Abstract
Naturally occurring CD4+ CD25+ regulatory T cells (nTreg) play a major role in controlling autoimmunity by suppressing self-reactive T cells. Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS), where T cells play a key role in orchestrating tissue damage. While CD4+ CD25+ nTreg have been investigated in peripheral blood from MS patients, little is known about their presence and possible function within the target organ, the CNS. In order to study whether these cells are present in the cerebrospinal fluid (CSF) under pathological conditions, we have analysed the frequency of CD4+ CD25+ nTreg in peripheral blood and CSF from MS patients (n = 14), patients with other neurological disorders (OND; n = 9) and compared peripheral levels with healthy controls (n = 40). We found that the frequency of CD4+ CD25+ forkhead transcription factor 3 (FOXP3)+ nTreg was significantly elevated in the CSF from MS patients (mean CSF = 4·05 ± 1·54% versus mean peripheral blood = 2·93 ± 0·94%) but not from patients with other neurological disorders (mean CSF = 3·78 ± 1·26% versus mean peripheral blood = 3·74 ± 1·4%). The frequency of nTreg in the periphery did not differ between MS patients and healthy donors; however, nTreg from MS patients showed reduced suppressive capacity.
Keywords: CSF, multiple sclerosis, regulatory T cells
Introduction
Autoimmune diseases result from the immune system's failure to maintain tolerance to self-structures. In multiple sclerosis (MS), the prototype autoimmune inflammatory disorder of the central nervous system, myelin-reactive T helper cells play a pivotal role in orchestrating self-reactive immune responses [1]. Peripheral regulatory mechanisms are necessary to control autoreactive cells which have escaped thymic tolerance. CD4+ CD25+ nT regulatory cells (nTreg) are critical players, exerting their potential via active suppression. Depletion of CD4+ CD25+ nTreg in mice results in autoimmune disease and the transfer of these cells, together with CD4+ CD25– T cells, can prevent autoimmunity in experimental models [2]. Although there is not yet a specific surface marker for this T cell subset, CD4 CD25+ nTreg cells can be characterized on the basis of their high expression of CD25 (in contrast to the intermediate expression in recently activated T cells), their memory phenotype (CD45RO) and the intracellular expression of the forkhead transcription factor 3 (FOXP3) [3].
As opposed to animal models, it is still unclear how nTreg contribute to the development of human autoimmune disease. Autoimmune diabetes was the first example where decreased frequency of peripheral nTreg was reported as a potential indicator for the impaired state of peripheral tolerance to islet antigens [4]. However, available data on the frequency of nTreg in the peripheral blood of MS patients has so far not revealed significant differences [5–7]. At first glance this is not surprising, given the fact that peripheral blood may only reflect insufficiently the pathological situation of the disseminating inflammation within the central nervous system (CNS). Because direct investigation of brain tissue in MS patients is difficult, cerebrospinal fluid (CSF) seems to be the best alternative to study the immune elements involved in CNS pathology. We thus addressed this issue by measuring the frequency of CD4+ CD25+ nTreg in blood and CSF of MS patients in comparison to healthy donors (HD) and patients with other neurological diseases (OND).
Materials and methods
Patients, CSF and blood specimens
Patients were diagnosed according to the criteria of McDonald et al. [8] and classified as clinically isolated syndrome (CIS), relapsing remitting MS (RRMS), secondary progressive MS (SPMS) and primary progressive MS (PPMS). All patients from whom CSF was analysed had a clinically isolated syndrome or relapsing remitting disease and were without corticosteroids for at least 2 months. CSF controls included patients with other neurological diseases of non-autoimmune origin such as dementia, normal pressure hydrocephalus and stroke (Table 1). All patients gave informed consent according to a protocol approved by the local ethics committee of the University of Tübingen. Eight to 20 ml of CSF were obtained by lumbar puncture from the patients. At the same time peripheral blood was collected by venous puncture. Forty age- and sex-matched healthy donors were used as controls for the analysis of peripheral blood.
Table 1.
