Abstract
T-bet is a novel transcription factor regulating lineage commitment of T helper (Th) lymphocytes to a predominant Th1 phenotype. Previous studies on T-bet and asthma focused mainly on bronchial biopsy specimens. This study assessed the relationship between T-bet expression and levels of selected chemokines in the peripheral blood of asthmatics. Blood was collected from 24 steroid-naive asthmatics, 39 asthmatics on inhaled corticosteroid and 32 age- and sex-matched controls for assay of T-bet expression, specific IgE and chemokines (interferon-gamma inducible protein-10 (IP-10/CXCL10), monokines induced by interferon-gamma (MIG/CXCL9), monocyte chemotactic protein-1 (MCP-1/CCL2), regulated upon activation normal T cell expressed and secreted (RANTES/CCL5) and interleukin-8 (IL-8/CXCL8) levels. T-bet mRNA expression was assessed by real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR). Chemokine levels were assessed by immunofluorescence flow cytometry. The mean (s.d.) age and forced expiratory volume in 1 s (FEV1)% predicted of the asthmatics were 43·6 (14·6) years and 85·9 (20·0)%, respectively. The median (IQR) T-bet expression after normalization with β-actin was suppressed in asthmatics versus controls [asthmatics 0·71 (0·59) versus controls 1·07 (1·14), P = 0·03].The median (IQR) of plasma RANTES was elevated, whereas IP-10 was suppressed in asthmatics versus controls (RANTES: 13 658·0 (13673·3) versus 6299·5 (19 407·8) pg/ml, P = 0·03; IP-10: 1047·6 (589·8) versus 1306·4 (759·9) pg/ml, P = 0·001). There was a weak and negative correlation between T-bet expression and RANTES level in the asthmatics (r = –0·29, P = 0·032). T-bet could be measured in peripheral blood and its expression was suppressed in asthmatics. This is in keeping with asthma being a predominantly Th2 disease and T-bet probably plays a role in the pathogenesis of asthma. Further studies are needed to explore the potential application of peripheral blood monitoring of T-bet.
Keywords: asthma, chemokines, peripheral blood, T-bet expression
Introduction
Asthma is a disease characterized by reversible airflow obstruction. Airway inflammation is a key factor in asthma that leads to increasing airway hyperresponsiveness and bronchospasm [1]. The regulation of T helper (Th) cells is important in the pathogenesis of asthma [2]. Th1 and Th2 cells develop from naive T cells during an immune response. Szabo et al. discovered a transcription factor that plays an important role in Th cell differentiation [3]. This transcription factor is named T-bet, a member of the T-box family, and its expression is limited primarily to the immune system in regulating lineage commitment of Th cells to a predominant Th1 type. T-bet not only induced interferon (IFN)-γ, the hallmark Th1 cytokines, to produce Th1 phenotype, but it also repressed interleukin (IL)-4 and IL-5 production from differentiated Th2 cells [4,5].
In animal studies of mice with deliberately disabled T-bet genes (T-bet–/– knock-out mice), the bronchi of the mice were infiltrated with eosinophils and lymphocytes and showed airway remodelling typical of allergic asthma even in the absence of allergic sensitization [6]. Bronchoalveolar lavage fluid (BAL) of these mice also contained increased amounts of Th2 cell-secreted cytokines. There was also a systemic deficiency of IFN-γ and a global increase in Th2 cells in these mice [4]. Finotto et al. also demonstrated a decreased expression of T-bet in lymphocytes of human asthmatic lung tissues when compared with control lungs using immunohistochemical staining with monoclonal antibody to T-bet [6].
