Abstract
Common variable immunodeficiency (CVID) is a heterogeneous syndrome characterized by defective immunoglobulin production and high frequency of bacterial infections, autoimmunity and manifestations of chronic inflammation. Abnormalities of CD4+CD25highforkhead box P3 (FoxP3)+ regulatory T cells (Treg) have been associated with autoimmune and inflammatory disorders, and we hypothesized that CVID might be characterized by Treg abnormalities. CD3+ cells from patients and controls were analysed for the expression of FoxP3 mRNA by real time reverse transcription–polymerase chain reaction (RT–PCR). Peripheral blood mononuclear cells from CVID patients and controls were stained for Treg markers, analysed by flow cytometry and compared to clinical characteristics. The main findings were: (i) CVID patients had significantly decreased expression of FoxP3 mRNA and decreased proportions of CD4+CD25highFoxP3+ cells compared to controls; (ii) CVID patients with splenomegaly had even lower proportions of Treg compared to other patients and controls; (iii) serum levels of the inflammatory marker neopterin were correlated negatively with the proportions of Treg within the CVID population, while there was no significant association with bronchiectasis. We have demonstrated decreased proportions of Treg in CVID patients, particularly in those with signs of chronic inflammation. Decreased proportions of TReg are suggested to be pathogenetically important in autoimmunity, and our results suggest that TReg may have a similar role in CVID.
Keywords: autoimmunity, chronic inflammation, Common variable immunodeficiency, FoxP3, regulatory T cells
Introduction
Common variable immunodeficiency (CVID) is a heterogeneous syndrome characterized by failure of B cell differentiation and defective immunoglobulin (Ig) production leading to recurrent bacterial infections, particularly in the respiratory tract. Although reduced Ig secretion from B cells is the hallmark of CVID, T cell abnormalities such as dysregulated cytokine production, impaired T cell proliferation in vitro and altered distribution of T cell subsets are seen in a considerable proportion of patients. These abnormalities may be of importance for both the B cell deficiency and for some of the clinical manifestations in these patients, which include autoimmunity and other manifestations of inflammatory and immunological hyperactivity such as splenomegaly and raised levels of inflammatory markers [1–3].
Several subgroups of T cells mediate regulatory mechanisms in the T cell immune response. The so-called regulatory T cells (Treg) are a subgroup of CD4+ T cells characterized by the up-regulation of the interleukin (IL)-2 receptor α-chain (CD25) and expression of the transcription factor protein forkhead box P3 (FoxP3) [4,5]. The biological role of Treg is thought to dampen immune responses through mechanisms which are not fully elucidated [4,6,7]. Treg are now characterized in both mice and humans, and their role in various clinical conditions such as transplant rejection reactions, cancer, autoimmunity and chronic infectious diseases is a subject of active investigation [8–11]. We hypothesized that CVID, with its high frequency of autoimmunity and signs of chronic inflammation, might be characterized by Treg abnormalities.
Materials and methods
Patients
Twenty-six patients with CVID, according to the criteria of the World Health Organization expert group for primary immunodeficiencies [1], attending our department were included consecutively in the study (Table 1). The patients did not have any clinically apparent infection when recruited, and did not receive treatment with antibiotics or corticosteroids. The diagnosis of splenomegaly was defined as spleen length > 13 cm on ultrasonographic or computed tomography (CT) scan examination and the diagnosis of bronchiectasis was based on typical findings on high-resolution CT scan of the thorax [12]. Eleven sex- and age-matched healthy individuals were included as controls. Informed consent for blood sampling was obtained from all subjects. The study was conducted according to the ethical guidelines at our hospital, which comply with the Helsinki declaration, and was approved by the hospital's authorized representative.
Table 1.
Characteristics of the study group.
| Controls (n = 11) | CVID (n = 26) | P-value | |
|---|---|---|---|
| Demographics | |||
| Gender (male/female, %) | 45/55 | 50/50 | 0·8 |
| Age (years, median and 25th −75th percentiles) | 45 (38–65) | 49 (39–58) | 0·92 |
| Clinical characteristics | |||
| Bronchiectasis (%) | 69 | ||
| Splenomegaly (%) | 58 | ||
| Idiopathic thrombocytopenic purpura (%)* | 34 | ||
| Granulomatous disease (%)** | 15 | ||
| Therapy | |||
| SCIG/IVIG (%)*** | 62/38 | ||
Cases include any history of idiopathic thrombocytopenic purpura.
