Abstract
Toll-like receptors (TLR) play an essential role in the activation of both innate and adaptive immune responses. Salivary gland epithelial cells (SGEC) may participate in the development of glandular inflammatory reactions that characterize primary Sjögren's syndrome (pSS). In this study we sought to assess the expression and function of several TLR molecules in cultured non-neoplastic SGEC obtained from pSS patients and disease controls. Long-term cultured non-neoplastic SGEC derived from pSS patients (SS-SGEC) and disease controls (control-SGEC), as well as the monocytic cell line THP-1 (positive control cell line), were examined by reverse transcription–polymerase chain reaction (RT–PCR) analysis and quantitative real-time PCR for mRNA expression of TLR1, -2, -3 and -4 molecules. TLR function was assessed by the induction of the expression (flow cytometry) of the immunoregulatory molecules CD54/intercellular adhesion molecule-1 (ICAM-1), CD40, CD86/B7·2, major histocompatibility complex (MHC) class I and MHC class II following treatment with the TLR ligands: Staphylococcus aureus peptidoglycan (TLR2), the synthetic dsRNA analogue polyinosinic:cytidylic acid (TLR3) and Escherichia coli lipopolysaccharide (TLR4). SGEC were found to express functional TLR2, -3 and -4 molecules, as attested by dose-dependent up-regulation of surface ICAM-1, CD40 and MHC-I expression (as well as of reciprocal TLR mRNA) following treatment with the respective TLR-ligands. SS-SGEC lines displayed significantly higher constitutive expression of TLR1 (P = 0·0027), TLR2 (P = 0·01) and TLR4 (P = 0·03) mRNA compared to control-SGEC. This study demonstrates that cultured SGEC express functional TLR molecules; the high constitutive TLR expression by SS-SGEC is probably suggestive of the intrinsic activation of epithelial cells in pSS and further supports the role of this type of tissue in pathogenesis of the disorder.
Keywords: epithelial cells, innate immunity, salivary gland, Sjögren's syndrome, Toll-like receptors
Introduction
The Toll-like receptors (TLR) belong to a conserved family of type I transmembrane receptors that consists of 11 mammalian members. Each member of the TLR family has been shown to recognize distinct elements of bacteria, fungi or viruses known as pathogen-associated molecular patterns (PAMPs) [1]. TLR are broadly expressed in the body, with apparently wide variation in the expression levels of each TLR type between different tissues [2,3]. This most probably reflects specific physiological roles of each tissue type in the innate immune system [4]; however, analytical studies of particular types of cells are limited.
The expression of various types of TLR molecules has been described previously in several types of epithelial tissues, such as gastrointestinal, bronchial and urinary epithelia, supporting the notion that the epithelium serves a critical function as the defensive front line of the innate immune system [5–7]. Upon ligation, TLR signalling results in the priming of the adaptive immune system and the initiation of inflammatory responses through the induction of proinflammatory cytokines, chemokines, co-stimulatory and adhesion molecules [8,9]. Furthermore, TLR triggering appears to have a role in the pathogenesis of autoimmune disorders, as suggested by the induction or the promotion of organ-specific autoimmune lesions in various experimental animal models [10–12].
Primary Sjögren's syndrome (pSS) or autoimmune epitheliitis is a relative common autoimmune exocrinopathy characterized by chronic dysfunction and destruction of salivary and lacrimal glands associated with chronic lymphocytic infiltrating lesions [13]. Glandular epithelia appear to have an active role in the induction and perpetuation of tissue inflammatory reactions in pSS patients. In fact, during the recent decade, using cultured non-neoplastic salivary gland epithelial cell (SGEC) lines, we have provided evidence that salivary epithelia, by virtue of constitutive or inducible expression of various immunoactive proteins, are inherently capable to trigger immune responses, whereas an intrinsic activation process appears to operate in the epithelia of pSS patients, as suggested by the high constitutive expression of various molecules in SGEC lines derived from pSS patients [14–19].
In this study, given the importance of epithelial cells in the pathogenesis of inflammatory reactions of pSS patients and the crucial role of TLR signalling in the development of inflammatory responses, we aimed to assess in parallel the expression and function of several TLR molecules in cultured non-neoplastic SGEC lines obtained from pSS patients and disease controls. Herein, we demonstrate that SGEC express functional TLR2, -3 and -4 molecules as illustrated by the up-regulation of the expression of the adhesion molecule CD54/intercellular adhesion molecule-1 (ICAM-1), the co-stimulatory molecule CD40 and the antigen-presenting molecule major histocompatibility complex-1 (MHC-I) following triggering with appropriate ligands. In addition, SGEC derived from pSS patients were found to display significantly higher constitutive mRNA expression of various TLR molecules compared to disease controls, a fact which supports the intrinsic epithelial activation in pSS [18].
