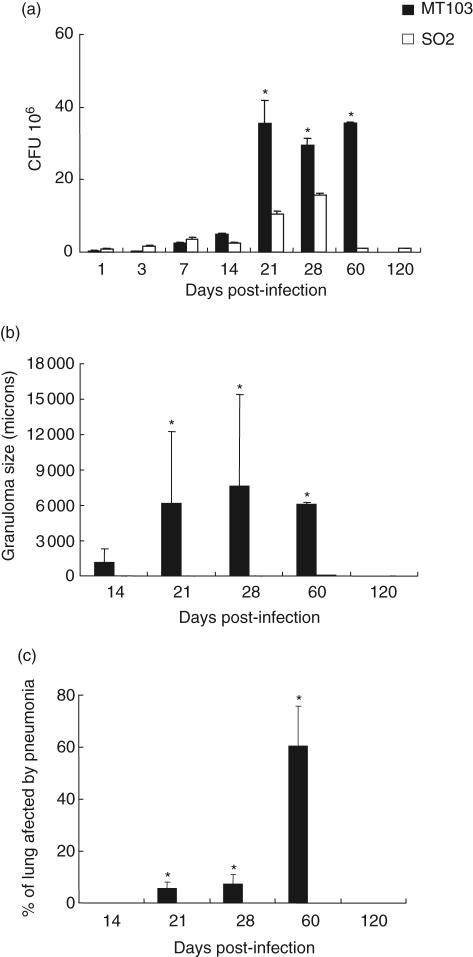
A total of 200 Balb/c mice were divided randomly into two groups; one group was infected with M. tuberculosis SO2 and the other with M. tuberculosis MT103, both by the intratracheal route. At time intervals of up to 120 days, eight mice were selected randomly from each treatment group and killed in order to assess the levels of infection and progression of disease. Mice infected with M. tuberculosis SO2 survived throughout the 120 days of the study. In contrast, mice infected with M. tuberculosis MT103 started to die at 21 days post-infection and all had died by 63 days (Fig. 1). During the first week of infection similar CFU numbers were seen in both groups. Between days 14 and 60 post-infection, significantly higher bacterial loads were recovered in mice infected with M. tuberculosis MT103 when compared with M. tuberculosis SO2 (Fig. 2a). A reduction of more than three orders of magnitude was found in CFU numbers cultured from the mice infected with the M. tuberculosis SO2 at day 60. The histological examination of lungs appeared to mirror the number of CFU in lung homogenates. Numerous isolated granulomas were observed in the mice infected with the M. tuberculosis MT103 strains as early as 14 days post-infection (Fig. 2b). By day 60 the granulomatous lesions had increased in size and coalesced to form consolidated areas of pneumonia throughout most of the lung (Fig. 2c). In contrast, lung histopathology following infection with the M. tuberculosis SO2 was limited; relatively few medium-sized granulomas were seen at day 60 post-infection, while at day 120 no granulomas were evident (Fig. 2b). Following infection with M. tuberculosis MT103, progressive pneumonia was produced, reaching a peak at day 60 when more than 60% of the lung surface was affected (Fig. 2c). In mice infected with M. tuberculosis SO2 there was no evidence of pneumonia, and only mild hyperplasia of the lymphoid tissue associated with bronchial mucosa was apparent at day 120 post-infection (Fig. 3).
Fig. 1.
Attenuation of Mycobacterium tuberculosis SO2 in Balb/c mice: survival of Balb/c mice infected with M. tuberculosis MT103 or the attenuated M. tuberculosis SO2 monitored over 120 days post-infection.
Fig. 2.
Lung bacillary burden and histomorphometry of Balb/c mice infected with Mycobacterium tuberculosis SO2 or MT103 parental strain by the intratracheal route. (a) Numbers of colony-forming units (CFUs) in the lungs of mice infected with either M. tuberculosis MT103 or M. tuberculosis SO2; (b) granuloma size (μ2) in M. tuberculosis MT103 or M. tuberculosis SO2-infected lungs; (c) percentage of lung surface area affected by pneumonia. Bars represent means ± s.d. from three mice per time-point. Asterisks represent statistical significance between groups at those time-points (P < 0·05).