Cases include granulomatous disease as confirmed by biopsy.
Some patients on IVIG received supplementary SCIG. IVIG: intravenous immunoglobulin therapy; SCIG: subcutaneous immunoglobulin therapy.
Isolation of cells
Peripheral blood mononuclear cells (PBMC) were obtained from heparinized blood by Isopaque-Ficoll (Lymphoprep; Nycomed Pharma, Oslo, Norway) gradient centrifugation. For flow cytometry, peripheral blood mononuclear cells (PBMC) were cryopreserved in liquid nitrogen. Further separation of CD3+ T cells (negative selection by monodisperse immunomagnetic beads; Dynal, Oslo, Norway) was performed as described elsewhere [13,14]. The negatively selected T cells consisted of > 90% CD3+ cells.
Flow cytometry
Cryopreserved PBMC were thawed and stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4, phycoerythrin (PE)-conjugated anti-FoxP3 and allophycocyanin (APC)-conjugated anti-CD25 antibodies with appropriate isotype controls (all from eBiosciences, San Diego, CA, USA). Flow cytometry was performed using a fluorescence activated cell sorter (FACS)Calibur instrument with CellQuest software (Becton Dickinson, San Diego, CA, USA). List mode files were collected for 200 000 cells from each sample. CD25high cells were defined as described elsewhere [15]. Cells from the lymphocyte gate was used for analysis and prior to permeabilization this gate contained less than 5% dead cells as measured by staining with propidium iodide.
Real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted from T cells using RNeasy columns (Qiagen, Hilden, Germany), subjected to DNase I treatment (RQI DNase; Promega, Madison, WI, USA) and stored at −80°C. Primers were designed using the Primer Express software, version 2·0 (Applied Biosystems, Foster City, CA, USA) for FoxP3 (forward primer: 5′-ACTGCCAGGCGGACCAT-3′, reverse primer: 5′-CTTCTCCAGCACCAGCTGCT-3′). Quantification of mRNA was performed using the ABI Prism 7000 (Applied Biosystems). Gene expression of the housekeeping gene α-actin (forward primer: 5′-AGGCACCAGGGCGTGAT-3′, reverse primer: 5′-TCGTCCCAGTTGGTGACGAT-3′) was used for normalization.
Cell cultures
Cell cultures were performed as described elsewhere [16]. Briefly, negatively selected T cells (106 cells/ml, 0·2 ml/well; Costar, Cambridge, MA, USA) were stimulated with anti-CD3 (clone SpvT3b, 40 ng/ml; Dynal) and anti-CD28 (clone 15E8, 50 ng/ml; CLB, Amsterdam, the Netherlands) for 48 h and supernatants were analysed for interleukin (IL)-10 levels by enzyme immunoassay (R&D Systems, Minneapolis, MN, USA).
Statistical methods
For comparison of two groups of individuals, the Mann–Whitney U-test was used. When more than two groups of individuals were compared, the Kruskal–Wallis test was used, and if significant a Mann–Whitney U-test was performed comparing differences between each pair of groups. Coefficients of correlation were calculated by the Spearman's rank test. P-values are two-sided and considered significant when < 0·05.
Results
The proportion of Tteg in CVID patients and healthy controls
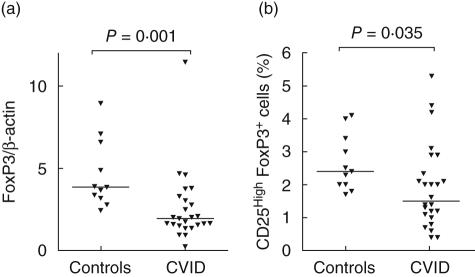
As shown in Fig. 1a, CVID patients (n = 25) had significantly decreased gene expression of the transcriptional factor FoxP3 in CD3+ cells compared to healthy controls (n = 11). This reduction of FoxP3 was extended and confirmed by flow cytometry, showing decreased proportions of CD4+CD25highFoxP3+ cells in the CD4+ cell population of the CVID patients (n = 24)(Figs 1b and 2).