Chemokines, also known as chemotactic cytokines, are small heparin-binding proteins that direct the movement of circulating leucocytes to sites of inflammation or injury [7]. There are currently about 50 different human chemokines, which are divided into four families based on their molecular structures and positions of the two cysteine (C) residues: CC, CXC (alpha), C and CX3C [8]. Previous studies have shown that chemokines and their receptors are related to asthma [9,10]. Chemokines such as IFN-γ inducible protein-10 (IP-10 or CXCL10) and monokines induced by IFN-γ (MIG or CXCL9) can attract Th1 cells through the CXCR3 receptors, thus enhancing the polarization of T lymphocyte recruitment [11,12]. Regulated upon activation normal T cell expressed and secreted (RANTES or CCL5) is an important attractant for eosinophils and is up-regulated in the BAL of asthmatic children when compared with controls [13]. Following endobronchial allergen challenge, levels of RANTES and monocyte chemotactic protein-1 (MCP-1 or CCL2) were found to increase in the allergen-challenge site when compared to the saline-challenged control site in the asthmatic airway [14]. IL-8 or CXCL8 is a potent chemoattractant for neutrophils and is related to airway inflammation in patients with asthma. A study on hypertonic saline challenge of the asthmatics found that neutrophils in the sputum correlated with the sputum IL-8 level [15]. We have assessed previously the above-selected chemokines (MCP-1, RANTES, IL-8, MIG and IP-10) and their receptors (CCR3, CCR5 and CXCR3) in asthmatic subjects and found that plasma concentration of IP-10 was significantly lower while that of RANTES and the expression of CCR3 were higher in asthmatic patients [16].
So far no there are data on T-bet expression in the peripheral blood of asthmatics and controls. As T-bet plays a role in the determination of the fate of naive Th cell differentiation to Th1 and Th2 cells, it is potentially a target for therapeutic intervention in asthma [17,18]. Because both T-bet [19] and chemokines are crucial for the trafficking of T cells, we would like to assess their relationship in the same study. Sampling T-bet from peripheral blood is more convenient and non-invasive than sampling from lung tissues. This study aimed to study whether T-bet expression could be assessed in the peripheral blood of asthmatic and control subjects. In addition, we examined any relationship between T-bet and chemokines in the peripheral blood of the asthmatics (including IP-10, MIG, RANTES, MCP-1 and IL-8). We hypothesized that T-bet could be measured in the peripheral blood of asthmatics and control subjects, and its level would be lower in asthmatics than controls as asthma is a predominantly Th2 disease.
Methods
This was a cross-sectional case–control study involving 63 asthmatics [34 steroid-naive and 39 on inhaled corticosteroid (ICS)] and 32 sex- and age-matched controls. Adult asthmatics aged ≥ 18 years were recruited from the asthma clinic of the Prince of Wales Hospital, a university teaching hospital. Asthma was diagnosed according to the British Thoracic Society criteria [20]. All asthmatic subjects should have a diagnosis of asthma established in the past based on symptoms (wheeze, shortness of breath, cough or chest tightness), together with lung function measurements showing significant reversibility to bronchodilator [forced expiratory volume in 1 s) (FEV1) ≥ 15% (and 200 ml) increase after 400 mg salbutamol given by metered dose inhaler with spacer]. The asthmatics should be either life-long non-smokers or ex-smokers for at least half a year and with a smoking history of less than 5 pack-years. Their asthma control was stable with no increase in usual asthma symptoms and no change of asthma medications for 8 weeks prior to the study. Current cigarette smokers or subjects on systemic corticosteroids were excluded from this study. Sex- and age-matched non-smoking volunteers free of respiratory diseases and without respiratory tract infections for at least 8 weeks prior to the assessment were recruited as control subjects. This study was approved by the clinical research ethics committee of The Chinese University of Hong Kong and informed consent was obtained from all participants.
Spirometry (Vitalograph, Model S, Buckingham, UK) of the asthma subjects was measured according to the American Thoracic Society standards [21]. Updated normograms were adopted to calculate the predicted lung function of the subjects [22]. Asthma symptoms were recorded and the asthma severity was graded from 1 to 4 according to the symptoms classification by the GINA guideline [1]. Patients with symptoms less than once a week or nocturnal symptoms less than twice a month were given a score of 1. A score of 2 was given to patients with symptoms > once/week or less than once/day. A score of 3 was given to patients with daily symptoms or nocturnal symptoms > once/week whereas patients with daily symptoms, or frequent nocturnal symptoms, or limitation of physical activity due to asthma were given a score of 4.
