Abstract
An important limitation in T cell studies of human autoimmune (type 1) diabetes is lack of direct access to cells infiltrating the pancreas. We hypothesized that cells recently released from the pancreas into the blood might express a characteristic combination of markers of activation. We therefore examined the recently activated circulating T cell population [CD3+, human leucocyte antigen D-related (HLA-DR+)] using cytokine production and 10 additional subset markers [CD69, CD25, CD122, CD30, CD44v6, CD57, CD71, CCR3 (CD193), CCR5 (CD195) or CXCR3 (CD183)], comparing newly diagnosed adult (ND) (age 18–40 years) patients (n = 19) to patients with diabetes for > 10 years [long-standing (LS), n = 19] and HLA-matched controls (C, n = 16). CD3+ DR+ cells were enriched by two-step immunomagnetic separation. No differences in basal or stimulated production of interleukin (IL)-4, IL-10, IL-13 or interferon (IFN)-γ by CD3+ DR+ enriched cells were observed between the different groups of subjects. However, among the CD3+ DR+ population, significant expansions appeared to be present in the very small CD30+, CD69+ and CD122+ subpopulations. A confirmatory study was then performed using new subjects (ND = 26, LS = 15), three-colour flow cytometry, unseparated cells and three additional subset markers (CD38, CD134, CD4/CD25). This confirmed the expansion of the CD3+ DR+ CD30+ subpopulation in ND subjects. We conclude that a relative expansion in the T cell subpopulation with the activated phenotype CD3+ DR+ CD30+ is seen in the peripheral blood of subjects with newly diagnosed type 1 diabetes. This subpopulation represents less than 0·7% of circulating T cells and may provide a rich source of disease-specific T cells that can be isolated from blood.
Keywords: activated T cells, CD30, diabetes, DR, human
Introduction
Type 1 diabetes is characterized by a selective autoimmune destruction of the insulin-producing β cells of the pancreatic islets of Langerhans [1]. Autoantibodies are present and can be detected 5 years or more before the onset of clinical disease [2]. Techniques for measuring islet cell antibodies are now well advanced and are useful in predicting those at risk of developing the disease [3,4]. However, evidence from animal models and circumstantial evidence in humans indicates that the β cell destruction is mediated by T cells rather than antibodies [5], and the recent development of new approaches to the immunotherapy of type 1 diabetes [6–9] has led to an urgent need to develop indicators of anti-islet T cell activity. Such markers would be of considerable value, not only to indicate which antibody-positive individuals are likely to progress rapidly to islet destruction and should be targeted for immunotherapy, but also as surrogate markers of successful immunomodulation following experimental interventions.
Antigen-specific T cells proliferate in regional lymph nodes, recirculate through the blood and concentrate at the site of disease due to the combined action of up-regulated adhesion molecule pairs, chemokines and engagement of their antigen receptor [10]. As a result, 1–10% or more (up to 70% in viral infections [11]) of cells at the site of disease may be specific for disease-related antigens [12–15]. Estimates based on the use of human leucocyte antigen (HLA)-tetramers directed towards a single T cell specificity may result in a significant underestimate of the total antigen-specific response [16,17]. In peripheral blood, the only easily accessible source for repeated sampling of T cells in humans, high frequencies of antigen-specific T cells (over 10% of CD8 cells) have been reported at the peak of viral infection or with persistent viral infection [11,18,19]. However, in autoimmune disease, estimates are very much lower – typically one in 103 (0·1%) of peripheral blood T cells or less are thought to be specific for any given epitope [13,20–22] and in many cases detection has not been possible with single specificity tetramers (frequencies < 0·01% [23,24]). In addition to this problem of low frequency, highly activated T cells released from the autoimmune process may have limited further proliferative potential and/or responses may be masked by the presence of regulatory T cells [5,22,25,26]. As a result, traditional T cell proliferation assays have proved very disappointing in autoimmune diseases such as type 1 diabetes [27]. Newer techniques have been developed using antigen-specific class II tetramers or sensitive cytokine enzyme-linked immunospot assays (ELISPOTs) and the latter in particular appear promising [5,20,22]. However, autoantigen-specific T cells can also be detected in healthy individuals [20–22,28] and, used in isolation, these antigen-specific techniques may fail to distinguish between naive (or memory) T cells and T cells involved actively in an autoimmune process.
To help overcome these limitations, we have developed an alternative but complementary approach. Antigen-specific T cells are released from draining lymph nodes 5–10 days after the onset of an immune response [10,16]. These cells must then recirculate through the blood before re-entering the inflamed tissue and, during their passage, are likely to express markers of recent activation such as HLA-DR, CD69 or CD25 [10]. We therefore hypothesized that a significant proportion of T cells recently released from the pancreatic lymph nodes would be present in the peripheral blood in the activated T cell subset. In humans, HLA-DR is the most convenient activation marker as it defines a more discrete population than CD25 or CD69, is expressed for longer than these markers (10–14 days) but is down-modulated more rapidly than CD38, CD62L or CD45 RO as immune responses subside. Many additional T cell surface antigens have been identified which are up-regulated on a subset of T cells following activation including CD134 (OX40), CD30 and CD122 [interleukin (IL)-2 β-chain], as well as many chemokine receptors [29].