Fig. 1.
Expression of forkhead box P3 (FoxP3) mRNA in T cells (a) and proportions of CD4+CD25highFoxP3+ cells in the CD4+ cell population (b) among healthy controls and common variable immunodeficiency patients.
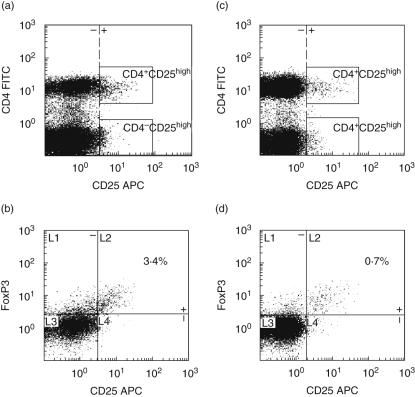
Fig. 2.
CD4+CD25high and CD4+CD25highforkhead box P3 (FoxP3)+ cells in one representative healthy control (a, b, respectively) and in one representative common variable immunodeficiency (CVID) patient with splenomegaly (c, d, respectively) as measured by flow cytometry. Boxes (a, c) indicate cells in the CD25high population. Quadrants (b, d) indicate CD25highversus CD25low/intermediate cells and FoxP3+versus FoxP3– cells.
The proportion of Treg in relation to clinical and immunological parameters in CVID
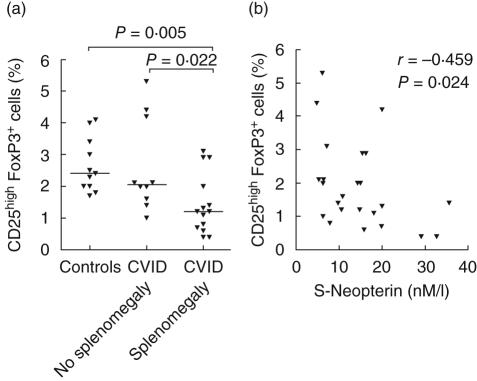
As shown in Fig. 3a, patients with splenomegaly had markedly decreased proportions of CD4+CD25highFoxP3+ cells compared to both healthy controls and other CVID patients. Moreover, CVID patients with idiopathic thrombocytopenic purpura (ITP) had a decrease in the CD4+CD25highFoxP3+ cell populations compared to other CVID patients, although the difference did not reach statistical significance (P = 0·086). CVID patients with granulomas as confirmed by biopsy also had a non-significant decrease in Treg compared to patients without confirmed granulomatous disease (P = 0·373). In contrast, there was no association between the proportions of CD4+CD25highFoxP3+ cells and the occurrence of bronchiectasis in the CVID population. Serum neopterin level is a reliable marker of monocyte/macrophage activation [17] previously shown to be elevated in CVID [18–20]. Herein we found that serum levels of neopterin were correlated negatively to the proportions of CD4+CD25highFoxP3+ cells within the CVID group (Fig. 3b).
Fig. 3.
Proportions of CD4+CD25highforkhead box P3 (FoxP3)+ cells in the CD4+ cell population among healthy controls and common variable immunodeficiency (CVID) patients with and without splenomegaly (a). Correlation between proportions of CD4+CD25highFoxP3+ cells and serum levels of neopterin in CVID patients (b).
We have described previously impaired IL-10 release by T cells from CVID patients [16], and IL-10 levels in T cell supernatants of these patients correlated significantly with the proportion of CD4+CD25highFoxP3+ cells found in the same patients in our study (r = 0·730, P = 0·046).
Discussion
In the present study we found decreased proportions of CD4+CD25highFoxP3+ cells in the CVID group, particularly in patients with splenomegaly and raised levels of serum neopterin, indicating low numbers of Treg in these patients.
While the co-expression of CD4 and CD25high has been used widely as a phenotype marker for Treg, the identification of FoxP3 now allows for a more strict and valid classification of Treg even in clinical studies [21]. Previous studies on Treg may have been confounded by a failure to separate regulatory cells that highly express CD25 from other CD4+CD25+ cells [22]. We believe that using the combination of CD4+CD25high with FoxP3 as a marker of Treg will reflect more accurately the true numbers of Treg. In this study, the decreased proportions of CD4+CD25highFoxP3+ cells in CVID patients compared to controls is supported by low expression of FoxP3 mRNA in CD3+ cells in the same patients.