Characteristics of multiple sclerosis (MS) patients and cerebrospinal fluid (CSF) controls.
| No. | Sex | Age (years) | Diagnosis/classification | Acute disease | CSF analysed |
|---|---|---|---|---|---|
| HD1 | F | 24 | Healthy donor | ||
| HD2 | M | 39 | Healthy donor | ||
| HD3 | M | 66 | Healthy donor | ||
| MS1 | F | 26 | CIS | Yes | No |
| MS2 | M | 34 | RRMS | Yes | No |
| MS3 | M | 60 | SPMS | Yes | No |
| MS4 | M | 42 | RRMS | No | Yes |
| MS5 | M | 37 | RRMS | No | Yes |
| MS6 | M | 38 | RRMS | yes | Yes |
| MS7 | F | 40 | CIS | Yes | Yes |
| MS8 | M | 37 | CIS | Yes | Yes |
| MS9 | M | 41 | CIS | No | Yes |
| MS10 | F | 54 | SPMS | Yes | Yes |
| MS11 | M | 50 | RRMS | Yes | Yes |
| MS12 | F | 39 | RRMS | Yes | Yes |
| MS13 | F | 28 | RRMS | No | Yes |
| MS14 | F | 42 | RRMS | Yes | Yes |
| MS15 | M | 44 | CIS | No | Yes |
| MS16 | F | 38 | RRMS | No | Yes |
| MS17 | F | 37 | RRMS | No | Yes |
| MS18 | F | 36 | RRMS | No | No |
| MS19 | F | 47 | RRMS | No | No |
| MS20 | M | 61 | SPMS | No | No |
| MS21 | F | 35 | SPMS | No | No |
| MS22 | M | 44 | CIS | Yes | No |
| MS23 | M | 36 | SPMS | No | No |
| MS24 | F | 25 | RRMS | No | No |
| MS25 | F | 31 | RRMS | No | No |
| MS26 | F | 33 | RRMS | No | No |
| MS27 | F | 35 | RRMS | No | No |
| MS28 | F | 37 | RRMS | Yes | No |
| MS29 | F | 40 | RRMS | No | No |
| MS30 | F | 42 | RRMS | No | No |
| MS31 | F | 50 | RRMS | No | No |
| MS32 | F | 55 | RRMS | No | No |
| MS33 | F | 49 | RRMS | Yes | No |
| MS34 | M | 21 | RRMS | No | No |
| MS35 | M | 25 | RRMS | No | No |
| MS36 | M | 26 | SPMS | Yes | No |
| MS37 | M | 62 | PPMS | No | No |
| MS38 | M | 38 | RRMS | No | No |
| MS39 | M | 47 | RRMS | Yes | No |
| OND1 | W | 24 | Stroke | Yes | |
| OND2 | M | 24 | Disturbance eye movements | Yes | |
| OND3 | W | 33 | Stroke | Yes | |
| OND4 | M | 56 | Polyneuropathy | Yes | |
| OND5 | M | 80 | Normal pressure hydrocephalus | Yes | |
| OND6 | M | 79 | Dementia | Yes | |
| OND7 | W | 66 | Dementia | Yes | |
| OND8 | M | 76 | Dementia | Yes | |
| OND9 | M | 74 | Normal pressure hydrocephalus | Yes | |
| OND10 | W | 24 | Myelopathy | Yes |
CIS: clinically isolated syndrome; OND: other neurological diseases; PPMS: primary progressive MS; RRMS: relapsing remitting MS, SPMS: secondary progressive MS.
Flow cytometry analysis of peripheral blood and CSF: quantitative analysis of CD4+ CD25bright nTreg
The percentage of CD4+ CD45RO+ cells expressing high levels of CD25 was quantified by flow cytometry on a fluorescence activated cell sorter (FACS) Calibur cytometer (BD Biosciences, Heidelberg, Germany). After lysis of the erythrocytes, 100 µl of peripheral blood were incubated with anti CD4-peridinin-chlorophyll-protein complex (PerCP), anti-CD45RO-fluorescein isothiocyanate (FITC), anti-CD25-phycoerythrin (PE) (BD Biosciences) or with the relevant isotype controls. CSF was processed at 4°C immediately after spinal tap. Cells were centrifuged after collection and resuspended in phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS) before staining with the corresponding antibodies. A minimum of 6000 cells per staining was required, with at least 1000 events measured.
For analysis, a first gate on a forward scatter/CD4-PerCP dot plot was set up to collect all CD4+ cells excluding the CD4low monocytes. A second dot plot (CD45RO-FITC/CD25-PE) gated on the CD4+ cells selected previously allowed us to visualize the different levels of expression of CD25 in the CD4-positive memory cells (CD45RO+). A quadrant was set on this second dot plot such that the percentage of CD25bright cells in the CD45RO-negative fraction (upper left quadrant) always contained 0·1% of CD25bright cells. The percentage of CD45RO+ CD25bright (upper right quadrant) was given as the frequency of CD4+ CD25+ nTreg in blood or CSF.