Materials and methods
Reagents
Phycoerythrin (PE)-conjugated monoclonal antibodies (MoAbs) against human CD54/ICAM-1 and CD86/B7·2 (clone FUN-1), the fluorescein isothiocyanate (FITC)-conjugated MoAbs against human leucocyte antigen (HLA)-antigen A, B, C (ABC) (MHC-I) and HLA-D-related (DR) (MHC-II) and the isotype matched-control MoAb were all purchased from Becton Dickinson (San Jose, CA, USA). The FITC-conjugated MoAb against human CD40 was purchased from Ancell (Batport, MN, USA). Peptidoglycan (PGN) (Staphylococcus aureus), polyinosinic:cytidylic acid (polyI:C) and lipopolysaccharide (LPS) (Escherichia coli, serotype O55:B5) were all purchased from Sigma (St Louis, MO, USA). To ensure against endotoxin contamination, all non-LPS culture reagents were tested routinely by the chromogenic Limulus amebocyte lysates assay (Sigma, St Louis, MO, USA). The human recombinant interferon-gamma-1b (IFN-γ, Imukin) was from Boehringer Ingelheim (Ingelheim, Germany).
Cell cultures
Labial minor salivary gland (MSG) biopsies were obtained with informed consent from 14 patients undergoing diagnostic evaluation for sicca symptoms indicative of SS, as approved by the regional hospital ethical committee. Patients studied included seven with pSS, diagnosed on the basis of the American–European classification criteria for SS [20], and seven disease controls who did not fulfil the SS classification criteria and did not have histopathological or serological evidence for SS. Non-neoplastic SGEC lines were established from each MSG biopsy by the explant outgrowth technique, as described previously [21]. At 70–80% confluence, each primary culture was trypsinized and passed serially onto culture vessels in serum-free keratinocyte basal medium (KBM; Clonetics, Walkersville, MD, USA) as described previously [21]. The epithelial origin of cultured SGEC was verified routinely by morphology, as well as by the uniform and consistent expression of epithelial markers and the absence of markers indicative of lymphoid/monocytoid cells. Previously published evidence from similarly established SGEC cultures are suggestive of their ductal epithelial origin [21]. SGEC lines obtained from pSS patients (SS-SGEC) and controls (control-SGEC) were treated in an identical fashion and were all subjected to two passages prior to analysis. The neoplastic TLR-expressing monocytic cell line, THP-1 (positive control cell line; kindly provided by Professor K. Karalis, Foundation for Medical Research of the Academy of Athens), was maintained in RPMI-10% fetal bovine serum (FBS) [22].
Functional assessment of TLR molecule expression
SS-SGEC and control-SGEC lines were cultured to confluence in 24-well plates in serum-free KBM medium and were exposed to medium alone or medium containing PGN (100 µg/ml, TLR2-ligand), polyI:C (5 µg/ml, TLR3-ligand), LPS (1 µg/ml, TLR4 ligand) or IFN-γ (500 IU/ml) for 24, 48 and 72 h. THP-1 cells were cultured in 24-well plates in RPMI-10% FBS and stimulated by PGN (100 µg/ml), polyI:C (5 µg/ml) or LPS (1 µg/ml) for 24, 48 and 72 h. The optimal doses of the TLR ligands were determined in preliminary experiments. The expression of CD54/ICAM.1, CD40, CD86/B7·2, MHC-I and MHC-II was assessed by flow cytometry using approximately 5 × 104 cells resuspended in ice-cold phosphate buffered saline containing 2·5% FBS and 0·3% NaN3. For staining the PE-conjugated MoAbs(to CD54/ICAM-1 or CD86/B7·2 proteins), the FITC-conjugated MoAbs (to CD40 or HLA-ABC or HLA-DR) or an isotype-matched control MoAb were applied. The cells were analysed using a FACSCalibur flow cytometer and cellquest software (Becton-Dickinson, San Jose, CA, USA). Mean fluorescence intensity (MFI) values obtained by staining with specific MoAbs were corrected by the subtraction of background values (isotype control MoAb).