Nine ml of ethylenediamine tetraacetic acid (EDTA) venous blood and 5 ml of clotted blood was taken from the subjects for measurement of T-bet, chemokines, specific IgE and total IgE assay. Peripheral blood mononuclear cells (PBMC) were isolated from EDTA blood by Ficoll-Paque density gradient centrifugation (Amersham Pharmacia Biotech Ltd, Uppsala, Sweden). Total RNA was extracted immediately from PBMC and stored for T-bet assay. Plasma and serum samples were preserved at −70°C for subsequent chemokines, specific IgE and total IgE assay.
T-bet expression measurement
Total RNA of blood was extracted by RNeasy Mini Kit (Qiagen Inc., Ontario, Canada), following the manufacturer's instructions. All specimens were pretreated with deoxyribonuclease I (Invitrogen TM, Life Technologies, Carlsbad, CA, USA) and stored at −70°C.
For each reaction, approximately 0·5 mg of RNA was reverse-transcribed to complementary DNA (cDNA) with Superscript II Rnase H– reverse transcriptase (Invitrogen TM, Life Technologies, Carlsbad, CA, USA). T-bet mRNA expressions were quantified by the real-time quantitive (Q)PCR with the use of ABI Prism 7700 Sequence Detector System (Applied Biosystems, Foster City, CA, USA). The primer and probe sequences of human T-bet were synthesized according to a published report [23]: forward primer: 5′-CAA CAA CCC CTT TGC CAA AG-3′, reverse primer: 5′-TCC CCC AAG CAG TTG ACA GT-3′ and the probe: 5′-6FAM-CCG GGA GAA CTT TGA GTC CAT GTA CGC-TAMRA-Q-3′ were synthesized by Applied Biosystems. In the primer sequence, 6FAM was 60 carboxyflurorescein and TAMRA was N,N,N′,N′-tetramethyl-6-carboxyrhodamine. PCR amplifications were performed in a 20 ml volume at 50° C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Each sample was run in duplicate. Results were analysed with the Sequence Detection Software version 1·7 (Applied Biosystems).
Relative standard curves of T-bet and β-actin were prepared separately in each PCR run to normalize data (with beta-actin) for quantifying and comparing the relative expression of T-bet across batches of reactions. Complementary DNA derived from phorbol 12-myristate 13-acetate (PMA)-stimulated human white cells was used for generating relative standard curves. For each sample, the relative amount was calculated using linear regression analysis from their respective standard curves. A relative T-bet expression value was then obtained by dividing T-bet value by the β-actin mRNA value. T-bet expression was expressed as a ratio of normalization with β-actin.
Serum allergen-specific IgE
The measurement of serum specific IgE to house dust mites (Dermatophagoides pteronyssinus and the allergen protein from the house dust mites Der p1), dog, cat, mixed cockroaches and mixed moulds was performed by fluorescence enzyme immunoassay (FEIA) (AutoCAP analyser; Pharmacia Diagnostics AB, Uppsala, Sweden) [24]. Subjects with one or more positive results on the above allergen-specific IgE were considered as atopic. Sensitization to local pollens was not tested due to its very low prevalence in our locality [25].
Plasma chemokine measurement
Plasma was stored at −70°C until measurement of chemokines. The chemokines IP-10, MIG, MCP-1, RANTES and IL-8 concentrations were assessed by flow cytometry using the human chemokine cytometric bead array (CBA) kit (Becton Dickinson Biosciences Pharmingen, Santa Fe, CA, USA), according to the manufacturer's instructions. Samples were read by a three-colour Becton Dickinson fluorescence activated cell sorter (FACS)CaliburTM flow cytometer and analysed using BD CellQuestTM software and BDTM CBA Software. The assay sensitivities of these five chemokines were 2·8, 2·5, 2·7, 1·0 and 0·2 ng/l, respectively. The coefficients of variation for all chemokine assays were less than 10%.