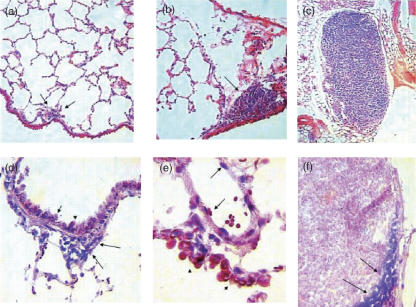
Fig. 3.
Representative histology and mycobacterial DNA (IS6110) detection by in situ polymerase chain reaction (PCR) in mice lung after intratracheal infection with Mycobacterium tuberculosis SO2. (a) Occasional and small granulomas (arrows) are seen after 60 days post-infection; (b) at 120 days post-infection, middle hyperplasia of lymphoid tissue associated with the bronchial wall (arrow) is the only distinctive histological abnormality; (c) large mediastinal lymph node with diffuse hyperplasia is generated after 120 days post-infection; (d) at 120 days post-infection, in situ PCR detection of mycobacterial DNA is positive (blue dots) in bronchial hyperplastic lymphoid tissue (arrows) and bronchial epithelium (arrow heads); (e) after 120 days of infection, mycobacterial DNA detection by in situ PCR also shows strong positivity in the venular endothelium (arrows) and bronchial epithelium (arrowheads); (f) positive PCR signal in macrophages located in the subcapsular sinus of mediastinal hyperplastic lymph node.
Samples of lung tissue were examined for the presence of lesions in mice killed during the course of the experiment. Numerous isolated granulomas were observed in the mice infected with the M. tuberculosis MT103 strains as early as 14 days post-infection. By day 60 the granulomatous lesions had increased in size and coalesced to form consolidated areas of pneumonia throughout most of the lung (data not shown). In contrast, lung histopathology following infection with the M. tuberculosis SO2 strain was very limited; only very few medium-sized granulomas were seen at day 60 post-infection (Fig. 3a), while at day 120 no granulomas were evident (Fig. 3b) and only diffuse hyperplasia of the mediastinal lymph nodes was seen (Fig. 3c). Progressive pneumonia was produced after infection with M. tuberculosis MT103, reaching a peak at day 60 when more than 60% of the lung surface was affected (data not shown). In mice infected with the M. tuberculosis SO2 strain there was no evidence of pneumonia, but mild hyperplasia of the lymphoid tissue associated with bronchial mucosa was apparent at day 120 post-infection (Fig. 3c). In these infected lungs the in situ PCR for detection of IS6110 was positive in bronchial epithelial cells, vascular endothelium and macrophages located in peribronchial hyperplastic lymphoid tissue, indicating the presence of M. tuberculosis SO2 at these sites (Fig. 3e,f).
Immunological responses of Balb/c mice infected with M. tuberculosis MT103 and M. tuberculosis SO2
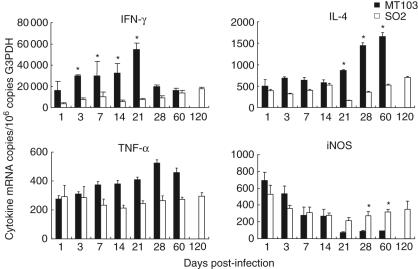
The expression of cytokines IFN-γ, IL-4 and TNF-α were determined by real-time PCR in the lungs of mice infected with the M. tuberculosis MT103 and the M. tuberculosis SO2 strain. Consistent with the extensive lung inflammation produced by the M. tuberculosis MT103 strain, the expression of IFN-γ, IL-4 and TNF-α was higher in lungs from animals infected with M. tuberculosis MT103 than in lungs from mice infected with M. tuberculosis SO2 (Fig. 4). Infection with M. tuberculosis MT103 induced a progressive increase in IFN-γ mRNA, peaking at day 21, followed by a decrease at days 28 and 60. In contrast, infection with M. tuberculosis SO2 induced a smaller increase in IFN-γ expression, peaking at day 120. The M. tuberculosis MT103 strain induced stable expression of IL-4 from days 1–21 post-infection. After this time-point, the levels of IL-4 increased 50% at day 28 and 100% at day 60. In contrast, M. tuberculosis SO2 induced lower IL-4 expression throughout the duration of the study (Fig. 4). The M. tuberculosis MT103 induced progressive increase in TNF-α expression, peaking at day 28, followed by a small decrease at day 60, whereas the M. tuberculosis SO2 induced stable TNF-α expression during the 4 months of study. The levels of iNOS were also determined in lung extracts. In mice infected with the M. tuberculosis MT103 and M. tuberculosis SO2, the expression of iNOS was rapid and peaked early in infection, declining thereafter until day 21, where the levels remained consistent throughout the remainder of the study (Fig. 4). In these later stages of infection, the iNOS levels generated in response to the M. tuberculosis SO2 strain infection were significantly greater than those measured in response to M. tuberculosis MT103.