In diabetes, increases of 0·5% or less in the activated T cell population, as might be expected from previous studies of antigen-specific T cells, would be hard to detect in standard flow cytometric assays and, indeed, only a minor increase in the total activated T cell population is seen in newly diagnosed diabetes [30]. We hypothesized that if the diabetic autoimmune process generates preferentially cells with a particular combination of additional activation markers, changes in this very small but characteristic population may be easier to detect in the peripheral blood and reflect changes in disease activity more readily in the target tissue. Furthermore, defining this phenotype may identify a polyclonal population rich in disease-specific cells which can be monitored and isolated easily for further study. We therefore examined the differences in 13 subsets of the activated (HLA-DR+) T cell population between patients with active islet destruction (as represented newly diagnosed (ND) type 1 diabetes) and those without disease activity [long-standing (LS) diabetes or healthy controls (C)] and report our results here.
Materials and methods
Subjects
Patients with ND type 1 diabetes (as defined by random blood glucose levels greater then 11 mmol/l, age < 40, body mass index < 30 kg/m2 and a clinical decision to start insulin therapy) aged between 18 and 40 years were identified from the South-west Newly diagnosed Diabetes Collection (SWENDIC) covering a network of 14 diabetes centres in South-West England and South Wales. Patients with LS type 1 diabetes (defined as > 10 years from diagnosis and with no significant comorbidity or complications other than background retinopathy) from the same age group, intended to represent inactive disease, were recruited from the diabetic clinics of three hospitals in the Bristol area of South-West England. HLA matched C individuals were identified from among laboratory, hospital or university staff. Up to 70 ml of venous blood was drawn from each individual (within 3 months of diagnosis in the case of ND) and processed within 4 h without cryopreservation. Note that ND patients were sampled a minimum of 1 week after diagnosis and longer if patients were admitted to hospital at the time of diagnosis, to ensure that sampling did not take place at a time when the subjects were unwell due to ketosis or intercurrent infection. Children were not used for this study in view of the large blood volumes required. The study was approved by the South-West Multicentre Research Ethics committee and written informed consent was obtained in all cases.
T cell isolation
Peripheral blood mononuclear cells (PBMC) were isolated by buoyant density centrifugation (Ficoll Hypaque; GE Healthcare, Little Chalfont, Buckinghamshire, UK). For T cell separation studies, 5–10 × 107 PBMC were first enriched in CD3+ T cells by immunomagnetic bead staining and negative selection using cell separation columns (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany). Following this, cells were enriched in HLA-DR+ cells by a further immunomagnetic staining step and positive column-based selection.
Flow cytometry
Aliquots of 2–10 × 105 cells were stained by a 20-min incubation with optimal concentrations of preconjugated antibodies at 4°C followed by washing with cold phosphate-buffered saline containing 1% fetal calf serum. The following antibodies were used (all Becton Dickinson, Oxford, UK): CD3-fluorescein isothiocyanate (FITC), CD3-phycoerythrin (PE), CD3-peridinin chlorophyll (PerCP), CD3-allophycocyanin (APC), HLA-DR-PE, CD4-FITC, CD4-PE, CD8-PE, CD25-FITC, CD30-FITC, CD38-FITC, CD44v6-FITC, CD57, CD69-FITC, CD71-FITC, CD122-FITC, CD134-FITC, CCR3 (CD193)-FITC, CCR5 (CD195)-FITC and CXCR3 (CD183)-FITC. 1 × 104 events were acquired on a fluorescence activated cell sorter (FACS)Calibur flow cytometer (Becton Dickinson) and analysed using cellquest© software (BD Biosciences, San Jose, CA, USA). For three-colour analysis of unseparated cells, 2 × 105 CD3+ live-gated events were acquired. Constant cytometer settings were used for all analyses and settings calibrated monthly using Calibrite© beads (Becton Dickinson).
Stimulation assays and cytokine enzyme-linked immunosorbent assays (ELISAs)
For cell stimulations, 2 × 105 cells were aliquoted into wells of a round-bottomed 96-well plate in 200 µl of 10% fetal calf serum in RPMI-1640 medium (Gibco, Paisley, UK). The same batch of serum was used throughout the study with aliquots stored at −20°C. One µmol/l of phorbol myristic acid (PMA; Sigma, Poole, UK) plus 5 µmol/l of ionomycin (Sigma) was used to stimulate cells in triplicate cultures. After 48 h incubation at 37°C in 5% CO2 plates were frozen at −20°C. Assays were batched and when required plates were thawed and 50–100 µl of supernatant removed for cytokine assays. Antibody pairs for ELISAs for IL-4, IL-10, IL-13 and interferon (IFN)-γ assays were purchased from R&D Systems (Mineapolis, MN, USA) and used according to the manufacturer's instructions. Well-coating antibodies were used at 2–4 µg/ml and biotinylated detection antibodies at 2 µg/ml. Avidin peroxidase was used to detect binding and colour was developed using tetramethilbenzidine (TMB) substrate (Sigma) before analysis in a plate spectrophotometer. Lower limits of detection were 10 pg/ml for IL-4, IL-10 and IL-13 and 50 pg/ml for IFN-γ.