Expression of FOXP3
Expression of FOXP3 was assessed by intracellular staining according to the manufacturer's protocol (eBioscience, San Diego, CA, USA). Briefly, cells were first surface-stained with CD25-allophycocyanin (APC) (BD Biosciences) and CD4-Pacific blue (Dako, Hamburg, Germany). After washing, cells were resuspended in fixation/permeabilization buffer, incubated for 30 min at room temperature (RT) in the dark and washed. Next, cells were blocked with 2% normal rat serum and subsequently the FOXP3 antibody (PCH101) or the isotype control (rat IgG2a) was added for 30 min incubation at 4 °C. After washing, cells were analysed with the Cyan, using the Summit software (Dako Cytomation).
Isolation of nTreg
nTreg cells were isolated according to the manufacturer's protocol (CD4+ CD25+ regulatory T cell isolation kit, Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, CD4 T cells were isolated by negative selection, incubated subsequently with CD25 microbeads and enriched by positive selection. To ensure highest purity, CD4+ CD25+ T cells were run over a second column. In all cases purity was > 92%.
Suppression assay for nTreg
Lymphocyte proliferation of responder T cells (CD4+ CD25– T cells) was assessed in the presence of nTreg. Briefly, 1 × 106 CD4+ CD25– responder T cells were incubated in 500 µl of PBS containing 10 µm carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE; Molecular Probes, Karlsruhe, Germany) and blocked subsequently with medium containing 15% FCS. To assess the suppressive nature of nTreg, CFDA-SE-labelled responder T cells (1 × 105 cells per well) were cultured in the presence of increasing numbers of nTreg, irradiated allogeneic PBMCs and 1 µg/ml soluble anti-CD3 monoclonal antibody (OKT3). We measured proliferation on the fourth day of culture by flow cytometry. Suppression was calculated after normalization of the values to a maximum given by the proliferation of responder T cells in the absence of nTreg.
Statistical analysis
Paired and unpaired t-tests were used to compare the frequency of CD25bright cells in (i) in peripheral blood CD4+ T cells from patients with MS (including subgroups) and healthy donors, and (ii) between CSF and blood from MS and OND patients. In all cases the P-values were calculated two-tailed and considered statistically significant if P < 0·05.
Results
MS patients and healthy donors have similar frequencies of nTreg in peripheral blood
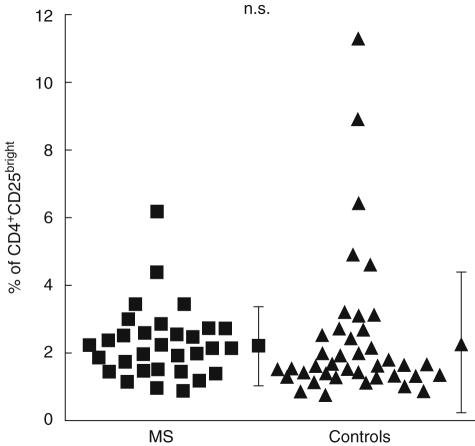
Thirty-six patients with MS (CIS, n = 5; RRMS, n = 25; SPMS, n = 5; PPMS, n = 1; ages 21–62, mean = 40) and 40 age- and sex-matched healthy controls (ages 23–80, mean = 40) were analysed for the frequency of CD4+ CD25bright cells in peripheral blood. In agreement with previous data [5–7], no significant differences were found in the frequency of nTreg between both groups. The average percentage of CD25bright cells in the CD4 compartment was 2·13 ± 1·19% for MS patients and 2·28 ± 2·18% for healthy donors (P = 0·71) (Fig. 1).
Fig. 1.
Comparison of nTreg frequencies in peripheral blood from multiple sclerosis (MS) patients and healthy donors. Percentages of CD4+ CD25bright T cells in peripheral blood of patients with MS (n = 36; black squares) in comparison to healthy controls (n = 40; black triangles) were assessed by flow cytometry after staining for CD4, CD45RO and CD25. Gates for CD4 cells were set on FSC/CD4 and dot plots to identify the desired subsets. A quadrant was set on a second dot plot (CD45RO/CD25), such that the percentage of CD25bright cells in the CD45RO-negative fraction (upper left quadrant) always contained 0·1% of CD25bright cells. Percentages of CD45RO+ CD25bright T cells (upper right quadrant) were given as the frequency of CD4+ CD25+ nTreg. The mean ± s.d. is shown for each group. The statistical difference is non-significant (n.s.).