Data were analysed by the spss software for Windows, version 11·5 (SPSS Inc., IL, USA). The levels of the T-bet and chemokines were compared between the asthmatics and controls by Mann–Whitney rank sum test or Kruskal–Wallis test as appropriate. Correlation of the T-bet expression, chemokine levels, lung function, total IgE and eosinophil count were assessed by Spearman's rank correlation test. A P-value of < 0·05 was considered as statistically significant.
Results
The demographic data of the subjects are shown in Table 1. The asthmatics and controls were age- and sex-matched. The subjects generally had satisfactory lung function with a mean FEV1% predicted value of > 80%. For those patients who were on ICS, the mean (s.d.) daily dosage was 1144·6 (661·2) μg beclomethasone dipropionate equivalent. Among the asthmatics on ICS, 11 (28·2%) were on inhaled long-acting beta-agonist (LABA). None of the steroid-naive asthmatics were on LABA and all the asthmatic subjects were on either theophylline or leukotriene antagonists. Sixty-seven per cent of the asthmatics were atopic as defined by positive specific IgE. The dominant allergen among atopic asthmatics in this study was house dust mite.
Table 1.
Demographic characteristics of the asthmatic patients and controls.
| Steroid-naive asthma (n = 24) | Asthmatics on ICS (n = 39) | All asthma subjects (n = 63) | Control (n = 32) | |
|---|---|---|---|---|
| Age (years) | 42·2 ± 12·3 | 44·5 ± 15·9 | 43·6 ± 14·6 | 41·9 ± 12·6 |
| Sex (male/female) | 9/15 | 20/19 | 29/34 | 11/21 |
| FEV1 (L) | 2·41 ± 0·81 | 2·34 ± 0·88 | 2·37 ± 0·85 | – |
| FVC (L) | 2·91 ± 0·98 | 2·83 ± 1·04 | 2·86 ± 1·01 | – |
| FEV1 (% predicted) | 90·1 ± 20·0 | 83·3 ± 20·0 | 85·9 ± 20·0 | – |
| FVC (% predicted) | 90·1 ± 18·5 | 81·8 ± 17·4 | 84·9 ± 18·2 | – |
| Symptoms score | 2·0 ± 0·9 | 1·9 ± 0·9 | 2·0 ± 0·9 | – |
| Atopy [n (%)]† | 15 (62·5) | 27 (69·2) | 42 (66·7) | 14 (43·8)* |
| Serum IgE level (kIU/l) | 253·8 ± 241·3 | 309·4 ± 437·1 | 287·9 ± 372·4 | 227·1 ± 430·1* |
| Serum esoinophils (× 109/l) | 0·30 ± 0·25 | 0·36 ± 0·49 | 0·34 ± 0·41 | – |
| % Eosinophils (%) | 4·50 ± 3·05 | 4·36 ± 3·26 | 4·42 ± 3·16 | – |
Data were presented as n (%) or mean (s.d.).
Atopy was defined by positive specific IgE assay from blood. ICS = inhaled corticosteroid.
P < 0·05 compared all asthma to control subjects.
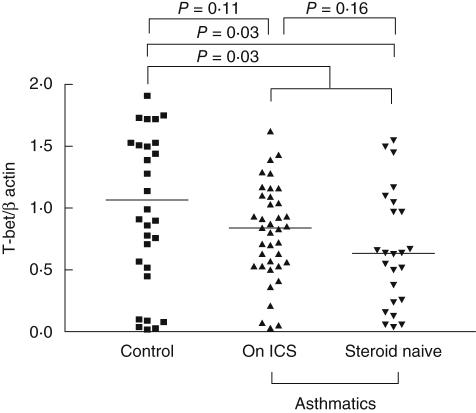
The T-bet expression and plasma IP-10, MIG, MCP-1, RANTES and IL-8 concentrations are shown in Table 2. As also illustrated in Fig. 1, T-bet expression was lower in the asthmatics than the controls [median (IQR) value 0·77 (0·50–1·09) versus 1·07 (0·53–1·67), P = 0·03], which was suppressed mainly in the steroid-naive asthmatics, with median values of 0·64 (0·25–1·03), P = 0·03. There was a trend for a lower T-bet level in the asthmatics on ICS compared to controls (median values for asthmatics on ICS and controls were 0·84 (0·53–1·10) and 1·07 (0·53–1·67), respectively). However, this did not reach statistical significance (P = 0·11). The raw PCR data for both β-actin and T-bet are shown in Fig. 2. The molecular size of the T-bet PCR product and β-actin were 105 and 295 base pairs, respectively. IP-10 concentration was found to be lower in the asthmatics than the controls [median 1047·6 (696·0–1285·7) versus 1306·4 (1059·9–1819·8) pg/ml, P = 0·001]. The reverse was noted for the RANTES level in which the asthmatics had a significantly higher level [13 657·9 (6593·1–20 266·4) versus 6299·5 (452·8–19 860·6) pg/ml, P = 0·03].