Islet cell antibody and HLA analysis
Antibodies to glutamic acid decarboxylase 65 (GAD65) and the intracellular portion of insulinoma-associated protein-2 (IA-2) were measured by immunoassay, using 35S-labelled in vitro-transcribed GAD65 and IA-2, as described previously [31]. Samples assayed for GAD and IA-2 antibodies were considered positive if they had levels above the 97·5th percentile of 2860 schoolchildren [3]. HLA class II analysis was carried out using polymerase chain reaction sequence-specific primers (PCR–SSP) for DRB1 and DQB1 using previously described methods [32].
Statistical analysis
Results are expressed as median values with ranges. Where there were three groups (ND, LS, C), groups were compared using the Kruskal–Wallis test. If significance was demonstrated for a trend across all three groups (P < 0·05), individual pairwise comparisons were performed using Dunn's multiple comparison test on GraphPad Prism© software (GraphPad, San Diego, CA, USA). This approach was taken to minimize the risks of a type 1 error due to multiple comparisons. Where only two groups were present (ND, LS) medians were compared using the Mann–Whitney U-test. No correction was made for multiple testing.
Results
Demographic comparison of study subjects
For the study of CD3+ DR+ cells after immunomagnetic separation (series A), 19 ND, 19 LS and 16 C subjects were recruited. Note that sufficient cells to perform analysis of the full panel of markers were not obtained from all individuals. For the confirmatory study (series B) a further unrelated 26 ND and 15 LS subjects were recruited. The demographics of the patient populations are shown in Table 1. Parameters were generally well matched, although the LS population had a higher percentage of HLA-DR3 and/or DR4 expression consistent with their earlier age of diagnosis (11·7 versus 28·2 years, series A; 15·4 versus 27 years, series B, Table 1).
Table 1.
Demographics of study populations.
| ND (n = 19) | LS (n = 19) | C (n = 16) | |
|---|---|---|---|
| (a) Series A | |||
| Mean age (range) when bled (years) | 28·2 (20–39) | 24·8 (23–41) | 28·1 (21–35) |
| Mean age (range) at diagnosis (years) | 28·2 (20–39) | 11·7 (3–27) | n.a. |
| Interval from diagnosis to bleed (range) (d = days, y = years) | 31·9 d (18–91 d) | 20·7 y (12–38 y) | n.a. |
| % male | 45 | 63 | 46 |
| % IA-2 or GAD antibody+ | 801 | n.d. | n.d. |
| % DR3+ or DR4+ | 932 | n.d. | 100 |
| (b) Series B | ND (n = 26) | LS (n = 15) | |
| Mean age (range) when bled (years) | 27·1 (17–36) | 33·1 (21–41) | |
| Mean age (range) at diagnosis (years) | 27·0 (17–36) | 15·4 (7–28) | |
| Interval from diagnosis to bleed (range) (d = days, y = years) | 44 d (8–120 d) | 17·7 y (12–27 y) | |
| % Male | 62 | 40 | |
| % IA-2 or GAD antibody+ | 70 | 923 | |
| % DR3+ or DR4+ | 77 | 92 | |
n.a: Not applicable; n.d.: not done.
In nine patients autoantibody results not available.
In four patients HLA typing not available.
Three of 15 not tested.
HLA: human leucocyte antigen; GAD: glutamic acid decarboxylase; IA-2: insulinoma-associated protein-2; ND: newly diagnosed; LS: long-standing; C: age-matched controls.
Comparisons of CD3+ DR+ subsets using immunomagnetic separation and 10 subset markers
Figure 1a shows the percentage of CD3+ DR+ cells in the peripheral blood population in series A prior to immunomagnetic separation. Median values were not significantly different between the groups (C : ND : LS = 5·2% : 6·3% : 4·6%, P = 0·68), although there was a trend to higher values in the ND population. After two-step immunomagnetic separation, the CD3+ DR+ population was enriched 10-fold to a median of 48% (range 7–83%, Fig. 1b) and the CD3+ DR– fraction contained 5% or less DR+ cells (Fig. 1c).
Fig. 1.
Immunomagnetic separation of CD3+ DR+ T cells in series A. (a) Comparison of percentage of CD3+ DR+ cells in unseparated peripheral blood lymphocytes enumerated by flow cytometry between human leucocyte antigen (HLA)-matched control (C, n = 16), newly diagnosed (ND, n = 19) and long-standing (LS, n = 19) subjects. Median level is shown as a horizontal line. (b) Flow cytometry profile of CD3+ DR+-enriched cells. (c) Flow cytometry profile of CD3+ DR– cells. (d) Forward and side scatter profile of CD3+ DR+ enriched cells showing regions R1 and R2 used for analysis.
Figure 1d shows the regions gated for two-colour analysis. The results for the CD3+ DR+ enriched population applying region R1 and using the 10-parameter panel of subpopulation makers are shown in Fig. 2. Significant differences in population size by Kruskal–Wallis statistics across all three groups of subjects were seen in three of the activated markers subsets – CD30, CD69 and CD122 (at P < 0·05 level). In post-hoc pairwise comparisons for these three markers, which showed significant trends across all three groups when analysed together, differences at the P < 0·05 level using Dunn's multiple comparison test were seen between ND and LS for CD30 and C and ND for CD69 and CD122 (as shown in Fig. 2). In contrast, no difference in expression of the 10 markers was seen in the CD3+ DR– cells that passed through the column on immunomagnetic separation (data not shown). Note that these results relate to the small- and medium-sized lymphocyte population (R1, Fig. 1d). The R2 population of large cells (comprising only around 3% of the total CD3+ DR+-enriched population) showed much higher levels of activation markers (for example, 23–39% CD30+, 26–39% CD69+ and 66–77% CD122+) and frequently contained high percentages of CD4+ CD8+ double-positive cells (around 60% of the population). No significant differences were seen between subject groups in the overall R2 population size or after subdivision with the 10 subpopulation markers (data not shown).