Furthermore, no significant differences were observed (i) in relation to disease subtype (CIS, RRMS, SPMS or PPMS) or (ii) disease activity (acute relapse versus stable disease) (data not shown). Similarly, patients with OND (n = 9) did not differ in their peripheral blood nTreg from MS or HD (data not shown).
Suppressive function of CD4+ CD25+ nTreg is impaired in MS patients
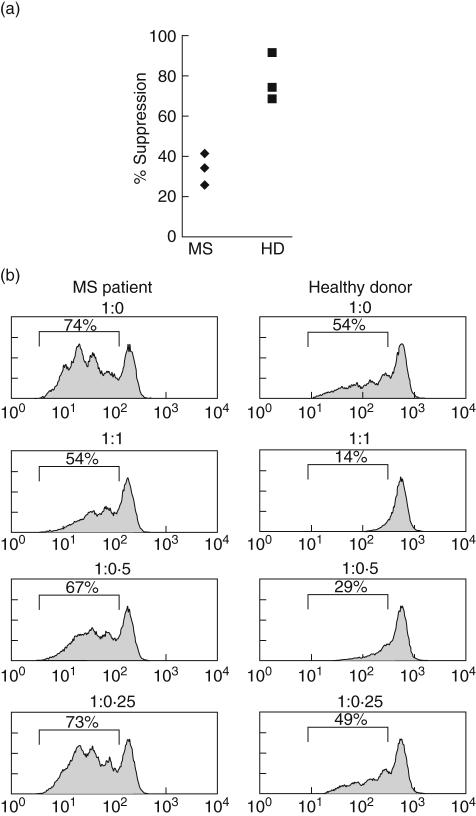
Two recent studies have demonstrated an impaired function of CD4+ CD25+ nTreg in MS [6,7], which might serve as an explanation for the loss of tolerance in CNS autoimmunity. To investigate if this was also the case in our patients, we selected a few patients and controls and performed suppression assays in which nTreg were titrated into stimulated responder T cells at different ratios. In the cases analysed (n = 3 for MS – acute and untreated – and HD, sex- and age-matched) we also observed a reduced capacity of nTreg from MS patients to suppress proliferation of the corresponding responder population. Suppression by nTreg at a 1 : 1 responder to suppressor ratio ranged between 34 and 41% in MS patients, whereas nTreg from healthy donors showed 68–91% suppression (Fig. 2). Thus, our data in a small series of cases is consistent with the reported reduced suppressive capacity of nTreg in MS patients.
Fig. 2.
Impaired suppressive function of nTreg in multiple sclerosis (MS) patients. Suppression assay with CD4+ CD25+ nTreg. CD4+ CD25+ nTreg were titrated into carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE)-labelled CD4+ CD25– responder T cells cultured in the presence of allogeneic peripheral blood mononuclear cells (PBMC) and soluble anti-CD3. Cell proliferation and influence of CD4+ CD25+ nTreg was quantified by fluorescence-activated cell sorter (FACS) analysis on day 4. (a) Graph shows a summary of suppression assays from three MS patients (left) and three healthy donors (HD) (right). Responder (CD4+ CD25–) to suppressor (CD4+ CD25+ nTreg) ratio is 1 : 1. (b) FACS analysis of a representative suppression assay from a MS patient (left) and HD (right) with percentages of proliferation stated in histogram. Responder to suppressor ratios are given on top of each histogram.
FOXP3+ CD4+ CD25bright nTreg infiltrate the CSF in patients with neuroinflammatory condition
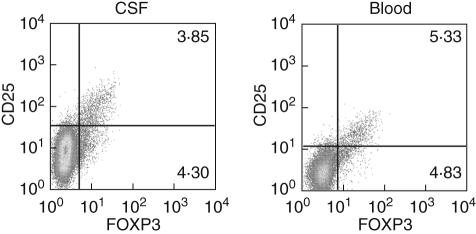
The paucity of T cells in the CNS of MS patients prevented us from performing suppression assays using CSF CD4+ CD25+ nTreg. However, to check if indeed these cells have the requirements to be regulatory, we assessed expression of FOXP3 in CSF samples from patients with neuroinflammatory disease (Fig. 3). FOXP3 is considered the lineage specification marker for nTreg, but until now no specific cell molecule for CD4+ CD25bright nTreg has been described. We therefore performed a combination of surface (CD4 and CD25) and intracellular staining (FOXP3) in both PBMC and CSF from several patients to check if the cells that are CD25bright are also expressing FOXP3. As seen in Fig. 3, in both PBMC and CSF all CD25bright cells are also positive for FOXP3, and thus they can be defined as nTreg. In addition, in both samples a similar percentage of FOXP3+ cells remain CD25 negative, likely to constitute the reservoir of committed regulatory cells that regain CD25 expression and suppressive function upon activation [9].