CVID is a heterogeneous group of disorders with a variety of clinical and immunological characteristics, including signs of chronic inflammation. Lymphoid hyperplasia in the form of splenomegaly is common in CVID [2], particularly in those subgroups of CVID characterized by low numbers of memory B cells [23,24], and often in association with raised levels of inflammatory cytokines [25,26]. Interestingly, we find even lower proportions of Treg in CVID patients with splenomegaly compared to both healthy controls and other CVID patients. Moreover, the negative correlation between levels of neopterin, reflecting the degree of monocyte/macrophage activation [17], and proportions of Treg further supports a link between chronic inflammation and a decrease of Treg in these patients. We have described previously a subgroup of CVID patients with chronic inflammation in vivo characterized by splenomegaly, signs of T cell dysfunction and raised neopterin levels [19,25,27,28] and our findings in this study suggest that low numbers of Treg could be added to the list of immunological features characterizing this group of CVID patients.
The reason for the low numbers of Treg in CVID patients with splenomegaly is unclear; while low numbers of circulatory CD4+CD25high cells have been observed in some other diseases [10], even without splenomegaly, this is less well documented for CD4+CD25highFoxP3+ cells [29,30]. The homeostasis of Treg is probably controlled by several factors, among them the differentiation of Treg in the thymus, induction and/or expansion of Treg in the periphery, half-life of Treg in the circulation and possible redistribution of Treg to extra-vascular tissue [31]. We can only speculate which factors contribute to our finding, but redistribution of Treg to the spleen is one of several possible explanations.
Both ITP and granulomatous disease are common complications of CVID, and although the decrease of Treg in these groups of patients did not reach statistical significance we cannot exclude a possible association. Interestingly, we found no correlation between the numbers of Treg and the occurrence of bronchiectasis, suggesting that the inflammatory burden imposed by the recurrent bacterial pulmonary infections associated with this condition is different from the chronic inflammation associated with splenomegaly.
The effector mechanisms of Treg are not clear, but cell contact appears to be of major importance. However, although the role of soluble cytokines has not been established firmly, IL-10 and transforming growth factor-α have been suggested to be involved in the Treg-mediated regulation of T cell responses in vivo [4,6,7]. We have reported previously decreased secretion of IL-10 in T cell cultures from CVID patients [16], and our finding in the present study with a correlation between secreted IL-10 levels in T cell cultures and Treg numbers in CVID may further support a possible link between Treg and IL-10.
The present study is, to our knowledge, the first demonstration of decreased proportions of Treg in CVID patients, showing a particularly low percentage in those with signs of chronic inflammation in vivo. Decreased proportions of Treg and a FoxP3 defect have been suggested to be pathogenetically important in autoimmunity [10]. Our findings in the present study, showing markedly decreased proportions of CD4+CD25highFoxP3+ cells in CVID patients with splenomegaly and a negative correlation to neopterin levels, suggest that Treg may have a similar role in subgroups of this immunodeficiency. However, further studies are needed to clarify whether this Treg abnormality contributes to the pathogenesis of CVID.
Acknowledgments
This work was supported financially by the Research Council of Norway. We thank Bodil Lunden for excellent technical assistance.