Table 2.
T-bet expression and plasma inducible protein-10 (IP-10), monocyte chemotactic protein-1 (MCP-1), monokines induced by interferon-γ (MIG), regulated upon activation normal T cell expressed and secreted (RANTES) and interleukin-8 concentrations in asthmatic patients and controls.
| Steroid-naive asthma | Asthma on ICS | All asthmatics | Control | |
|---|---|---|---|---|
| T-bet | 0·64 (0·25–1·03)** | 0·83 (0·53–1·10) | 0·71 (0·50–1·09)† | 1·07 (0·53–1·67) |
| IP-10 (pg/ml) | 1234·7 (955·4–1518·4)* | 865·9 (679·5–1073·8)† | 1047·6 (696·0–1285·7)‡ | 1306·4 (1059·9–1819·8) |
| MCP-1 (pg/ml) | 85·6 (55·4–117·6) | 76·7 (40·6–115·6) | 81·35 (50·0–113·8) | 67·6 (43·5–90·1) |
| MIG (pg/ml) | 331·0 (183·2–580·8) | 270·5 (194·5–349·5) | 278·1 (190·3–438·7) | 271·6 (194·5–465·4) |
| RANTES (pg/ml) | 12959·1 (7021·4–20240·0)** | 14734·1 (5631·3–20361·1) | 13658·0 (6593·1–20266·4)‡ | 6299·5 (452·8–19860·6) |
| IL-8 (pg/ml) | 5·23 (3·77–16·26) | 5·28 (3·53–17·76) | 5·27 (3·56–17·60) | 5·57 (4·01–18·22) |
Values are presented as median (interquartile range). ICS = inhaled corticosteroid.
Comparison of the steroid-naive asthmatics and asthmatics on ICS: P < 0·05;
comparison of the steroid-naive asthmatic and controls: P < 0·05;
comparison of asthma on ICS versus controls: P < 0·05;
comparison of the all asthmatic and control group: P < 0·05. IL: interleukin.
Fig. 1.
T-bet expression in asthmatic patients and controls. ICS: inhaled corticosteroid.
Fig. 2.
Scatter plots (with medians) showing the raw data of T-bet and β-actin. *P < 0·05 when compared to the asthma group with controls and when compared with the steroid-naive group with controls. ICS: inhaled corticosteroid.
Among the measured parameters, T-bet expression in asthmatics was found to correlate negatively with RANTES concentration (r = –0·29, P = 0·03) (Fig. 3). When the asthmatics were stratified into the steroid-naive and asthmatics in the ICS group, no statistically significant correlation was observed between T-bet and RANTES in either group. The level of T-bet also did not correlate with the level of IP-10, MIG, MCP-1 and IL-8 in all the asthmatics, steroid-naive asthmatics or asthmatics on ICS. In addition, T-bet expression in the asthmatics had no correlation with the lung function (FEV1, FEV1% predicted and ratio of FEV1/FVC), eosinophil count and symptom score. When the asthmatics were divided into atopic and non-atopic groups, no correlations between the T-bet concentration and the chemokines, lung function, eosinophil count and symptom score were observed in both the atopic or non-atopic group, except that in the atopic group T-bet had a negative correlation with RANTES (r = – 0·39, P = 0·02).
Fig. 3.
Correlation of T-bet expression and regulated upon activation normal T cell expressed and secreted (RANTES) concentration in the asthma subjects.