Fig. 2.
Comparison of percentages of CD3+ D+ subpopulations in R1 between human leucocyte antigen (HLA)-matched healthy control (C), newly diagnosed (ND) and long-standing (LS) subjects in series A. Median level is indicated by horizontal line. Sufficient CD3+ DR+ cells for analysis of the complete panel of markers was not available on all individuals and hence the number of subjects for whom data are available in each marker group varies. Significant trends across all three groups (C, ND, LS) were seen only for CD30 (P = 0·01), CD69 (P = 0·03) and CD122 (P = 0·03). These parameters were then analysed post-hoc for pairwise comparisons between the groups. For clarity, only the results of the pairwise comparisons are shown in the figure.
Comparison of cytokine production between DR+ and DR – populations
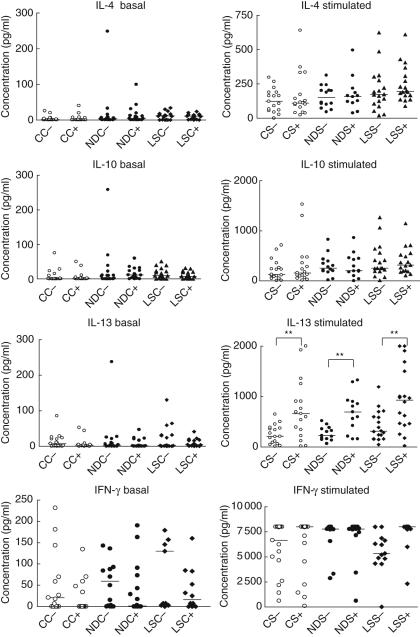
Cytokine production with and without PMA and ionomycin stimulation was compared between the immunomagnetically enriched DR+ and DR– T cells and across the three groups of subjects (Fig. 3). No intergroup differences were seen, but higher levels of IL-13 were produced by DR+ than DR– cells in all three subject groups.
Fig. 3.
Comparison of basal and phorbol myristic acid (PMA)/ionomycin stimulated cytokine production from CD3+ DR– and CD3+ DR+ T cells comparing the three groups of subjects [healthy control (C), newly diagnosed (ND) and long-standing (LS)] in series A. DR– unstimulated cells: CC–, NDC–, LSC–; DR+ unstimulated cells: CC+, NDC+, LSC+ DR– stimulated cells: CS–, NDS–, LSS–-; DR+ stimulated cells: CS+, NDS+, LSS+ 0. Median values are indicated by horizontal lines. **P ≤ 0·05.
Design of a confirmatory study of the increase in the CD3+ DR+ CD30 population in subjects newly diagnosed with type 1 diabetes – series B
More than 30 statistical comparisons were performed in the study of subpopulation markers in series A. It is therefore possible that the significant differences in the CD30, CD122 and CD69 populations seen at the P < 0·05 level were observed by chance due to the multiple comparisons. To address this, we considered series A as an exploratory study and designed a second, confirmatory study using new subjects. Of the three subpopulations that had shown differences in newly diagnosed patients (CD30, CD69 and CD122), the CD3+ DR+ CD30 was the most discrete using flow cytometry. For the confirmatory study we therefore designated changes in this population as the ‘primary outcome’ and performed analysis of samples for this parameter in triplicate. Series B was studied using unseparated cells studied by three-colour flow cytometry. The non-separation approach has the additional advantage of providing information on absolute population sizes. Cell acquisition was live-gated and only CD3+ events collected.
The largest differences in CD30 expression were observed between newly diagnosed and long-standing patients (rather than versus healthy controls) and we therefore concentrated on the comparison of these two groups in the confirmatory study to give the maximum power to detect a difference (ND n = 26, LS n = 15, Table 1b). Relatively uninformative markers [CD71, CD44v6, CD57, CCR3 (CD193) and CXCR3 (CD183)] were omitted from series B and three new markers (CD38, CD134 and CD4/25 in combination) were added. Finally, series A had demonstrated that the expression of activation markers increased markedly with increasing T cell size (consistent with cell enlargement that follows activation). For the flow cytometry in series B, we therefore used forward and side scatter to divide the CD3+ cells into three populations of increasing size/granularity. The regions used to define these populations are referred to as R*1, R*2 and R*3 to distinguish them from R1 and R2 used in the DR+-enriched populations in series A.