References
- 1.Rosen FS, Eibl MM, Roifman CM, et al. Primary immunodeficiency diseases. Report of an IUIS Scientific Committee. Int Union Immunol Soc Clin Exp Immunol. 1999;118(Suppl. 1):1–28. doi: 10.1046/j.1365-2249.1999.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Renzo M, Pasqui AL, Auteri A. Common variable immunodeficiency: a review. Clin Exp Med. 2004;3:211–7. doi: 10.1007/s10238-004-0027-2. [DOI] [PubMed] [Google Scholar]
- 3.Brandt D, Gershwin ME. Common variable immune deficiency and autoimmunity. Autoimmun Rev. 2006;5:465–70. doi: 10.1016/j.autrev.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6.Levings MK, Roncarolo MG. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol. 2005;293:303–26. doi: 10.1007/3-540-27702-1_14. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–76. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 8.Waldmann H. Regulatory T cells in transplantation. Semin Immunol. 2006;18:111–19. doi: 10.1016/j.smim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavan S, Holmgren J. CD4+CD25+ suppressor T cells regulate pathogen induced inflammation and disease. FEMS Immunol Med. 2005;44:121–7. doi: 10.1016/j.femsim.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Fevang B, Mollnes TE, Holm AM, et al. Common variable immunodeficiency and the complement system; low mannose-binding lectin levels are associated with bronchiectasis. Clin Exp Immunol. 2005;142:576–84. doi: 10.1111/j.1365-2249.2005.02951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aukrust P, Aandahl EM, Skalhegg BS, et al. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J Immunol. 1999;162:1178–85. [PubMed] [Google Scholar]
- 14.Stylianou E, Aukrust P, Muller F, Nordoy I, Froland SS. Complex effects of interferon-alpha on the cytokine network in HIV infection − possible contribution to immunosuppression. Cytokine. 2001;14:56–62. doi: 10.1006/cyto.2000.0850. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+) CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 16.Holm AM, Aukrust P, Aandahl EM, Muller F, Tasken K, Froland SS. Impaired secretion of IL-10 by T cells from patients with common variable immunodeficiency − involvement of protein kinase A type I. J Immunol. 2003;170:5772–7. doi: 10.4049/jimmunol.170.11.5772. [DOI] [PubMed] [Google Scholar]
- 17.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 18.Harland C, Shah T, Webster AD, Peters TJ. Serum neopterin patients with X-linked and acquired ‘common variable’ hypogammaglobulinaemia. Int Arch Allergy Appl Immunol. 1989;88:471–3. doi: 10.1159/000234734. [DOI] [PubMed] [Google Scholar]
- 19.Aukrust P, Froland SS, Muller F. Raised serum neopterin levels in patients with primary hypogammaglobulinaemia; correlation to other immunological parameters and to clinical and histological features. Clin Exp Immunol. 1992;89:211–6. doi: 10.1111/j.1365-2249.1992.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilic SS, Kezer EY, Ilcol YO, Yakut T, Aydin S, Ulus IH. Vitamin a deficiency in patients with common variable immunodeficiency. J Clin Immunol. 2005;25:275–80. doi: 10.1007/s10875-005-4090-6. [DOI] [PubMed] [Google Scholar]
- 21.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 22.Graca L. New tools to identify regulatory T cells. Eur J Immunol. 2005;35:1678–80. doi: 10.1002/eji.200526303. [DOI] [PubMed] [Google Scholar]
- 23.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+) IgM(-) IgD(-) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 24.Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 25.Aukrust P, Lien E, Kristoffersen AK, et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency − possible immunologic and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]
- 26.Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159:6236–41. [PubMed] [Google Scholar]
- 27.Aukrust P, Muller F, Froland SS. Elevated serum levels of interleukin-4 and interleukin-6 in patients with common variable immunodeficiency (CVI) are associated with chronic immune activation and low numbers of CD4+ lymphocytes. Clin Immunol Immunopathol. 1994;70:217–24. doi: 10.1006/clin.1994.1032. [DOI] [PubMed] [Google Scholar]
- 28.Aukrust P, Svardal AM, Muller F, Lunden B, Berge RK, Froland SS. Decreased levels of total and reduced glutathione in CD4+ lymphocytes in common variable immunodeficiency are associated with activation of the tumor necrosis factor system: possible immunopathogenic role of oxidative stress. Blood. 1995;86:1383–91. [PubMed] [Google Scholar]
- 29.Barath S, Sipka S, Aleksza M, et al. Regulatory T cells in peripheral blood of patients with mixed connective tissue disease. Scand J Rheumatol. 2006;35:300–4. doi: 10.1080/03009740600709790. [DOI] [PubMed] [Google Scholar]
- 30.Longhi MS, Hussain MJ, Mitry RR, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–91. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 31.Wei S, Kryczek I, Zou W. Regulatory T cell compartmentalization and trafficking. Blood. 2006;108:426–31. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]