Fig. 4.
Immunological responses of Balb/C mice infected with Mycobacterium tuberculosis MT103 and M. tuberculosis SO2: mean ± s.d. values of quantitative expression of mRNA encoding cytokines and nitric oxide synthase (iNOS) determined by real-time polymerase chain reaction (PCR) in lung homogenates from three mice per group infected with either M. tuberculosis MT103 or M. tuberculosis SO2. Asterisks represent statistical significance between groups at those time-points (P < 0·05).
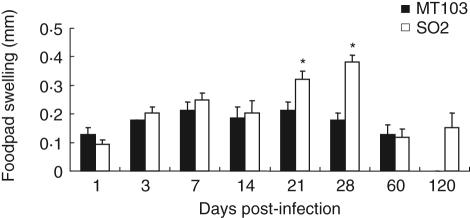
Prior to euthanasia, the DTH response to culture filtrate proteins of M. tuberculosis H37Rv was measured. The only significant differences in DTH responses between both treatment groups were measured at days 21 and 28 post-infection when a significant increase (P < 0·05) in skin induration was observed in the mice infected with M. tuberculosis SO2 strain compared to the mice infected with M. tuberculosis MT103 (Fig. 5). By day 60 the DTH responses had decreased in both infected groups. At day 120 post-infection the DTH response was maintained in the mice infected with M. tuberculosis SO2.
Fig. 5.
Delayed-type hypersensitivity response of Mycobacterium tuberculosis MT103 and M. tuberculosis SO2 in Balb/c mice: delayed-type hypersensitivity response measured in mouse footpads following injection of mycobacterial antigens. Bars represent means ± s.d. from three mice per time-point in two different experiments. Asterisks represent statistical significance between groups at those time-points (P < 0·05).
Protective immunity against M. tuberculosis H37Rv intratracheal challenge of Balb/c mice vaccinated with M. tuberculosis SO2 or M. bovis BCG
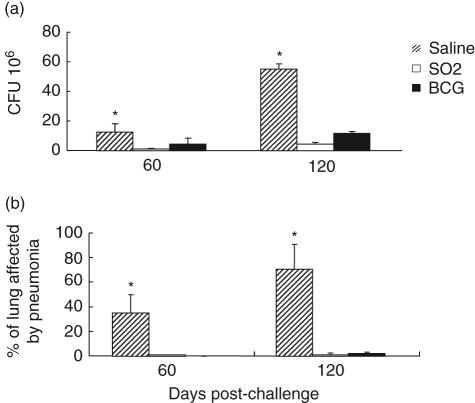
The vaccine efficacy of the M. tuberculosis SO2 was compared in groups of Balb/c mice vaccinated subcutaneously with 2·5 × 103 CFU of either the M. tuberculosis SO2 strain or M. bovis BCG-Phipps. At 60 days post-vaccination, all mice were challenged with M. tuberculosis H37Rv by the intratracheal route, and the protective response was assessed by evaluating the numbers of viable M. tuberculosis H37Rv recovered from the lungs and histopathology at days 60 and 120 post-challenge. Mice vaccinated with the M. tuberculosis SO2 or M. bovis BCG had significantly reduced numbers of viable M. tuberculosis in lungs compared with saline-treated control mice at days 60 and 120 (Fig. 6a). However, there were no statistically significant differences in M. tuberculosis CFUs in mice vaccinated with M. tuberculosis SO2 strain or M. bovis BCG (P > 0·05).