Fig. 3.
Identification of CD25 and forkhead transcription factor 3 (FOXP3)-expressing CD4 cells from peripheral blood and cerebrospinal fluid (CSF). Fluorescence activated cell sorter (FACS) staining of a paired CSF/blood sample from a patient with neuroinflammatory disease for surface CD4 and CD25, and intracellular FOXP3. A gate for CD4+ T cells was set on forward scatter/CD4 dot plots to identify the desired subset. In a second dot plot, co-expression of FOXP3 and CD25 was assessed in comparison to the control isotype staining. A representative staining is shown for CSF cells (left) and blood (right).
The frequency of nTreg in the CSF of MS patients is higher than in peripheral blood
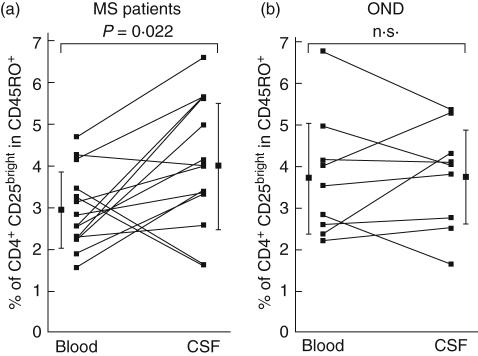
We next compared the frequency of nTreg in paired samples of CSF and peripheral blood in 14 patients with MS and nine with OND. Nearly all T cells in the CSF show a memory (CD45RO+) phenotype [10]. Thus, in order to obtain a more relevant comparison between blood and CSF, we analysed the content of CD25bright in the CD4+ CD45RO+ compartment. Interestingly, the percentage of nTreg was elevated significantly in the CSF of MS patients in comparison to their peripheral blood (mean CSF = 4·05 ± 1·54% versus mean peripheral blood = 2·93 ± 0·94%, n = 14, P = 0·022) (Fig. 4). An increase of nTreg in the CSF compartment in comparison to the periphery was noticeable in 11 of the 14 MS cases analysed (79%). This is in contrast to other neurological diseases of non-inflammatory, non-autoimmune origin, where the percentages of CD4+ CD25+ nTreg in CSF remained very similar to blood (mean CSF = 3·78 ± 1·26% versus mean peripheral blood = 3·74 ± 1·48, n = 9, P = 0·92) (Fig. 4). Of note, in the CSF we could not find significant differences in the frequency of nTreg in MS patients separated according to their disease course. Taken together, our results show that nTreg exist in the CSF, are elevated in MS patients in comparison to peripheral blood, and therefore have the potential to modulate immune responses in the target organ.
Fig. 4.
Comparison of nTreg frequencies in the blood and cerebrospinal fluid (CSF) from multiple sclerosis (MS) patients and other neurological disorders (OND). (a) Percentages of CD4+ CD25bright T cells in the CD4+ CD45RO+ compartment of blood and CSF of MS patients (n = 14). Direct comparison of blood versus CSF for each analysed individual is represented by connected dots. The difference, indicated by the mean ± s.d., is statistically significant (P = 0·022). (b) Percentages of CD4+ CD25bright T cells in the CD4+ CD45RO+ compartment of blood and CSF are represented for patients with OND (n = 9). No significant differences were detected. For all groups, flow cymetry analysis for CSF and blood was conducted according to Fig. 1.
Discussion
The investigation of nTreg in human autoimmunity has attracted considerable attention. Several studies have recently analysed the frequency and function of CD4+ CD25bright nTreg in multiple sclerosis, the prototype of an autoimmune inflammatory CNS disease [6–7,11,12]. All these studies, however, assessed peripheral lymphocytes. Assuming that the CSF represents the fluid compartment that is closest to reflect the immunopathogenic situation in MS, this study is the first to demonstrate the presence and enrichment of FOXP3+ CD4+ CD25bright regulatory T cells in close proximity to the autoimmune target CNS.
Similar to the enrichment of functionally active CD4+ CD25+ nTreg in the synovial fluid of inflamed joints that has been observed in rheumatoid arthritis [13,14], our results show that there is an imbalance in the proportion of nTreg in peripheral blood compared to the target organ of inflammation. An increase in nTreg in the CSF was found in 79% of patients with neuroinflammation when compared to their own peripheral compartment, which was accompanied by a modest decrease in the percentage of nTreg in blood.