Discussion
To the best of our knowledge, this is the first controlled study of the T-bet gene mRNA expression in the peripheral blood of asthmatics quantitatively by RT–PCR. We have shown that patients with asthma had a significantly lower level of T-bet. Previous studies have assessed T-bet in bronchial biopsy specimens in both humans and mice [4]. Apart from the lungs, T-bet mRNA could be assayed in urinary sediment, and the elevated T-bet expression in patients with active lupus nephritis was found to decrease in the urinary sediment in parallel with a reduction in lupus activity after treatment by immunosuppressants [26]. Our finding of a lower T-bet level in the peripheral blood of asthmatics was consistent with the finding of a previous study using the mouse model. The knock-out of T-bet would lead to spontaneous development of airway hyperresponsiveness and other changes consistent with asthma in the animal's airway [6]. It is interesting that, for asthma, the changes of T-bet mRNA expression occurred not only in lung tissues, but could also be measured in the systemic circulation.
We observed a lower T-bet mRNA expression in the steroid-naive asthmatics than in controls. The same trend occurred in those asthmatics on ICS, but the difference did not reach statistical significance when compared with controls. There was also a trend for a higher T-bet level in the asthmatics on ICS when compared to the steroid-naive asthmatics. In vitro study of stimulated human CD4 cells found that dexamethasone could inhibit the induction of T-bet [27]. As our study was a cross-sectional study that sampled only blood from patients without taking any specimens from the airway, we could not assess whether ICS had any direct effect on T-bet expression in the lungs and thus in the systemic circulation. Previous studies have found that T-bet polymorphism was associated with airway hyperresponsiveness in children with asthma [28]. In addition, strong genetic influence on T-bet expression was observed in a twin study. Intrapair T-bet variability was high among dizygotic twins, whereas variability was very low among monozygotic twins [29]. A recent study has found that the genetic variation of T-bet (non-synonymous variation in the T-box21 coding for replacement of histidine 33 with glutamine) was associated with greater ICS responsiveness and lower bronchial hyperresponsiveness (as measured by the concentration of PC20) in the asthmatic children [27]. It is thus possible that T-bet expression is determined by genetics, and the genetic variants may affect both the degree of bronchial hyperresponsiveness and steroid responsiveness in the asthmatic airway.
We have assessed several chemokines in this study. Previous studies have found that mice undergoing Th1-polarized inflammation showed preferential expression of T-bet and other chemokines such as IP-10 and macrophage inflammatory protein 1 alpha (MIP-1-α) [30]. We noted that IP-10 was suppressed in the asthmatics in this study when compared with controls and this finding is consistent with our previous study [16]. As asthma is believed to be a Th2-related disease, with the Th1/Th2 balance, the finding of lower IP-10 in asthmatics was consistent with previous observations that the levels were higher in Th1 diseases. However, no correlation between T-bet and IP-10 was observed in this current study. A recent bronchoscopic study from patients with moderate-to-severe asthma has reported that 2 weeks of systemic steroid therapy could up-regulate the expression of IP10 and IL-8 in the endobronchial biopsy specimens [31]. Such a trend of up-regulation of IP-10 or IL-8 was not observed in our asthmatics who were on ICS.
Plasma RANTES was found to be elevated in the asthmatics when compared to controls. In addition, RANTESconcentration had a negative correlation with T-bet expression in the peripheral blood of asthmatics. RANTES is a member of the CC chemokine family, and is chemotatic for T cells and monocytes [32]. In addition, it is a potent eosinophil chemoattractant [33–35]. Previous studies have shown that RANTES were increased in the bronchoalvelar lavage fluid of children with asthma [13] and in sputum of adult asthmatics whose asthma control was poor [36]. In addition, the level of RANTES was found to correlate with the asthma severity score [16]. As RANTES is so closely related to asthma and allergic diseases, which are Th2-dominated diseases, it is not surprising that that its level correlated negatively with the level of T-bet which is important for development of the Th1 phenotype. In this current study, T-bet levels correlated negatively with atopic asthmatics but not in the non-atopic asthmatics. Systemic steroid and antihistamine could modulate the release of RANTES from the cultured peripheral mononuclear cells from atopic asthmatics [37]. It is possible that the role of RANTES in the atopic asthmatic airway is more dominant when compared to the non-atopic asthmatics.