Fig. 6.
Protective efficacy of Mycobacterium tuberculosis SO2 and BCG-Phipps in Balb/c mice: (a) numbers of colony-forming units (CFUs) of M. tuberculosis H37Rv recovered from lungs of challenged mice that were vaccinated with either M. tuberculosis SO2 or M. bovis bacille Calmette–Guérin (BCG), compared with saline-treated controls; (b) percentage of pneumonia in Balb/c mice vaccinated subcutaneously with either M. tuberculosis SO2 or M. bovis BCG at 60 and 120 days following intratracheal challenge with M. tuberculosis H37Rv. Asterisks denotes where results from saline-treated mice controls are significantly different (P < 0·05) from mice receiving either vaccine.
Samples of lung tissue were examined for the presence of lesions in each of the mice vaccinated with M. tuberculosis SO2 or M. bovis BCG and in the control group. Numerous granulomas were observed in control mice infected with the M. tuberculosis H37Rv strain. By days 60 and 120 the granulomatous lesions had increased in number and size and coalesced to form generalized necrotizing pneumonia throughout most of the lung (mean approximately 70% at day 120). In contrast, after 60 days, animals vaccinated with M. tuberculosis SO2 and M. bovis BCG showed less than 2% of pneumonia (Fig. 6b). At day 120 post-infection, where disease was progressive in non-vaccinated mice, only 1·1% of pneumonia was detected in mice vaccinated with M. tuberculosis SO2 strain, which was similar although less than that observed in animals vaccinated with M. bovis BCG (2·3%).
Discussion
In this study, we used M. tuberculosis SO2 as a prototype vaccine and showed that, in addition to being highly attenuated, it elicited high levels of protection in Balb/c mice. The phoP gene is an integral part of a two-component regulatory system that responds to environmental changes by altering transcription patterns of other genes, including virulence factors [6]. In M. tuberculosis the inactivation of phoP transcription factor causes changes in the shape and size of the bacteria as well as the morphology of the colonies when compared with the wild-type parental M. tuberculosis MT103 strain, indicating that PhoP is a global regulator of many different genes [6]. The M. tuberculosis SO2 also exhibits reduced bacillary multiplication in mouse macrophages and in vivo using the mouse intravenous-infection model, suggesting an essential role of phoP regulating M. tuberculosis virulence genes [6].
The survival and immunogenicity of the M. tuberculosis SO2 and M. tuberculosis MT103 was compared in a well-characterized model of progressive pulmonary tuberculosis in immunocompetent Balb/c mice [14]. By studying the rates of bacterial multiplication in the lungs and the extent of tissue damage (pneumonia) and mortality, this mouse model is effective for comparative studies on virulence and immune responses induced by different mycobacterial strains [13]. Our results indicated a strong attenuation of M. tuberculosis SO2 strain as all Balb/C mice survived intratracheal infection with this strain in comparison with mice infected with the parental M. tuberculosis MT103. This was associated with a reduction in orders of magnitude of the bacterial burden in the lungs of the mice infected with M. tuberculosis SO2 strain. Although perivascular and peribronchial inflammation was evident from the first day of infection, only few and small granuloma were seen at day 60 post-infection, and no pneumonic lesions were observed in the M. tuberculosis SO2-infected animals. In a previous study we reported that the M. tuberculosis SO2 was highly attenuated in the SCID mouse model when delivered by the aerosol or intravenous route [10]. We also demonstrated that the M. tuberculosis SO2 strain was more attenuated than BCG in the SCID mouse model, even when delivered at a 10-fold higher dose. This is particularly relevant in the context of developing a superior vaccine to BCG for use in immunocompromised patients, where complications associated with BCG vaccination have been reported [15]. A more attenuated vaccine with enhanced safety may contribute to resolving this problem. The results of our studies demonstrate that the M. tuberculosis SO2 strain shows superior safety in immunocompetent and immunocompromised mice when delivered by multiple routes.