Quantitative assessment of TLR molecule expression
Total RNA was extracted from 1 × 106 THP-1 cells and 1·5 × 106 SGEC (cultured to confluence in 24-well plates in serum-free KBM medium) using the Trizol RNA isolation system (Invitrogen Life Technologies, Paisley, UK) and the RNeasy Mini kit (Qiagen, Valencia, CA, USA), respectively, according to the manufacturer's instructions. One µg of total RNA was reverse-transcribed into cDNA using the ImProm-II Reverse Transcriptase kit (Promega, Madison, WI, USA). Quantitative real-time–PCR (Q–PCR) analysis was performed using SYBR Green I (Molecular Probes, Eugene, OR, USA) and specific primers for TLR1, -2, -3, -4 and 18S ribosomal RNA in a LightCycler by Roche Diagnostics (Mannheim, Germany); 18S rRNA was used as a house-keeping gene. The following primers were used to amplify a specific fragment of the following genes: TLR1, forward, 5′-ATAAAAGCAGGGGACAATCC-3′, reverse, 5′-GGCACACCATCCTGAGATAC-3′; TLR2, forward, 5′-TGCTCCTGTGAATTCCTCTC-3′, reverse, 5′-TCCCGCTCACTGTAAGAAAC-3′; TLR3, forward, 5′-GGGTCTGGGAACATTTCTCT-3′, reverse, 5′-AAAGGCACCTATCCGTTCTT-3′; TLR4, forward, 5′-AGTCAAGGAACCCATGACAA-3′, reverse, 5′-GAGAATGACCAGGATGGTTG-3′; 18S ribosomal RNA (rRNA), forward, 5′-AACCAGACAAATCGCTCCAC-3′, reverse, 5′-GTTCCGACCATAAACGATGC-3′. The amplification conditions were as follows: 95°C (30 s), 35 cycles of 95°C (1 s), 60°C (5 s) and 72°C (10 s). Standard curves were obtained by using serial dilutions of PCR products and 18S rRNA. The amounts of TLR1, -2, -3 and -4 amplicons were normalized against 18S rRNA transcripts. In preliminary experiments, normalization against 18S rRNA transcripts was applied on the basis of lowest variability of measurements among other housekeeping genes in different cell types and experimental conditions (data not shown), a fact also reported previously [23,24]. All samples were run in duplicate in three independent experiments. The absence of non-specific PCR products was confirmed by melting curve analysis and gel electrophoresis.
Statistical analysis
The Mann–Whitney U-test was used for the comparison of data; P-values less than 0·05 were considered significant.
Results
Detection and functional assessment of TLR expression by SGEC
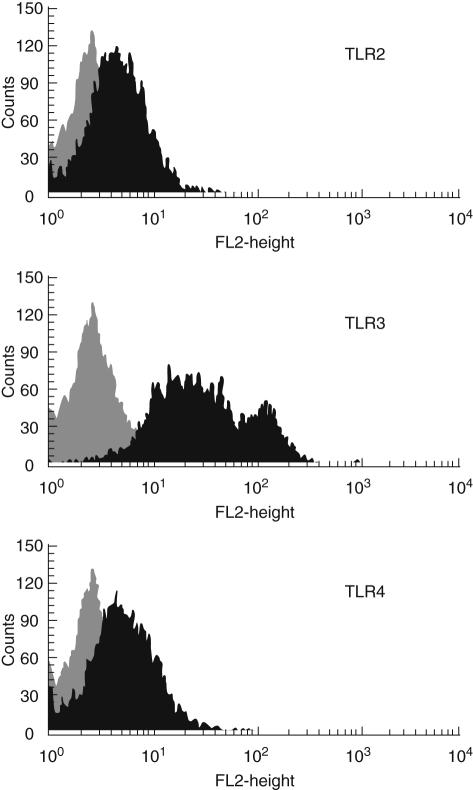
In preliminary experiments, SGEC lines derived from SS patients (SS-SGEC) and controls (control-SGEC) as well as THP-1 cells were found to display significant constitutive expression of TLR1, -2, -3 and -4 mRNA as assessed by RT–PCR analyses (data not shown), as well as of TLR2, -3 and -4 protein as assessed by flow cytometry (Fig. 1). The induction of the immunoregulatory molecules CD54/ICAM-1, CD40, CD86/B7·2, MHC-I and MHC-II expression in response to TLR triggering [8] was employed for the functional assessment of TLR expression in the various types of cells studied.