Changes in the overall activated cell population sizes seen using unseparated analysis in series B
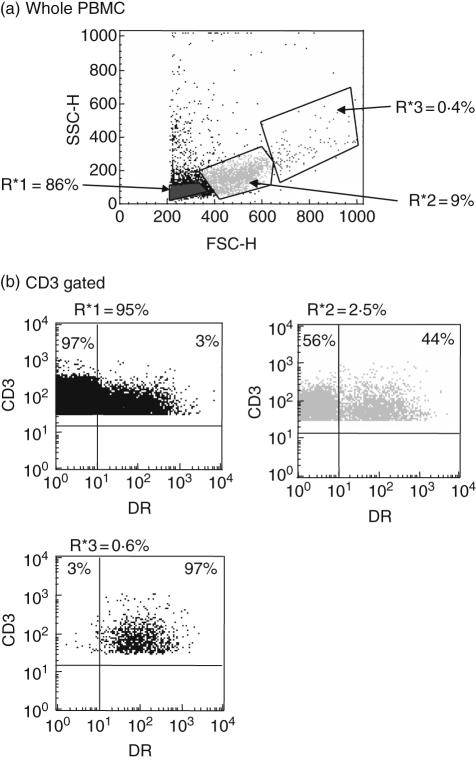
Figure 4a shows the regions applied to three populations of different size/granularity during analysis. The marked increase in levels of DR expression between the three gated populations is also demonstrated clearly in Fig. 4b (3% in R*1, 44% in R*2 and 97% in R*3).
Fig. 4.
Flow cytometry regions used for analysis in series B. (a) Regions used for analysis shown on forward and side scatter profile of whole peripheral blood mononuclear cells (PBMC). (b) Regions used for analysis of CD3 gated cells. Insets show the CD3+ DR+ double-staining profile of cells from each of the three regions, showing an increasing percentage of CD3+ DR+ cells on moving from R*1 to R*2 and R*3.
We were surprised to observe a significant reduction in the overall size of the CD3+ R*2 and R*3 populations in ND subjects when compared with LS subjects (R*2: 2·4% versus 3·4%; R*3: 0·4% versus 0·9%; Fig. 5b,c), respectively). In contrast, however, within both the R*2 and R*3 populations, the percentage of CD3+ DR+ cells was significantly greater in ND subjects than in LS subjects (R*2: 39% versus 31%, R*3: 95% versus 83%, Fig. 5e,f, respectively). In the R*1 population there was a small but non-significant trend towards an increase in CD3+ DR+ cells as a percentage of total T cells in ND subjects compared with LS subjects (3·0% versus 2·0%, Fig. 5d), comparable with the trend towards an increase in CD3+ DR+ cells seen in the whole peripheral blood population in ND subjects in series A (Fig. 1a).
Fig. 5.
CD3+ (a–c) and CD3+ DR+ (d–f) cells shown as a percentage of total peripheral blood mononuclear cells (PBMC) (a–c) or total CD3+ cells (d–f) in the different analysis regions (R*1, R*2, R*3) in series B. Newly diagnosed (ND) and long-standing (LS) subjects are compared. Median values are indicated by horizontal lines.
Comparison of CD3+ DR+ CD30+ and other subpopulation sizes in series B
Table 2 shows the median absolute triple-positive population sizes as a percentage of all CD3+ cells (Table 2a) and as a percentage of CD3+ DR+ cells (Table 2b) in the blood sample. Comparison is made between ND and LS subjects across all eight markers and all three gated regions. The CD3+ CD4+ CD25+ population was excluded from the latter analysis as it is not a subfraction of the CD3+ DR+ population. Significant increases in the triple-positive CD30+, CD25+ and CD134+ populations were seen in the R*1 population as well as significant reductions in most populations in the large cell (very activated) R*3 population (Table 2a). However, after correction for changes in the total CD3+ DR+ population between ND and LS subjects (as seen in Fig. 5) significant increases in the triple positive population size were apparent only for the CD30+ subset (Table 2b). Differences were seen in R*1 and R*2 but did not reach significance in R*3.
Table 2.
Comparison of T cell subsets in series B.
| CD30 | CD69 | CD25 | CD122 | CD38 | CD134 | CCR5 (CD195) | CD4/CD25 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ND | LS | ND | LS | ND | LS | ND | LS | ND | LS | ND | LS | ND | LS | ND | LS | |
| (a) Absolute numbers of T cellsa | ||||||||||||||||
| R*1 | 0·17** | 0·07 | 0·11 | 0·08 | 0·56* | 0·33 | 0·29 | 0·15 | 0·91 | 0·63 | 0·07* | 0·06 | 0·18 | 0·06 | 1·8 | 1·56 |
| R*2 | 0·29 | 0·25 | 0·32 | 0·33 | 0·33 | 0·36 | 0·53 | 0·43 | 0·80 | 0·81 | 0·18 | 0·19 | 0·18 | 0·20 | 0·24 | 0·18 |
| R*3 | 0·23† | 0·38 | 0·26 | 0·45 | 0·22* | 0·46 | 0·37** | 0·55 | 0·44** | 0·91 | 0·21 | 0·15 | 0·18* | 0·52 | 0·12 | 0·21 |
| (b) Percentage of the CD3+ DR+ population in each regionb | ||||||||||||||||
| R*1 | 6·23* | 4·56 | 3·52 | 3·86 | 24·61 | 19·1 | 8·57 | 6·84 | 30·71 | 34·8 | 3·12 | 2·77 | 5·63 | 3·54 | 3·49 | 3·11 |
| R*2 | 30·1** | 23·2 | 35·3 | 29·2 | 33·02 | 29·46 | 53·0 | 49·71 | 80·47 | 73·5 | 16·44 | 14·05 | 17·72 | 12·71 | 9·18 | 8·46 |
| R*3 | 62·3 | 53·7 | 86·9 | 86·07 | 64·56 | 64·51 | 87·63 | 90·12 | 98·36 | 97·36 | 43·36 | 42·2 | 58·41 | 52·94 | 14·96 | 12·92 |
ND: newly diagnosed; LS: long-standing; C: age-matched controls.