There are several limitations in this study. First, we assessed only the peripheral blood of the subjects without concomitant assessment of the airway by invasive methods such as bronchoalveolar lavage and biopsy. We thus could not correlate the changes in T-bet and chemokine levels in blood directly with the changes within the airway. Secondly, as this was a cross-sectional study, the effect of ICS on T-bet and chemokines could not be assessed. Th1 and Th2 immune responses have been studied traditionally by measuring intrinsic or extrinsic cytokine production. Instead of examining the downstream cytokine production, the main objective of our present study focuses on investigating upstream transcription factors of T-bet that control the spectrum cytokine/chemokine productions. We have thus used RT–PCR measurement of T-bet and chemokine assays to provide an immediate snapshot of chemokine status reflecting the in vivo physiological condition.
As T-bet is an important transcription factor in determining the differentiation of Th cells, it is a potential therapeutic target in asthma. Oral administration of pulverized Konjac glucomannan (PKGM) in mice has been shown to attenuate the induction of epsilon germline transcription and also the mRNA for IFN-γ and T-bet in the spleen [38]. Our finding that T-bet mRNA expression could be measured in peripheral blood has suggested a potential role of monitoring the blood T-bet level in asthmatics as a non-invasive research tool instead of using lung biopsy.
In conclusion, this study has demonstrated that T-bet mRNA expression could be measured in the peripheral blood of human subjects. The T-bet mRNA expression was lower in asthmatics than in controls. In addition, T-bet correlated negatively with RANTES in the peripheral blood of asthmatics, particularly in atopic subjects. As T-bet is the master control for the differentiation of Th cells into Th1 or Th2 subtypes, it is a potential therapeutic target for asthma. Further studies are needed to assess the correlation of T-bet expression in the peripheral blood and airway of asthmatic subjects.
Acknowledgments
The authors would like to thank Miss Mabel Tong for performing the lung function tests for the subjects and Miss Doris Chan for performing the statistical analysis.
References
- 1.National Heart, Blood and Lung, World Heath Organisation. Maryland: National Institutes of Health; Global Strategy for asthma management and prevention. NIH Publication no. 02–3659, 2005 update. 2005. [Google Scholar]
- 2.Romagnani S. T-cell responses in allergy and asthma. Curr Opin Allergy Clin Immunol. 2001;1:73–8. doi: 10.1097/01.all.0000010988.60715.c8. [DOI] [PubMed] [Google Scholar]
- 3.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 4.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 5.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J Immunol. 2002;168:1566–71. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 6.Finotto S, Neurath MF, Glickman JN, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–8. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 7.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 8.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 9.Smit JJ, Lukacs NW. A closer look at chemokines and their role in asthmatic responses. Eur J Pharmacol. 2006;533:277–88. doi: 10.1016/j.ejphar.2005.12.064. [DOI] [PubMed] [Google Scholar]
- 10.Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulmon Med. 2005;11:35–42. doi: 10.1097/01.mcp.0000144502.50149.e0. [DOI] [PubMed] [Google Scholar]
- 11.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–14. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rojas-Ramos E, Avalos AF, Perez-Fernandez L, Cuevas-Schacht F, Valencia-Maqueda E, Teran LM. Role of the chemokines RANTES, monocyte chemotactic proteins-3 and -4, and eotaxins-1 and -2 in childhood asthma. Eur Respir J. 2003;22:310–16. doi: 10.1183/09031936.03.00084802. [DOI] [PubMed] [Google Scholar]