Fig. 1.
Representative demonstration of constitutive surface protein expression of Toll-like receptor (TLR)-2, - 3 and -4 in a salivary gland epithelial cell (SGEC) as detected by flow cytometry (light grey-filled plots: isotype control stainings; dark grey-filled plot surface molecule expression).
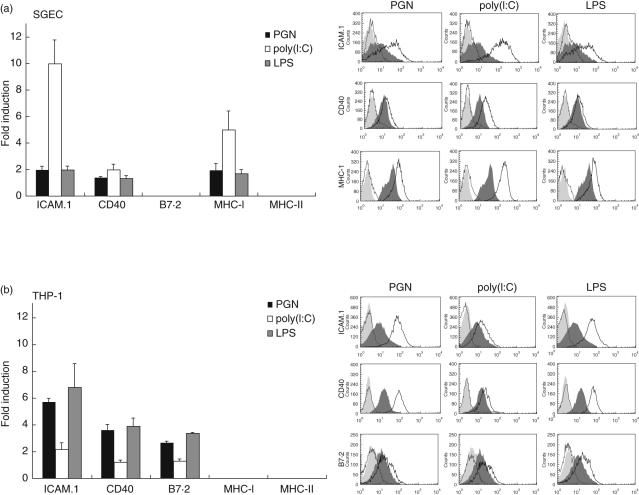
In all SGEC lines tested, TLR triggering by various ligands was found to induce the up-regulation of surface ICAM-1, CD40 and MHC-I molecules expression (but not B7·2 or MHC-II) that uniformly reached highest levels at 48 h of stimulation (Fig. 2). TLR3 triggering showed the most pronounced effect, that was largely comparable to that obtained following treatment of these cells with IFN-γ (data not shown). No significant differences were observed between SS-SGEC and control-SGEC lines (data not shown). THP-1 cells were also found to respond to stimulation of TLR2, -3 and -4 by inducing surface ICAM-1, CD40 and B7·2 expression, but not the expression of MHC-I or MHC-II molecules (Fig. 2).
Fig. 2.
Induction of intercellular adhesion molecule-1 (ICAM-1), CD40, MHC-I and B7·2 expression in salivary gland epithelial cell (SGEC) lines: (a) four control-SGEC and three SS-SGEC and (b) THP-1 cells following 48-h treatment by peptidoglycan (PGN) [Toll-like receptor-2 (TLR2) ligand, 100 µg/ml], polyinosinic:cytidylic acid (polyI:C) (TLR3 ligand, 5 µg/ml) and lipopolysaccharide (LPS) (TLR4 ligand, 1 µg/ml), as detected by flow cytometry. Left panels: fold-induction (mean ± s.e.m.) of surface molecules expression from baseline expression of untreated cells. Right panels: representative flow cytometry analyses demonstrating the inducible expression of surface molecules by TLR stimulation (dotted line and light grey-filled plots: isotype control stainings of stimulated and unstimulated cells, respectively; dark grey-filled plot: surface molecule expression on unstimulated cells; black line: surface molecule expression on stimulated cells).
In addition, triggering of cultured non-neoplastic control-SGEC lines (n = 3) by TLR2, -3 and -4 ligands was found to result in the significant up-regulation of the expression of the respective mRNA TLR molecules, as assessed by Q–PCR [mean-fold induction (±s.e.m.) over values in cells treated with medium alone; TLR2: 3·58 ± 1·44, TLR3: 2·38 ± 0·06 and TLR4: 4·19 ± 0·17].
Quantitative analysis of TLR mRNA levels in SGEC lines
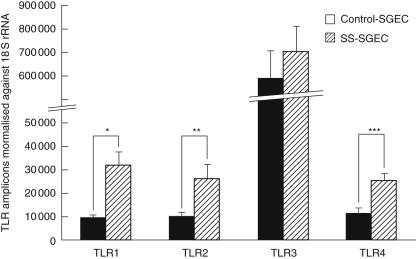
Q–PCR analysis was employed to address the level of constitutive expression of TLR1, -2, -3 and -4 mRNA in cultured SGEC lines derived from seven pSS patients and seven disease controls. Such assessment revealed statistically significantly higher levels of TLR1 (P = 0·0027), TLR2 (P = 0·01) and TLR4 (P = 0·03) mRNA in SS-SGEC, compared to control-SGEC lines, whereas both SS-SGEC and control-SGEC lines displayed statistically significantly higher TLR3 mRNA than THP-1 cells, both for P < 0·0001 (Fig. 3).