The immunological response to infection with the M. tuberculosis SO2 strain was accompanied by less severe histological lesions, and by longer-lasting cellular immune stimulation when compared with M. tuberculosis MT103 infection. Immunity to tuberculosis is generally mediated through a Th1 response involving the secretion of IFN-γ by sensitized lymphocytes, activation of macrophages and concomitant expression of iNOS and release of TNF-α [4]. There is evidence that the pattern of cytokines produced in the lung during tuberculosis infection changes over time and correlates with the type and magnitude of tissue injury. In murine models, specifically in Balb/c mice infected by the intratracheal route with a high-challenge dose, the progression to the disease state is associated with an early post-exposure release of IFN-γ and later by superimposed release of the Th2 cytokine, IL-4 [4,12]. The severity of the disease correlates strongly with IL-4 working in concert with TNF-α which elicits strong inflammatory activity resulting in exacerbated tissue damage [16]. In this study, the kinetics of cytokine expression in Balb/c mice were broadly similar after infection with both strains. However, the levels of expression of IFN-γ, IL-4 and TNF-α were generally higher in the lungs of mice infected with the M. tuberculosis MT103 strain. It was also noted that the M. tuberculosis MT103 infection produced progressive disease with more inflammation than did the M. tuberculosis SO2. The M. tuberculosis MT103 evoked a rapid increase in IFN-γ expression during the second and third weeks of infection, while IL-4 expression was low. This phase was followed by a progressive decrease in IFN-γ expression and a twofold increase in IL-4 expression, associated with high CFU counts and extensive pneumonia. Thus, it appears that bacilli proliferation was partially controlled while a Th1 response was maintained. In contrast, infection with the M. tuberculosis SO2 induced a lower but progressive increase in IFN-γ expression, peaking at day 120 post-infection, together with few CFU, lower and stable IL-4 expression and higher DTH responses after 21 days post-infection, with almost normal histology. The detection of elevated iNOS transcriptional activity can be seen as an indicator of IFN-γ-induced activation of macrophage effector function [17]. The production of nitric oxide has been implicated in growth inhibition of mycobacteria inside macrophages and iNOS gene expression can also be down-regulated by IL-4 [18]. The expression of iNOS in the M. tuberculosis MT103-infected mice was consistent with this pathway of regulation. Relatively high IFN-γ and low IL-4 mRNAs were associated with high iNOS expression early in infection and vice versa as disease progressed. Apart from very early post-infection, the levels of iNOS expression were consistently higher in the M. tuberculosis SO2-infected mice. This was associated with less inflammation and tissue damage facilitating persistence of the M. tuberculosis SO2 strain at low levels, as demonstrated by long survival, low CFUs and in-situ PCR IS6110 detection in macrophages located in the hyperplastic lymphoid tissue associated to bronchi and mediastinal lymph nodes, as well as in non-professional phagocytic cells such as bronchial epithelium and endothelial cells. However, without directly measuring an effect of NO production, the expression of iNOS may not be a reliable indicator of a specific NO-dependent regulation of intracellular growth of M. tuberculosis SO2.
In another study, where the M. tuberculosis H37Rv strain was delivered to C57BL/6 mice by the aerosol route (2 × 102 CFU), most of the same Th1 cytokine gene expression patterns were investigated [19]. However, unlike the present study, the cytokine mRNA levels were sustained until the end of the experiment (day 50 post-infection). Both studies examined the response to infection in the lung, the only organ in the mouse that develops progressive disease following tuberculosis infection [20]. The difference in cytokine expression patterns over the course of the studies may reflect the different inoculum doses used (2·5 × 105 CFUs in this study) or the genetic background of the mouse strains [21]. Apart from two successive time-points, the DTH responses did not differ significantly between mice infected with M. tuberculosis SO2 or M. tuberculosis MT103. Previous studies have shown that the DTH responses of Balb/C mice vaccinated with different BCG strains did not correlate with progression of histological lesions or with levels of protective immunity [13]. However, it was noted in this study that at the two time-points (21 and 28 days post-infection) where significant differences were recorded, this was associated with the greatest difference in CFUs recovered from the lungs of both groups of mice.