CD3+ DR+ CD‘X’+ populations in each region shown as a percentage of the total number of T cells in the whole sample.
CD3+ DR+ CD‘X’+ populations in each region shown as a percentage of the CD3+ DR+ cells in that region.
P < 0·05 ND versus LS;
P < 0·005 ND versus LS;
P = 0·05 ND versus LS.
Characterization of the CD3+ DR+ CD30+ subset by four-colour flow cytometry
Four-colour flow cytometry in a subset of ND subjects (n = 4) indicated that the CD3+ DR+ CD30+ population is > 70% CD4+ in subjects with type 1 diabetes in each of the three gated regions (data not shown). In addition, the CD3+ DR+ CD30+ population has limited overlap with other activated populations in regions R*1 and R*2 (for example, 0–12% CD69+, 0–20% CD122+). CD3+ DR+ CD30+ cells in R*3 were more frequently positive for additional activation markers (for example, 60–90% CD69+ and 25–90% CD122+).
Discussion
The main finding of the current study is a consistent expansion of 30–50% in the CD3+ DR+ CD30+ activated T cell population in patients with newly diagnosed diabetes compared to patients with long-standing disease (> 10 years). Expansions were seen in the standard lymphocyte population (R*1), but also in the moderately enlarged population (R*2) when correction was made for the fall in total cell numbers in this population.
The CD3+ DR+ CD30+ population in the R*1 and R*2 gated regions comprises around 0·36% of all circulating T cells and increases by 0·1% to a median value of 0·46% in ND patients. A further population of CD3+ DR+ CD30+ cells is present in the very large-cell R*3 population accounting for 0·3% of circulating T cells but this does not expand and shows a trend to reduction in size in ND patients (P = 0·05, Table 2a). The increase in population size in small- and medium-sized T cells (0·1%) is modest but consistent across both study series and represents the expected population expansion if five to 10 clones each of frequency one in 104 (0·01%) were to double in size. Such frequencies are consistent with recent results from ELISPOT studies [22] and studies with class II tetramers on the clonal frequency of antigen-reactive T cells [23] (Peakman et al. personal communication). The finding of an expansion in the CD3+ DR+ CD30+ population in the enlarged T cell population, R*2, is perhaps not surprising as enlargement in cell size typically indicates recent activation [33], but it emphasizes that this population (Fig. 4b) should not be excluded by gating in the analysis of lymphocytes from patients with diabetes, as is often performed.
The small size of the subpopulations under study, and the changes in total size of the larger T cell populations observed in newly diagnosed patients (Fig. 5), emphasize the complexity of studying activated T cell populations in the peripheral blood. A 37% rise in the overall percentage of DR+ T cells was seen in ND versus LS subjects (Fig. 1a) and although it did not reach statistical significance in our adult population, it is consistent with previous studies in younger populations [34–36]. It is possible that larger differences in the CD3+ DR+ CD30+ population would also be seen if the study were repeated in newly diagnosed children rather than adults, and this would certainly be of interest. The findings of an increase in the CD3+ DR+ CD69+ and CD3+ DR+ CD122+ populations in ND subjects versus LS subjects in series A (Fig. 2) were not confirmed in series B and may represent limitations in the statistical power of the study. No change in the CD4+ CD25+ population was seen in newly diagnosed diabetes, consistent with other reports, although there are qualitative changes in this population [37].
The observation that CD30 is expressed preferentially on an activated T cell population that appears to expand in newly diagnosed diabetes is interesting. CD30 (Ki-1) is a 120-kDa type 1 membrane glycoprotein of the TNF-receptor superfamily (TNFRSF8 [38]) identified originally on the tumour cells of Hodgkin's disease [39]. The exact physiological function of CD30 remains uncertain, but it binds to CD153 (CD30L) a 26–40 kDa member of the TNF family which is expressed on resting B cells and signal transduction via CD30 has been reported to co-stimulate T cells under some circumstances [40,41]. Initial reports associated CD30 with the Th2 phenotype but more recent reports have detracted from this [38]. CD30 may also play a role in thymic negative selection as CD30 knock-out mice have enlarged thymuses and increased numbers of thymocytes [42]. In peripheral T cells, CD30 is not expressed in the resting state. Although it is up-regulated after T cell activation, it is a relatively late activation marker with expression not appearing until 2 days after activation and persisting for more than 7 days [43]. We believe that such time–course considerations probably best explain the apparent coexpansion of the DR+ CD30+ population in the peripheral blood in newly diagnosed diabetes. Both markers appear to have a similar time–course of expression (10–14 days [44]) and hence would tend to be co-expressed after activation. Furthermore, the time–course of expression of both markers is not so brief so that any changes would not be apparent in the peripheral blood (as is the case with CD69) and not so prolonged that levels would be insensitive to changes in disease activity (as seen with CD45RO, for example). It is also possible that features of the autoimmune process in the islets favour CD30 expression, as induction of CD30 has been shown to be highly dependent on the method of T cell activation used [44] and expression segregates separately from other activation markers of the TNF–TNFR superfamily, for example CD134 (OX40), CD27 and 4-1BB (CD137) [44]. Immunocytochemistry of islets from active type 1 diabetes would be required to resolve this issue. It is interesting that our CD3+ DR+ CD30+ cells appear to be predominantly CD4+, as previous reports have suggested that the majority of CD30+ cells in healthy controls are CD8+ [45], but it should be emphasized that our analysis of this parameter was performed in only a small subgroup of subjects (n = 4).