Fig. 3.
Quantitative real-time polymerase chain reaction (PCR) analysis of constitutive Toll-like receptor-1 (TLR1), -2, -3 and -4 mRNA expression in cultured non-neoplastic control-salivary gland epithelial cell (SGEC) (black bars, n = 7) and Sjögren's syndrome (SS)-SGEC lines (striped bars, n = 7). The results are presented as the amounts of TLR1, TLR2, TLR3 and TLR4 amplicons (mean ± s.e.m.) normalized against 18S rRNA transcripts. Statistical comparison of SS-SGEC versus control-SGEC; *P = 0·0027, **P = 0·01, ***P = 0·03. THP-1 cells were used as positive control cell line for the TLR mRNA expression; the relative amounts of TLR amplicons (mean ± s.e.m.) normalized against 18S rRNA transcripts were found to be: TLR1: 33 229 (± 1247), TLR2: 34 056 (± 2067), TLR3: 6305 (± 331) and TLR4: 8522 (± 376).
Discussion
The establishment of non-neoplastic SGEC lines provides a valuable means for the study of the physiology of salivary epithelia, as well as the pathophysiological role of these cells in disorders affecting the salivary glands, such as pSS [21]. Using this approach we have previously indicated the putative immunological function of SGEC, as attested by the capacity of these cells to express constitutively or following stimulation various immunoactive molecules, including antigen-presenting, costimulatory, adhesion, apoptosis-related, cytokines and chemokines [17,18]. In corroboration with this notion, the present study provides the first evidence that SGEC also express constitutively various TLR molecules. The mRNA expression of various TLR species has been reported previously in whole human salivary gland tissue containing heterogeneous cell populations [2,3]. However, to our knowledge a quantitative and functional study in human non-neoplastic salivary epithelial cell lines has never been accomplished. In fact, in this study, the actual presence of functional TLR2, -3 and -4 molecules on SGEC was confirmed by demonstrating the up-regulation of CD54/ICAM-1, CD40 and MHC-I expression on these cells in response to treatment with the reciprocal TLR ligands. In addition, as described previously in other types of cells [25], TLR triggering in SGEC was found to result in the significant up-regulation of the expression of the reciprocal mRNA TLR molecules. Also, in agreement with previous studies in monocytic cells [22,26–28], the neoplastic monocytic THP-1 cell line was also found to up-regulate ICAM-1, CD40 and B7·2 but not MHC molecule expression in response to TLR2, -3 and -4 ligands.
Probably on account of the central role of epithelial cells in tissue immunity and particularly in innate immune responses [29–32], the quantitative analysis of TLR mRNA expression revealed considerable constitutive expression of TLR1, -2, -3 and -4 in all SGEC lines tested. TLR signalling may be an essential component of salivary gland function that apparently extends beyond immune responses. In fact, signalling via TLR molecules in the intestinal epithelium has been shown to be important for the homeostasis of these tissues, as it is protective from injurious stimuli by inducing tissue reparative and cytoprotective factors [33]. Notably, as also demonstrated previously in various other types of epithelial cells [2,3], SGEC were found to display particularly elevated constitutive TLR3 expression, a fact which probably suggests epithelial tissue-specific roles of this TLR molecule.
In a similar manner to several other molecules [15,16], SS-SGEC were found to display significantly higher constitutive expression of TLR1, -2 and -4 mRNA compared to control-SGEC, a finding which lends further support to the notion of intrinsic activation of epithelia in pSS and the central role of these cells in the pathogenesis of SS [18]. Although the precise nature of intrinsic epithelial activation in pSS remains obscure, cytokines constitutively produced by SS-SGEC may be directly responsible for autocrine induction of the expression of TLR and other molecules. Nevertheless, the increased constitutive TLR expression by SS-SGEC may indicate the active participation of these molecules in the pathophysiology of pSS. Interestingly, TLR3 triggering in experimental animals has been found to induce the development of lesions mimicking human autoimmune epithelitis syndromes, including autoimmune pancreatitis, gastritis or primary biliary cirrhosis [11,12,34]. Furthermore, TLR molecules have been thought to play a role in the pathogenesis of various human autoimmune inflammatory diseases, as suggested by aberrant TLR expression in the inflamed epithelial tissues. The synovial tissues of patients with rheumatoid arthritis have been shown to express up-regulated protein levels of TLR2, -3 and -7 compared to those from patients with osteoarthritis and healthy controls [35,36]. Up-regulated expression of TLR4 and TLR2 proteins has been also found in the intestinal tissues of patients with Crohn's disease (CD) and ulcerative colitis (UC), whereas TLR3 protein expression is reportedly down-regulated in patients with CD but not in those with UC [37]. In addition, significantly higher levels of TLR3, -4 and -7 mRNA have been demonstrated recently in the liver parenchyma of patients with primary biliary cirrhosis compared to patients with autoimmune hepatitis and chronic viral hepatitis [38].