Besides FOXP3+ CD25high T cells, a similar proportion of FOXP3+ CD25low cells can be found in CSF and blood (see Fig. 3). Studies of FOXP3gfp mice have already pointed to the existence of different populations according to FOXP3 and CD25 expression being the CD25 and FOXP3 double-positive T cells, those with a secure regulatory phenotype [3]. In addition, Zelenay et al. reported the presence of FOXP3+ CD25low cells, and have suggested that these cells constitute a reservoir of differentiated regulatory cells that can up-regulate CD25 expression upon activation [9]. Therefore all CD25+ cells that we have considered as regulatory T cells are FOXP3+, and it can therefore be assumed that they represent cells with a truly regulatory phenotype. Our results suggest that nTreg from the periphery are recruited into the CSF; thus we see the slight decrease in the periphery, rather than expanding once some nTreg reach the CSF or the target organ. Furthermore, de novo generation of nTreg in the target organ in MS is a possible but unlikely explanation for the observed distribution of nTreg.
It is not known how this enriched population of nTreg in the CSF relates to the lesion pathogenesis in MS. In contrast to animal studies, it is very difficult to assess the ‘antigen specificity’ of CD4+ CD25+ nTreg and their migration patterns into the CNS in humans. Similarly, functional analysis of nTreg from CSF is virtually impossible, due to the low frequency of CSF cells, as well as the availability of CSF itself.
Our data suggest that the enrichment of nTreg in the CSF is related selectively to the autoimmune inflammatory state in MS, because other neurological diseases of non-autoimmune, non-inflammatory origin (dementia, normal pressure hydrocephalus and stroke) did not show significant differences. It may even seem counterintuitive that, despite the presence (and increase) of regulatory T cells in the CSF, autoimmune inflammatory activity in the CNS occurs. Obviously, in spite of enrichment, nTreg are not sufficient to combat the ongoing inflammation. Insufficiency may either be a matter of quantity or functional capability. Strong arguments voting for the latter hypothesis are studies which show impaired suppressive capacity of CD4+ CD25+ nTreg in MS patients [6,7]. This impairment seems to be limited to RRMS patients, as suppressive function in SPMS patients is unaffected [12]. However, this difference in function of nTreg in different disease courses is not reflected in their frequency. Therefore, our hypothesis would be that nTreg are up-regulated in the CSF of MS patients because they try to combat and down-regulate ongoing chronic (auto)immunity. However, functional impairment of nTreg in MS patients could have contributed (i) to the development of the disease and (ii) the inefficiency in turning down T cell activity towards CNS structures.
The idea of local immunosuppression by functional nTreg affecting disease activity and progression is appealing, both from an immunopathogenetic and a therapeutic view. It remains to be shown whether an increase in the number or function of nTreg and a parallel decrease in number or function of pathogenic T cells could possibly account for the phases of relapses and remissions in MS.
Acknowledgments
This work was supported by grants from the German Research Foundation (to H. W., Wi 1722/3–1), the IZKF Würzburg (48–0 to H. W.), the SFB 685 (to E. T. and A. M.), the Hertie Foundation and the fortüne program of the Medical Faculty of the University of Tübingen (to H. W. and A. M.). E. T. is supported by a Margarete von Wrangell fellowship from Land Baden-Württemberg. We are grateful to all our blood and CSF donors.
References
- 1.Hemmer B, Cepok S, Nessler S, Sommer N. Pathogenesis of multiple sclerosis: an update on immunology. Curr Opin Neurol. 2002;15:227–31. doi: 10.1097/00019052-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–98. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–40. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putheti P, Pettersson A, Soderstrom M, Link H, Huang YM. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J Clin Immunol. 2004;24:155–61. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 6.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas J, Hug A, Viehover A, et al. Reduced suppressive effect of (CD4+) CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 8.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 9.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci USA. 2005;102:4091–6. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivisakk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389–94. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro J, Aristimuno C, Sanchez-Ramon S, et al. Circulating dendritic cells subsets and regulatory T-cells at multiple sclerosis relapse: differential short-term changes on corticosteroids therapy. J Neuroimmunol. 2006;176:153–61. doi: 10.1016/j.jneuroim.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Venken K, Hellings N, Hensen K, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4(+) CD25(+) regulatory T-cell function and FOXP3 expression. J Neurosci Res. 2006;83:1432–46. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- 13.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 14.de Kleer IM, Wedderburn LR, Taams LS, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]