Although the present study is the first evidence of a role of CD30-expressing T cells in human type 1 diabetes, several associations have been reported between CD30 and autoimmune diabetes in mice. In 1999 Kurts et al. reported that CD30-deficient mice were more susceptible to diabetes on adoptive transfer of islet-reactive CD8 cells, but later modified their conclusion after segregating disease susceptibility from CD30-deficiency in further back-crosses [46]. None the less, CD30 has been reported as a possible diabetes susceptibility gene in the non-obese diabetic (NOD) mouse [47] and a recent study indicated that treatment with anti-CD30L monoclonal antibody prevents disease development and disease transfer in NOD mice [48].
The strength of the current report lies in the consistent demonstration of the expansion in CD3+ DR+ CD30 in two independent studies, using different techniques and involving a total of over 40 newly diagnosed patients. However, further confirmation in other studies is required. We predict that the CD3+ DR+ CD30+ population will be rich in disease-specific T cells that have recently been released from the pancreas. If so, it should be possible to confirm this with HLA-tetramers and antigen-specific assays [22,36]. If confirmed, the identification of DR+ CD30+ as a specific activated T cell surface phenotype associated with disease will be of great value for the rapid isolation and characterization of T cells involved in type 1 diabetes.
Acknowledgments
We are extremely grateful to Professor Mark Peakman and Dr Sefina Arif for HLA-DR typing, to Professor Polly Bingley, Dr Kathleen Gillespie and Mr Alistair Williams for islet autoantibody assays, and to physicians and diabetes specialist nurses in all the collaborating centres for referring newly diagnosed patients. This work was supported by grants from Diabetes UK and the United Bristol Healthcare Trust Special Trustees.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Gardner SG, Gale EA, Williams AJ, et al. Progression to diabetes in relatives with islet autoantibodies. Is it inevitable? Diabetes Care. 1999;22:2049–54. doi: 10.2337/diacare.22.12.2049. [DOI] [PubMed] [Google Scholar]
- 3.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes. 1997;46:1701–10. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 4.Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53:384–92. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- 5.Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia. 2003;46:305–21. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- 6.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–53. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 7.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 8.Schatz DA, Bingley PJ. Update on major trials for the prevention of type 1 diabetes mellitus: the American Diabetes Prevention Trial (DPT-1) and the European Nicotinamide Diabetes Intervention Trial (ENDIT) J Pediatr Endocrinol Metab. 2001;14(Suppl. 1):619–22. doi: 10.1515/jpem.2001.14.s1.619. [DOI] [PubMed] [Google Scholar]
- 9.Greenbaum CJ, Harrison LC. Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes. 2003;52:1059–65. doi: 10.2337/diabetes.52.5.1059. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Ebisuno Y, Kanemitsu N, et al. Molecular determinants controlling homeostatic recirculation and tissue-specific trafficking of lymphocytes. Int Arch Allergy Immunol. 2004;134:120–34. doi: 10.1159/000078497. [DOI] [PubMed] [Google Scholar]
- 11.Doherty PC, Christensen JP. Accessing complexity: the dynamics of virus-specific T cell responses. Annu Rev Immunol. 2000;18:561–92. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 12.Wong FS, Karttunen J, Dumont C, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–31. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 13.Trudeau JD, Kelly-Smith C, Verchere CB, et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111:217–23. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayan CM, Londei M, Corcoran AE, et al. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci USA. 1991;88:7415–19. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullins RJ, Cohen SB, Webb LM, et al. Identification of thyroid stimulating hormone receptor-specific T cells in Graves' disease thyroid using autoantigen-transfected Epstein–Barr virus-transformed B cell lines. J Clin Invest. 1995;96:30–7. doi: 10.1172/JCI118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischof F, Hofmann M, Schumacher TN, et al. Analysis of autoreactive CD4 T cells in experimental autoimmune encephalomyelitis after primary and secondary challenge using MHC class II tetramers. J Immunol. 2004;172:2878–84. doi: 10.4049/jimmunol.172.5.2878. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda S, Van de Water J, Ansari A, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–40. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein–Barr virus in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bieganowska K, Hollsberg P, Buckle GJ, et al. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11–19 tetramer complexes in patients with human T cell lymphotropic virus-associated myelopathy. J Immunol. 1999;162:1765–71. [PubMed] [Google Scholar]