It remains unclear whether aberrantly increased expression of TLR molecules in these chronic autoimmune disorders, as well as in the epithelial cells of pSS patients, may actually denote distinct responsiveness to the specific TLR ligands. In our study, despite increased TLR expression, triggering of SS-SGEC lines with the various synthetic TLR ligands was not found to induce significantly higher expression levels of the immunoactive molecules tested; however, more thorough investigation with the application of limited amounts of TLR ligands in titrated experiments may be needed to clarify the issue.
Despite the fact that TLR2, -3 and -4 protein expression by SGEC was found readily detectable by flow cytometry, in our study the application of commercially available TLR-specific monoclonal or polyclonal antibodies has not been found suitable for the immunohistochemical evaluation of TLR expression in salivary gland epithelial tissues (data not shown). Moreover, studies are under way in order to address the differences regarding the TLR protein expression levels between SS-SGEC and control-SGEC lines.
In conclusion, the present study provides the first evidence of the constitutive expression of functional TLR molecules on cultured SGEC lines. SGEC are able to respond to stimulation by TLR2, -3 and -4 ligands via the up-regulation of certain immunoregulatory molecules expression, most probably reflecting the role of epithelial cells in tissue immunity and particularly in innate immune responses. Moreover, SGEC lines derived from patients with pSS display significantly higher constitutive expression of TLR1, -2 and -4 mRNA compared to control-SGEC, a finding that is in line with previous reports from this laboratory that indicate the incidence of intrinsic activation mechanisms in the epithelia of patients, and further supports the active participation of epithelia in SS pathogenesis.
Acknowledgments
The present study was supported by grants from the Hellenic Secretariat for Research and Technology (PENED 01ED324) and the Lilian Voudouri Foundation.
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–92. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 3.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343(5):338–44. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 5.Birchler T, Seibl R, Buchner K, et al. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31(11):3131–7. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Bocker U, Yezerskyy O, Feick P, et al. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis. 2003;18:25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 7.Hertz CJ, Wu Q, Porter EM, et al. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J Immunol. 2003;171:6820–6. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 8.Han DC, Huang GT, Lin LM, Warner NA, Gim JS, Jewett A. Expression of MHC Class II, CD70, CD80, CD86 and pro-inflammatory cytokines is differentially regulated in oral epithelial cells following bacterial challenge. Oral Microbiol Immunol. 2003;18:350–8. doi: 10.1046/j.0902-0055.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T, Takeuchi O, Fujita T, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–94. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 10.Anders HJ, Vielhauer V, Eis V, et al. Activation of Toll-like receptor-9 induces progression of renal disease in MRL-Fas (lpr) mice. Faseb J. 2004;18:534–6. doi: 10.1096/fj.03-0646fje. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Murakami H, Akbar SM, Matsui H, Onji M. A novel and effective approach of developing aggressive experimental autoimmune gastritis in neonatal thymectomized BALB/c mouse by polyinosinic:polycytidylic acid. Clin Exp Immunol. 2004;136:423–31. doi: 10.1111/j.1365-2249.2004.02467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu WM, Miyazaki T, Terada M, et al. A novel autoimmune pancreatitis model in MRL mice treated with polyinosinic:polycytidylic acid. Clin Exp Immunol. 2002;129:27–34. doi: 10.1046/j.1365-2249.2002.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moutsopoulos HM. Sjogren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–5. doi: 10.1006/clin.1994.1123. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Helu RF, Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. Induction of salivary gland epithelial cell injury in Sjogren's syndrome: in vitro assessment of T cell-derived cytokines and Fas protein expression. J Autoimmun. 2001;17:141–53. doi: 10.1006/jaut.2001.0524. [DOI] [PubMed] [Google Scholar]