- 14.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1 alpha, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–83. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–36. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 16.Lun SW, Wong CK, Ko FW, Ip WK, Hui DS, Lam CW. Aberrant expression of CC and CXC chemokines and their receptors in patients with asthma. J Clin Immunol. 2006;26:145–52. doi: 10.1007/s10875-006-9003-9. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DS, Lloyd CM. Asthma: T-bet − a master controller? Curr Biol. 2002;12:R322–4. doi: 10.1016/s0960-9822(02)00830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz RS. A new element in the mechanism of asthma. N Engl J Med. 2002;346:857–8. doi: 10.1056/NEJM200203143461114. [DOI] [PubMed] [Google Scholar]
- 19.Lord GM, Rao RM, Choe H, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–9. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.British Thoracic Society. Scottish Intercollegiate Guidelines Network (SIGN) British guideline on the management of asthma. Thorax. 2003;58(Suppl. 1):1–94. doi: 10.1136/thorax.58.suppl_1.1i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Standardization of spirometry − 1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136:1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- 22.Ip MS, Ko FW, Lau AC, et al. Updated spirometric reference values for adult chinese in Hong Kong and implications on clinical utilization. Chest. 2006;129:384–92. doi: 10.1378/chest.129.2.384. [DOI] [PubMed] [Google Scholar]
- 23.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam CW, Fung HK, Vrijmoed LL. Aetiology of allergic rhinitis in Hong Kong. Allergol Int. 1998;47:23–8. [Google Scholar]
- 25.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99:594–9. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 26.Chan RW, Lai FM, Li EK, et al. The effect of immunosuppressive therapy on the messenger RNA expression of target genes in the urinary sediment of patients with active lupus nephritis. Nephrol Dial Transplant. 2006;21:1534–40. doi: 10.1093/ndt/gfk102. [DOI] [PubMed] [Google Scholar]
- 27.Tantisira KG, Hwang ES, Raby BA, et al. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc Natl Acad Sci USA. 2004;101:18099–104. doi: 10.1073/pnas.0408532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raby BA, Hwang ES, Van Steen K, et al. T-bet polymorphisms are associated with asthma and airway hyperresponsiveness. Am J Respir Crit Care Med. 2006;173:64–70. doi: 10.1164/rccm.200503-505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hohler T, Reuss E, Adams P, et al. A genetic basis for IFN-gamma production and T-bet expression in humans. J Immunol. 2005;175:5457–62. doi: 10.4049/jimmunol.175.8.5457. [DOI] [PubMed] [Google Scholar]
- 30.Ritz SA, Cundall MJ, Gajewska BU, et al. The lung cytokine microenvironment influences molecular events in the lymph nodes during Th1 and Th2 respiratory mucosal sensitization to antigen in vivo. Clin Exp Immunol. 2004;138:213–20. doi: 10.1111/j.1365-2249.2004.02618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukakusa M, Bergeron C, Tulic MK, et al. Oral corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115:280–6. doi: 10.1016/j.jaci.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 33.Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–92. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukacs NW, Strieter RM, Warmington K, Lincoln P, Chensue SW, Kunkel SL. Differential recruitment of leukocyte populations and alteration of airway hyperreactivity by C-C family chemokines in allergic airway inflammation. J Immunol. 1997;158:4398–404. [PubMed] [Google Scholar]
- 35.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–95. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romagnoli M, Vachier I, Tarodo de la Fuente P, et al. Eosinophilic inflammation in sputum of poorly controlled asthmatics. Eur Respir J. 2002;20:1370–7. doi: 10.1183/09031936.02.00029202. [DOI] [PubMed] [Google Scholar]
- 37.Di Gioacchino M, Verna N, Cavallucci E, et al. Steroid and antihistamines modulate RANTES release in cultured peripheral blood mononuclear cells of atopic patients. Int J Immunopathol Pharmacol. 2002;15:27–34. doi: 10.1177/039463200201500104. [DOI] [PubMed] [Google Scholar]
- 38.Oomizu S, Onishi N, Suzuki H, et al. Oral administration of pulverized Konjac glucomannan prevents the increase of plasma immunoglobulin E and immunoglobulin G levels induced by the injection of syngeneic keratinocyte extracts in BALB/c mice. Clin Exp Allergy. 2006;36:102–10. doi: 10.1111/j.1365-2222.2005.02405.x. [DOI] [PubMed] [Google Scholar]