When the M. tuberculosis SO2 strain was compared with M. bovis BCG in a vaccine study, similar levels of protection were generated in Balb/c mice challenged with H37Rv by the intratracheal route. The M. bovis BCG substrain Phipps was used because this particular substrain has been shown to confer the highest levels of protection when compared against 10 other different BCG substrains in the Balb/c model of progressive pulmonary tuberculosis [13]. The pathological changes in the vaccinated mice indicated that both vaccines did not prevent establishment of infection (granuloma formation), but both protected against disease progression (pneumonia). Lung tissue was examined for the presence of lesions in each of the mice vaccinated with M. tuberculosis SO2 or BCG. The lung histopathology was similar in mice vaccinated with either M. tuberculosis SO2 or BCG, but in both cases was significantly less when compared with the control non-vaccinated animals. It was interesting to note that levels of pneumonia and bacillary load increased between 60 and 120 days in both the controls and vaccinated groups of mice, and that the differences were more pronounced at the latter time-point. This suggests that both vaccines, while not preventing infection, were better able to control bacterial multiplication at the later stage of infection. In a previous study we showed that M. tuberculosis SO2 was more protective than BCG when both were compared in the guinea pig model [10]. The variation in protective efficacy between the mouse and guinea pig model may reflect fundamental differences in how each species controls the disease. As a model system, mice are considered resistant to tuberculosis and generate strong cellular immune responses to infection. This results in protective responses that limit the establishment of infection to the lungs. In contrast, although guinea pigs also develop strong immune responses, the disease becomes progressive and can cause significant tissue damage. This makes the guinea pig model relatively easier to measure subtle differences between vaccine candidates that afford immune protection to tuberculosis [22].
Other studies have also compared the vaccine efficacy of attenuated mutant strains of M. tuberculosis [23–26]. A purine auxotroph of M. tuberculosis has been constructed that was unable to grow in macrophages and was also attenuated for growth in mice [25]. However, vaccination of immunocompetent mice with the M. tuberculosis leucine mutant did not significantly restrict the growth of the virulent challenge organism in the lungs or spleen when compared to the control mice vaccinated with BCG [24]. In other studies, attenuated M. tuberculosis proline and tryptophan auxotrophs conferred protection against intravenous challenge with M. tuberculosis that was comparable to that afforded by vaccination with BCG when measured by the mean survival times [23]. Two intravenous vaccine doses of a lysine auxotrophic mutant of M. tuberculosis H37Rv were required to generate protection equivalent to that of the BCG vaccine against aerosol challenge of mice [12]. It is probable that much of the variabilty in protective efficacy encountered in these studies were attributable to the parental M. tuberculosis strains used to construct the attenuated vaccine candidates as well as the different challenge organisms and delivery routes used. It was noted in this study that the mice inoculated with M. tuberculosis MT103 succumbed to infection and died earlier than mice inoculated with M. tuberculosis H37Rv. This may reflect different virulence properties of either strain in this mouse model, but does serve to highlight the high degree of attenuation associated with the phoP mutation. When combined with the levels of protection achieved in the Balb/C mice and guinea pigs [10], it suggests that vaccines based on M. tuberculosis phoP mutation may have potential to progress to trials on vaccine efficacy in other animal models such as primates and in humans. A vaccine that reduces infection in the lung and reduces dissemination to other organs is likely to have a significant impact on control of tuberculosis.