- 15.Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. CD40 on salivary gland epithelial cells: high constitutive expression by cultured cells from Sjogren's syndrome patients indicating their intrinsic activation. Clin Exp Immunol. 2002;127:386–92. doi: 10.1046/j.1365-2249.2002.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapsogeorgou EK, Dimitriou ID, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Activation of epithelial and myoepithelial cells in the salivary glands of patients with Sjogren's syndrome: high expression of intercellular adhesion molecule-1 (ICAM.1) in biopsy specimens and cultured cells. Clin Exp Immunol. 2001;124:126–33. doi: 10.1046/j.1365-2249.2001.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoussakis MN, Dimitriou ID, Kapsogeorgou EK, et al. Expression of B7 costimulatory molecules by salivary gland epithelial cells in patients with Sjogren's syndrome. Arthritis Rheum. 1999;42:229–39. doi: 10.1002/1529-0131(199902)42:2<229::AID-ANR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Manoussakis MN, Kapsogeorgou EK. The role of epithelial cells in the pathogenesis of Sjogren's syndrome. Clin Rev Allergy Immunol. 2007 doi: 10.1007/s12016-007-8007-4. in press. [DOI] [PubMed] [Google Scholar]
- 19.Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. Functional expression of a costimulatory B7.2 (CD86) protein on human salivary gland epithelial cells that interacts with the CD28 receptor, but has reduced binding to CTLA4. J Immunol. 2001;166:3107–13. doi: 10.4049/jimmunol.166.5.3107. [DOI] [PubMed] [Google Scholar]
- 20.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimitriou ID, Kapsogeorgou EK, Abu-Helu RF, Moutsopoulos HM, Manoussakis MN. Establishment of a convenient system for the long-term culture and study of non-neoplastic human salivary gland epithelial cells. Eur J Oral Sci. 2002;110:21–30. doi: 10.1034/j.1600-0722.2002.00152.x. [DOI] [PubMed] [Google Scholar]
- 22.Remer KA, Brcic M, Sauter KS, Jungi TW. Human monocytoid cells as a model to study Toll-like receptor-mediated activation. J Immunol Meth. 2006;313:1–10. doi: 10.1016/j.jim.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Bas A, Forsberg G, Hammarstrom S, Hammarstrom ML. Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol. 2004;59:566–73. doi: 10.1111/j.0300-9475.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Mulero S, Montanya E. Selection of a suitable internal control gene for expression studies in pancreatic islet grafts. Transplantation. 2005;80:650–2. doi: 10.1097/01.tp.0000173790.12227.7b. [DOI] [PubMed] [Google Scholar]
- 25.Andreakos E, Foxwell B, Feldmann M. Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunol Rev. 2004;202:250–65. doi: 10.1111/j.0105-2896.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 26.Katsuno G, Takahashi HK, Iwagaki H, et al. The effect of ciprofloxacin on CD14 and toll-like receptor-4 expression on human monocytes. Shock. 2006;25:247–53. doi: 10.1097/01.shk.0000208803.50914.a2. [DOI] [PubMed] [Google Scholar]
- 27.Ryan KA, Smith MF, Sanders MK, Jr, Ernst PB. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun. 2004;72:2123–30. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang FX, Kirschning CJ, Mancinelli R, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–4. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 29.Guillot L, Le Goffic R, Bloch S, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem. 2005;280:5571–80. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- 30.Guillot L, Medjane S, Le-Barillec K, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways. evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–18. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 31.Hornef MW, Bogdan C. The role of epithelial Toll-like receptor expression in host defense and microbial tolerance. J Endotoxin Res. 2005;11:124–8. doi: 10.1179/096805105X35224. [DOI] [PubMed] [Google Scholar]
- 32.Melmed G, Thomas LS, Lee N, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host–microbial interactions in the gut. J Immunol. 2003;170:1406–15. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Okada C, Akbar SM, Horiike N, Onji M. Early development of primary biliary cirrhosis in female C57BL/6 mice because of poly I:C administration. Liver Int. 2005;25:595–603. doi: 10.1111/j.1478-3231.2005.01043.x. [DOI] [PubMed] [Google Scholar]
- 35.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, et al. The expression of Toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of Toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52:2313–22. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 36.Seibl R, Birchler T, Loeliger S, et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;16:1221–7. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–17. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takii Y, Nakamura M, Ito M, et al. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest. 2005;85:908–20. doi: 10.1038/labinvest.3700285. [DOI] [PubMed] [Google Scholar]