- 20.Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV. Quantification of self-recognition in multiple sclerosis by single-cell analysis of cytokine production. J Immunol. 2000;165:1641–51. doi: 10.4049/jimmunol.165.3.1641. [DOI] [PubMed] [Google Scholar]
- 21.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–8. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reijonen H, Novak EJ, Kochik S, et al. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–82. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 24.Kotzin BL, Falta MT, Crawford F, et al. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:291–6. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan LC, Gudgeon N, Annels NE, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–35. [PubMed] [Google Scholar]
- 26.Dosch HM, Becker DJ. Measurement of T-cell autoreactivity in autoimmune diabetes. Diabetologia. 2000;43:386–7. doi: 10.1007/s001250050060. [DOI] [PubMed] [Google Scholar]
- 27.Roep BO. Autoreactive T cells in endocrine/organ-specific autoimmunity: why has progress been so slow? Springer Semin Immunopathol. 2002;24:261–71. doi: 10.1007/s00281-002-0109-8. [DOI] [PubMed] [Google Scholar]
- 28.Mazza G, Ponsford M, Lowrey P, Campbell MJ, Zajicek J, Wraith DC. Diversity and dynamics of the T-cell response to MBP in DR2+ve individuals. Clin Exp Immunol. 2002;128:538–47. doi: 10.1046/j.1365-2249.2002.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 30.De Berardinis P, Londei M, Kahan M, et al. The majority of the activated T cells in the blood of insulin-dependent diabetes mellitus (IDDM) patients are CD4+ Clin Exp Immunol. 1988;73:255–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Bingley PJ, Bonifacio E, Shattock M, et al. Can islet cell antibodies predict IDDM in the general population? Diabetes Care. 1993;16:45–50. doi: 10.2337/diacare.16.1.45. [DOI] [PubMed] [Google Scholar]
- 32.Gillespie KM, Valovin SJ, Saunby J, et al. HLA class II typing of whole genome amplified mouth swab DNA. Tissue Antigens. 2000;56:530–8. doi: 10.1034/j.1399-0039.2000.560607.x. [DOI] [PubMed] [Google Scholar]
- 33.Biswas P, Galli A, Capiluppi B, Ciuffreda D, Lazzarin A, Tambussi G. Selective enrichment of CD30-expressing cells within the blast region of lymphocytes from patients with primary HIV infection (PHI) J Biol Regul Homeost Agents. 2002;16:33–6. [PubMed] [Google Scholar]
- 34.Hehmke B, Michaelis D, Gens E, Laube F, Kohnert KD. Aberrant activation of CD8+ T-cell and CD8+ T-cell subsets in patients with newly diagnosed IDDM. Diabetes. 1995;44:1414–19. doi: 10.2337/diab.44.12.1414. [DOI] [PubMed] [Google Scholar]
- 35.Peakman M, Leslie RD, Alviggi L, Hawa M, Vergani D. Persistent activation of CD8+ T-cells characterizes prediabetic twins. Diabetes Care. 1996;19:1177–84. doi: 10.2337/diacare.19.11.1177. [DOI] [PubMed] [Google Scholar]
- 36.Kadziela K, Kowalska H, Rymkiewicz-Kluczynska B, et al. Changes in lymphocyte subsets in children with newly diagnosed type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16:185–91. doi: 10.1515/jpem.2003.16.2.185. [DOI] [PubMed] [Google Scholar]
- 37.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+) CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 38.Bengtsson A. The role of CD30 in atopic disease. Allergy. 2001;56:593–603. doi: 10.1034/j.1398-9995.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 39.Schwab U, Stein H, Gerdes J, et al. Production of a monoclonal antibody specific for Hodgkin and Sternberg–Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982;299:65–7. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- 40.Smith CA, Gruss HJ, Davis T, et al. CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–60. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 41.Horie R, Watanabe T. CD30: expression and function in health and disease. Semin Immunol. 1998;10:457–70. doi: 10.1006/smim.1998.0156. [DOI] [PubMed] [Google Scholar]
- 42.Amakawa R, Hakem A, Kundig TM, et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–62. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]
- 43.Ellis TM, Simms PE, Slivnick DJ, Jack HM, Fisher RI. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol. 1993;151:2380–9. [PubMed] [Google Scholar]
- 44.Chan KW, Hopke CD, Krams SM, Martinez OM. CD30 expression identifies the predominant proliferating T lymphocyte population in human alloimmune responses. J Immunol. 2002;169:1784–91. doi: 10.4049/jimmunol.169.4.1784. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal B, Reddish M, Longenecker BM. CD30 expression on human CD8+ T cells isolated from peripheral blood lymphocytes of normal donors. J Immunol. 1996;157:3229–34. [PubMed] [Google Scholar]
- 46.Kurts C, Carbone FR, Krummel MF, Koch KM, Miller JF, Heath WR. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 1999;398:341–4. doi: 10.1038/18692. [DOI] [PubMed] [Google Scholar]
- 47.Siegmund T, Armitage N, Wicker LS, Peterson LB, Todd JA, Lyons PA. Analysis of the mouse CD30 gene: a candidate for the NOD mouse type 1 diabetes locus Idd9. 2 Diabetes. 2000;49:1612–16. doi: 10.2337/diabetes.49.9.1612. [DOI] [PubMed] [Google Scholar]
- 48.Chakrabarty S, Nagata M, Yasuda H, et al. Critical roles of CD30/CD30L interactions in murine autoimmune diabetes. Clin Exp Immunol. 2003;133:318–25. doi: 10.1046/j.1365-2249.2003.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]