Acknowledgments
This work was supported by CONACyT (grant G36923-M), the Spanish MEC (BIO2005-07949) CIBER CB06/06/0020 and the EU FP6 TB-VAC Project (LSHP-CT2003-503367). We thank Alberto Cebollada for the statistical analyses of data figure preparation.
References
- 1.World Health Organization (WHO) Global Report tuberculosis. Geneva: WHO; 2005. Global tuberculosis control - surveillance, planning, financing. [Google Scholar]
- 2.Young D, Dye C. The development and impact of tuberculosis vaccines. Cell. 2006;124:683–7. doi: 10.1016/j.cell.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Pando R, Orozco H, Sampieri A, et al. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology. 1996;89:26–33. [PMC free article] [PubMed] [Google Scholar]
- 5.Behr MA. BCG − different strains, different vaccines? Lancet Infect Dis. 2002;2:86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 6.Perez E, Samper S, Bordas Y, et al. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol. 2001;41:179–87. doi: 10.1046/j.1365-2958.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 7.Ludwiczak P, Gilleron M, Bordat Y, et al. Mycobacterium tuberculosis phoP mutant: lipoarabinomannan molecular structure. Microbiology. 2002;148:3029–37. doi: 10.1099/00221287-148-10-3029. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalo Asensio J, Maia C, Ferrer N, et al. The virulence-associated two-component PhoP–PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem. 2006;281:1313–16. doi: 10.1074/jbc.C500388200. [DOI] [PubMed] [Google Scholar]
- 9.Walters SB, Dubnau E, Kolesnikova I, et al. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol. 2006;60:312–30. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin C, Williams A, Hernandez-Pando R, et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006;24:3408–19. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Arriaga AK, Orozco EH, Aguilar LD, et al. Immunological and pathological comparative analysis between experimental latent tuberculous infection and progressive pulmonary tuberculosis. Clin Exp Immunol. 2002;128:229–37. doi: 10.1046/j.1365-2249.2002.01832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rook GA, Hernandez-Pando R, Dheda K, et al. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25:483–8. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Castillo-Rodal AI, Castanon-Arreola M, Hernandez-Pando R, et al. Mycobacterium bovis BCG substrains confer different levels of protection against M. tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect Immun. 2006;74:1718–24. doi: 10.1128/IAI.74.3.1718-1724.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez B, Aguilar D, Orozco H, et al. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol. 2003;133:30–7. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesseling AC, Rabie H, Marais BJ, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Clin Infect Dis. 2006;42:548–58. doi: 10.1086/499953. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Pando R, Aguilar D, Hernandez ML, et al. Pulmonary tuberculosis in BALB/c mice with non-functional IL-4 genes: changes in the inflammatory effects of TNF-alpha and in the regulation of fibrosis. Eur J Immunol. 2004;34:174–83. doi: 10.1002/eji.200324253. [DOI] [PubMed] [Google Scholar]
- 17.Ehrt S, Schnappinger D, Bekiranov S, et al. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194:1123–40. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdan C, Vodovotz Y, Paik J, Xie QW, et al. Mechanism of suppression of nitric oxide synthase expression by interleukin-4 in primary mouse macrophages. J Leukoc Biol. 1994;55:227–33. doi: 10.1002/jlb.55.2.227. [DOI] [PubMed] [Google Scholar]
- 19.Jung YJ, Ryan L, LaCourse R, et al. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201:1915–24. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 21.Medina E, North RJ. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology. 1999;96:16–21. doi: 10.1046/j.1365-2567.1999.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldwin SL, D'Souza C, Roberts AD, et al. Bacille Calmette–Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Infect Immun. 1998;66:2951–9. [Google Scholar]
- 23.Smith DA, Parish T, Stoker NG, et al. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun. 2001;69:1142–50. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hondalus MK, Bardarov S, Russell R, et al. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2000;68:2888–98. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson M, Phalen SW, Lagranderie M, et al. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect Immun. 1999;67:2867–73. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavelka MS, Jr, Chen B, Kelley CL, Collins FM, et al. Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2003;71:4190–2. doi: 10.1128/IAI.71.7.4190-4192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]